Abstract
Color patterns play central roles in the behavior of insects, and are important traits for taxonomic studies. Here we report striking and stable structural color patterns—wing interference patterns (WIPs)—in the transparent wings of small Hymenoptera and Diptera, patterns that have been largely overlooked by biologists. These extremely thin wings reflect vivid color patterns caused by thin film interference. The visibility of these patterns is affected by the way the insects display their wings against various backgrounds with different light properties. The specific color sequence displayed lacks pure red and matches the color vision of most insects, strongly suggesting that the biological significance of WIPs lies in visual signaling. Taxon-specific color patterns are formed by uneven membrane thickness, pigmentation, venation, and hair placement. The optically refracted pattern is also stabilized by microstructures of the wing such as membrane corrugations and spherical cell structures that reinforce the pattern and make it essentially noniridescent over a large range of light incidences. WIPs can be applied to map the micromorphology of wings through direct observation and are useful in several fields of biology. We demonstrate their usefulness as identification patterns to solve cases of cryptic species complexes in tiny parasitic wasps, and indicate their potentials for research on the genetic control of wing development through direct links between the transregulatory wing landscape and interference patterns we observe in Drosophila model species. Some species display sexually dimorphic WIPs, suggesting sexual selection as one of the driving forces for their evolution.
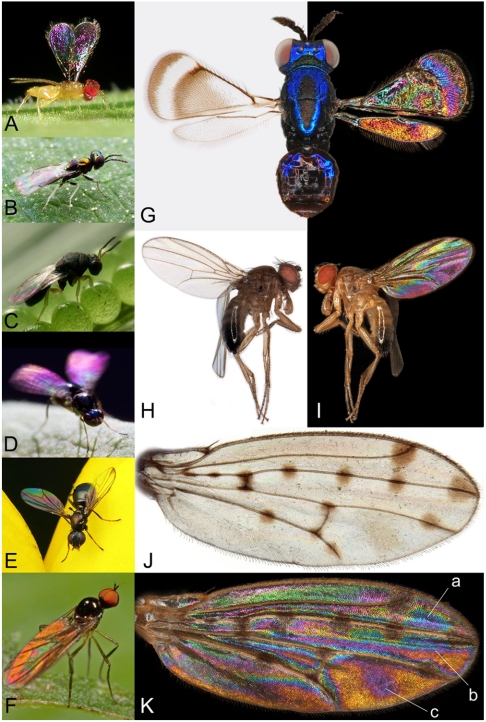
Generation of complex pigmentation patterns by insects is currently an active research front (1–3), with insights into the morphogenetic control of pigment spots in wings of a Drosophila model species (4) (Fig. 1 J and K) underpinning principles for coloration and repeated regulatory evolution that are potentially broadly applicable beyond insects (5–7). Parallel studies of structural insect colors with repeated functional morphology and multiple functions of simple structures (8–10) have recently expanded into a major research area (11–14) that is predominantly focused on larger organisms such as butterflies (14–16), beetles (17), and damselflies (18). Here we merge these two fields by showing structural wing color patterns in the transparent wings of small wasps (Hymenoptera) and flies (Diptera). Given favorable light conditions, they display a world of brightly patterned wings (Fig. 1) that are apparently unnoticed by contemporary biologists. The color patterns are the effect of thin film interference; about 20% of incoming light beams are reflected from a single extremely thin and transparent layer with a refractive index of chitin (13). The remaining 80% of the light goes through the wing. Any animal with color vision can see these color patterns when the wing reflections are not overpowered by strong background reflections. The strength of their appearance in natural conditions depends on the balance between light reflections from the wing and from the background. The intensity of the background reflections in nature varies from 0% (pitch black background, Fig. 1 A, D, and E) to 100% (pure white background or toward a light source), but will normally be similar to a green leaf, where the wing reflections are readily observed (Fig. 1 B, C, and F). In laboratory conditions most wings are studied against a white background (Fig. 1 G, H, and J), or the wings are embedded in a medium with a refractive index close to that of chitin (e.g., ref. 19). In both cases the color reflections will be faint or invisible.
Fig. 1.
WIPs in Hymenoptera and Diptera. (A–F) Examples of WIP displays under natural conditions. Note that the habit of the majority of small wasps and flies to fold their wings over each other and over the dark-colored abdomen at rest will aid to create a darker background for the wing on top. (A) This Chrysonotomyia sp. (Eulophidae) from Costa Rica exposes its forewings and displays the WIP against a black background. (B) A resting Chrysocharis sp. (Eulophidae), USA. (C) A resting Neorileya sp. (Eurytomidae), Costa Rica. (D) This Archisepsis diversiformis (Sepsidae) from Costa Rica creates a strong visual communicative signal in colors by active and specific wing movements, a typical behavior for members of the family. (E) Another unidentified Sepsidae from USA displaying a very different WIP. (F) A male Ocydromia glabricula (Hybotidae) from the Netherlands displaying its WIP in a green environment. (G) This greatly enlarged composite image of Closterocerus coffeellae (Eulophidae, female collected in Colombia) illustrates the dramatic effect of changing background reflections on WIP visibility. The left side wing displays its pigmentation pattern against a light reflecting white background whereas the right side wing displays its WIP reflection against a light absorbing black background. (H–I) A freshly killed wild male Drosophila melanogaster from Sweden shows the same effect. Only the background is changed between photos in (H) and (I) (reflected). (J–K) Right wing of the model taxon Drosophila guttifera (Drosophilidae). This wing is of the male holotype, collected in Florida and described by F. Walker in 1849 (Courtesy of NHM London). (J) The distinct spots along the veins and weak intervein color shades are currently being subjected to intensive morphogenetic research (4). (K) WIP image of the same wing as it appears simply by viewing it against a black background. A relevant question is whether the pigmentation is formed partly or mainly to control the WIP, such as the blue preapical spot (a) that is framed and demarcated by three pigment spots. The longitudinal division of the wing disc into anterior and posterior compartments associated with the regulators engrailed and hedgehog is visible as a distinct color transition (b). The intervein shade cis-regulatory element (see ref. 4) can be directly associated with the distinct magenta spot (c). (Photo A, C, and D courtesy of Kenji Nishida; B and E courtesy of Alex Wild; and F courtesy of Klaas van der Veen).
Insects are an exceedingly diverse and ancient group and their signal-receiver architecture of thin membranous wings and color vision was apparently in place before their huge radiation (20–22). The evolution of functional wings (Pterygota) that can be freely operated in multidirections (Neoptera), coupled with small body size, has long been viewed as associated with their extreme diversity (20). With selection acting to decrease the size of wing membranes that are reinforced for aerodynamic function by membrane corrugations, hair placement, and venation, there has been simultaneous reinforcement of an optically refracted and stable color reflection. This reflection, coupled with the early evolution of trichromatic UV-blue-green perception by the insect compound eye (22), has along with pigmentation (2, 4, 23), transformed wings into visual communication posters for those who can see their colors.
The color sequence reflecting from transparent insect wings was discovered and published before Darwin’s theory of evolution (24), but it has later been disregarded as a soap bubble iridescence effect, with randomly changing colors flashing over the wing surface (25). Taxonomic monographs for Hymenoptera and Diptera typically describe wings as transparent, with or without pigmented areas, but with no mention of structural color patterns (e.g., refs. 26, 27). However, we have found that these small transparent wings almost universally display stable and essentially noniridescent structural color patterns that are often taxon-specific. The patterns are visible and stable at various angles of view in live insects in nature (Fig. 1 A–F) as well as on 100-year-old dry museum specimens (Fig. 1K).
Discussion
Two-Beam Wing Interference Patterns (WIPs).
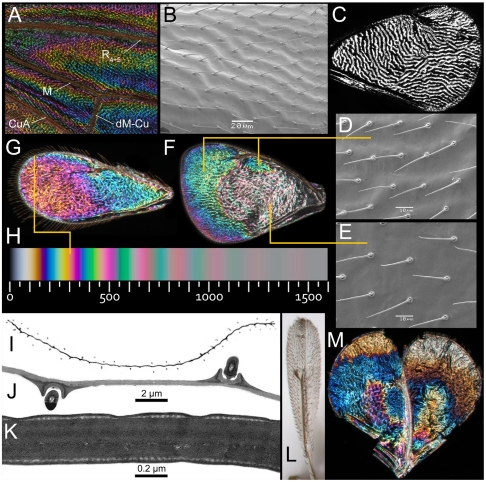
The wings of most insects are mainly composed of two layers of transparent chitin compressed to a single membrane (Fig. 2 I–K) with a refractive index of approximately 1.57 (14). In air, these dimensions are ideal for two-beam thin film interference, whereby light beams reflect from the upper and lower surfaces of the membrane (13). The thickness of the composite chitinous membrane varies with the topography of the wing, and the areas of different thickness reflect different interference colors that together produce a specific color pattern, the WIP. The sequence of colors in WIPs of Hymenoptera (Fig. 2 F, G, and M) and Diptera (Fig. 2A) is regular and identical to the Newton series reflected from a thin film of oil on water (25, 28). The Newton series is a very characteristic sequence of repeated color bands grouped into orders. The first three Newton orders (up to 550 nm wing membrane thickness, see Fig. 2H) display a near complete scale of spectral colors, except for pure red, whereas the next higher orders (with increasing wing thickness) reflect a repeated sequence of nonspectral (to the human eye) magentas and greens that gradually fade into uniform pale gray. Those of the second and third order are the brightest in the scale. This ordered color sequence makes it possible to reciprocally calculate and map membrane thickness in the range between ca. 50 and 1500 nanometres when compared with a Newton series scale (Fig. 2 A, F–H, and M).
Fig. 2.
Structural features of the wings in small chalcidoid wasps and Drosophila that produce strong noniridescent WIPs. (A) Midsection of wing membrane of D. melanogaster showing reflecting ridges along rows of microtrichia with nonreflecting troughs between them. Dense microtrichia produce a pebbled membrane surface. Vein abbreviations are taken from ref. 27. (B–C) Forewing of Chrysocharis sp. B. SEM image displaying its corrugation ridges with rows of setae. (C) Duotone image enforcing the topography of the membrane corrugations (as in a fingerprint). Note how the arrangement of corrugations gradually changes from anterior-posterior in the basal part to radial in the apical part—most likely for aerodynamic purposes and to give functional strength to the different wing parts. The corrugations simultaneously serve to reinforce the reflected WIP and eliminate the iridescence due to a dioptric stabilization of the convex ridges. (D–F) Forewing of a male Achrysocharoides latreillei demonstrating structural differentiation and the resulting WIP. (D) SEM image of corrugated parts. (E) SEM image of smooth central part. (F) Resulting WIP with colorful parts where the wing membrane is thin and corrugated, and the weakly reflecting central part where the wing membrane is thick and smooth. (G) WIP of female Asecodes congruens. The yellow line shows where the cross section was made for TEM images (J–K) and the corresponding match on the Newton scale (H). (H) Computer generated (Adobe® 1998 RGB rendered) Newton series scale of two-beam interference colors (28) calibrated for the refractive index of chitin (1.57) viewed in air at perpendicular angle of light incidence. The scale gives approximate thickness of a wing membrane in nanometres. (I) Composite duotone image of a whole apical cross section of the forewing of Achrysocharoides atys, showing the waves of sinuous corrugations with dots of crossed hairs on both sides. (J–K) TEM images of cross sections of the apical part of a forewing from the same species as in G, a freshly killed specimen was used for the TEM images to avoid possible artificial changes in the wing structure caused by drying or alcohol treatment. (J) Wing section showing how the wing membrane is uniform and extremely thin compared with two hair sockets on upper and lower membranes. (K) Enlarged section showing how the dorsal and ventral membranes are fused together to form a single thin film. The membrane thickness is between 308–317 nm, which is a perfect match with the color transition yellow-magenta as observed and in the Newton scale (compare to panels G and H). (L) Left “balloon” forewing of male Omphale sp. in frontal view. This condition sometimes occurs when recently emerged insects are killed in alcohol before the membranes are properly fused together and alcohol penetrates between them; this makes it possible to “open” the wing and observe the dorsal and ventral membranes separately. (M) Unequal organisation of dorsal (Left) and ventral (Right) membranes and resulting WIPs in an opened left forewing of a male Achrysocharoides platanoidae. The dorsal membrane produces the main WIP (as normally observed) whereas the ventral membrane has an unclear pattern that reinforces the main pattern when merged with the dorsal membrane. See Fig. 3B for a forewing of A. platanoidae in its natural condition where a distinct blue spot with yellow border is found along the apical margin. In the opened wing the color of the spot switches to yellow due to approximately half thickness of the dorsal membrane. A switch of colors occurs throughout the wing due to reduced thickness (compare to scale in panel H).
The fore- and hindwings of Hymenoptera are coupled together into one functional unit during flight and during what we suspect are WIP displays. The hindwing pattern usually forms an extension of the WIP from the forewing or sometimes displays its own characteristic details (Fig. 3), just as is the case with the pigment-based and scale-based patterns on Lepidoptera wings. In wings of small chalcidoid wasps (body length less than 3 mm) there are membrane corrugations that form regularly spaced parallel ridges about 20 μm apart (Fig. 2 B and D), with rows of setae along the tops of ridges. Diptera have only one pair of functional wings and the corrugation ridges usually occur in association with regular rows of microtrichia. These are typically spaced about 10–15 μm apart in the middle of spherical cell structures, as in Drosophila (29). The more pebbled interference patterns suggest spherical reflection around each microtrichium (Fig. 2A).
Fig. 3.
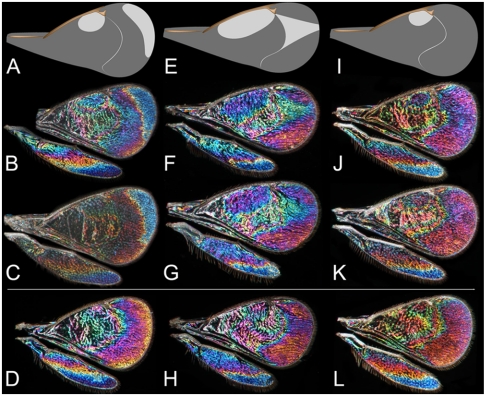
WIPs of three species of genus Achrysocharoides (Hymenoptera: Eulophidae). Male wings (above the line) and female wings (below the line). While sorting a collection of Achrysocharoides, several males with a distinct blue spot in the WIP were discovered. Further investigations revealed a case of two cryptic species (A. acerianus and A. platanoidae) and extending the investigation resolved another case of three cryptic species (A. gahani, A. robiniae, A. robinicolus) (38). All species were initially separated using male WIPs, but other morphological differences in combination with acquired new biological data confirmed the hypothesis of species delimitation. A. platanoidae and A. robiniae have sexually dimorphic WIPs despite having transparent wings without any pigmentation and from the classical point of view males and females have identical wings. (A–D) A. platanoidae. (A) Schematic illustration of the distinctive small spot in the corner between the marginal vein and the stigmal vein and the larger marginal spot along the apical edge, which is blue in the WIP. (B) WIP of male (UK, 1999). (C) WIP of male (Sweden, 2007). (D) WIP of female (Sweden, 1981). (E–H) A. robiniae; all collected in Hungary, 2002. (E) Schematic illustration of the distinctive large spot along marginal vein and the extended spot in the apical part, which is green in the WIP. (F–G) WIP of males. (H) WIP of female. (I–L) A. robinicolus; all collected in USA, 2002. (I) Schematic illustration of the distinctive small spot in the corner between the marginal and the stigmal veins and lack of pattern in the apical part. (J–K) WIP of males. (L) WIP of female.
Whereas the microstructures of the wing membrane are somewhat different in Hymenoptera and Diptera, the resulting effect is the same: essentially noniridescent coherent scattering (cf. 8). The old report (25) of highly variegated colorings randomly mingled, with housefly wing changing color as the angle of vision changes, is wrong. We find almost no iridescence unless the light is narrowly concentrated in one direction at a slight angle to the surface. The stable noniridescent patterns that we see can be explained by the convex ridges of a corrugated (Fig. 2 C and I) or pebbled (Fig. 2A) wing membrane that act as diopters to stabilize the interference reflection and eliminate the iridescence effect over a large range of light incidences (8, 9). Contrary to the iridescence of a flat thin film, the strongly microstructured wing membrane appears noniridescent, both under different ring light illuminations and in natural outdoor light.
Pigmented areas and the rigidity of wing veins contribute to stabilize the wing color pattern, contributing frames for the WIPs of different wing segments and the wing overall. The WIPs may reciprocally display the vein system and emphasize the pigment patterns (Figs. 1, 4, 5, and 6). In species with smoky or semitransparent pigmented wings, the WIP loses its characteristic metallic shine (e.g., Fig. 6G), and it may not appear if pigments are capturing the light (e.g., Fig. 5 P–R). In species with large individuals, the reticulate system of veins compartmentalizes and supports the wing such that it remains strong while simultaneously being thin enough to produce WIPs in the areas framed by the veins. For example, wings of some Braconidae and Ichneumonidae wasps display cell-specific WIPs that are different from those of other adjacent compartments (Fig. 6 N–P). As a species evolves smaller individuals, the wing vein system is commonly reduced. In the smallest wasps, but those having a wing large enough to display a WIP, the veins are confined to the anterior wing margin, leaving the wing membrane as a seemingly large empty space (about the size of a wing cell on a larger wasp or fly). To stabilize such a vein-free wing there are extensive supporting corrugations and thickenings of the membrane (Fig. 2 B–E). These features form structural patterns that display WIPs based on the three first Newton orders, which are created by membrane thickness from 100 to about 600 nm (Fig. 2H). In sum a taxon-specific WIP reflects a complex of micromorphological features of the wing (uneven membrane thickness, corrugations, setae arrangement, pigmentation, venation) framed by a specific wing shape (Fig. 5, 6).
Fig. 6.
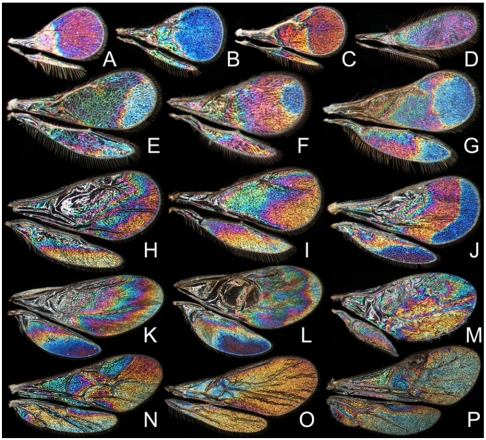
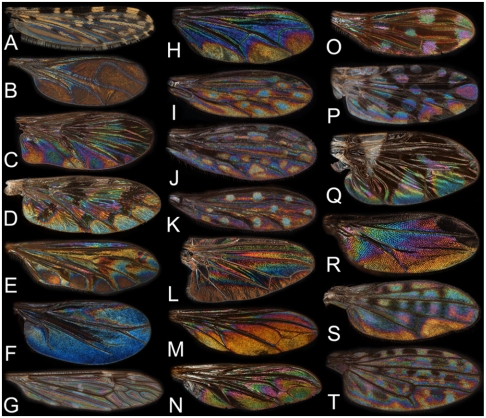
WIP diversity in small Hymenoptera. (A–C) Trichogrammatidae, three unidentified species (females, Costa Rica). (D) Mymaridae (male, Sweden). (E) Aphelinidae (female, Malaysia). (F) Aphelinidae (female, Costa Rica). (G) Aphelinidae (female, Malaysia). (H) Pteromalidae, Pteromalus sp. (male, United States). (I) Eulophidae, Omphale clypealis (female, Spain). (J) Torymidae, Idiomacromerus sp. (male, Turkey). (K) Encyrtidae, Cerchysius sp. (female, Canada). (L) Encyrtidae, Microterys sp. (female, Greece). (M) Agaonidae (female, Brazil). (N) Ichneumonidae (female, Sweden). (O) Braconidae, Dacnusiini (female, Sweden). (P) Braconidae, Meteorus sp. (female, Sweden).
Fig. 5.
WIP diversity across Diptera. The first row (A–G) displays lower Diptera (“Nematocera”), the second row (H–N) displays lower flies whereas the last row (O–T) displays higher flies (Acalyptrata). (A) Culicidae, Anopheles melas (female, Ghana). (B) Sciaridae, Zygoneura sp. (male, Japan). (C) Keroplatidae, Macrocera fascipennis (male, Sweden). (D) Keroplatidae, Proceroplatus sp. (male, Honduras). (E) Lygistorrhinidae, Lygistorrhina pictipennis (male, Japan, note that the M fork appears complete here despite the veins being gone except for apical parts of M1 and M2). (F) Scatopsidae, Swammerdamella brevicorne (female, Sweden). (G) Tipulidae, Tipula confusa (male, Sweden). (H) Dolichopodidae, Condylostylus sp. (female, Canada). (I) Empididae, Dolichocephala guttata (female, Sweden). (J) Empididae, Dolichocephala irrorata (female, Sweden). (K) Empididae, Dolichocephala ocellata (male, Sweden). (L) Platypezidae, Paraplatypeza atra (male, Sweden). (M) Pipunculidae, Eudorylas obscurus (male, Sweden). (N) Pipunculidae, Nephrocerus scutellatus (male, Sweden). (O) Diopsidae, Teleopsis rubicunda (male, Philippines). (P) Tephritidae, Actinoptera discoidea (male, Sweden). (Q) Tephritidae, Rhagoletis pomonella (male, USA). (R) Chloropidae, Chloropsina sp. (female, Malaysia). (S) Ephydridae, Paralimna sp. (female, Ghana). (T) Ephydridae, Limnellia quadrata (male, Sweden).
Genetic Control of the WIP.
The complex black pigment patterns that are repeatedly evolved in many groups of Diptera are formed and controlled by a set of spatiotemporal on/off switches for the single gene yellow (6, 7) and sometimes also involve other genes and physical wing traits (2, 4). An increasing body of evidence demonstrates direct parallels between development and regulation of wing patterns in distantly related groups such as Drosophila and butterflies (2, 7, 23, 30).
WIPs add an additional dimension and morphological diversity palette to the now emerging “repeated regulating evolution” model (5). WIPs mean that wing pigmentation (4) is only a part of the story. Other morphogenetic elements are responsible for the regulation of membrane thickness, formation of membrane corrugations, hair placement (29), venation pattern (31), and other traits. The transregulatory wing landscape (32, 33) illustrates how different genes, cis-regulatory elements (33), and wing landmarks (4) (e.g., veins, bumps, troughs, slopes, hairs) may work together to form the wing and create/stabilize the size, location and nature of a specific WIP. A specific WIP may be the analogue to a pigment field or complex that performs a specific function, such as are false eye spots (34). For example, the longitudinal division of the wing disc into the anterior-posterior compartments associated with the regulators engrailed (32) and hedgehog (23), is directly reflected in WIPs. There is a distinct color shift indicating a transition line in membrane thickness as observed in Drosophila guttifera, Drosophila melanogaster and several related species (Fig. 1K, 4 A–E, G, L, and M). In chalcidoids a proximal-apical division of the WIP is commonplace (Figs. 2 D–F and 3). In the case of “balloon wings” (Fig. 2L), where the dorsal and ventral wing membranes are unfused, there are clear differences between the WIPs of these two surfaces (Fig. 2M). The dorsal membrane is thicker and produces the actual interference pattern, whereas the ventral membrane displays a vague gradient. It appears that genetic control of the dorsal membrane has an active role in producing the WIP whereas the ventral membrane appears more passive, a parallel with the higher evolutionary rates of dorsal wing patterns found in butterflies (35).
Fig. 4.
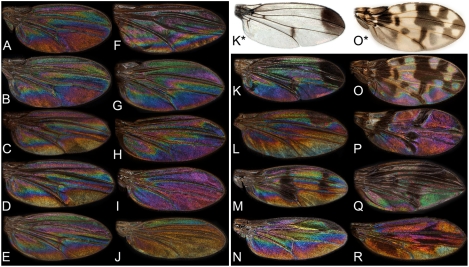
WIP diversity across Drosophilidae. With wing lengths from 1.5 to 3.5 mm, these WIPs are mainly found within the three first Newton orders; i.e. membrane thickness up to about 600 nm (compare to Fig. 2H). The left half (A–J) shows wings without pigment patterns whereas the right half (K–R) shows wings with pigment patterns. (A–B) Drosophila melanogaster (Laboratory breed Canton-S, A = male, B = female). (C–D) Drosophila obscura (Sweden, C = male, D = female). (E) Drosophila kuntzei (female, Germany). (F) Amiota magna (female, Japan). (G) Mycodrosophila gratiosa (female, Japan). (H) Sphaerogastrella javana (male, Sri Lanka). (I) Liodrosophila globosa (male, Sri Lanka). (J) Scaptomyza sp. (male, Peru). (K) Zygotricha sp. (female, Ecuador, K∗ = on white background). L. Drosophila pulchrella (male, Japan, only the males have this preapical pigment spot). (M) Chymomyza amoena (male, Canada). (N) Drosophilidae indet. (male, Sierra Leone). (O) Drosophila calloptera (female, Peru, O∗ = on white background). (P) Mycodrosophila sp. (female, Peru). (Q) Leucophenga digmasoma (female, Borneo). (R) Threocephala inornata (male, Sri Lanka).
WIP Diversity and Stability.
The majority of the more than 17,000 species of butterflies can be distinguished by their wing color patterns (16, 30), though it is also the case that many of these seemingly species-specific color patterns may be in common throughout complexes of visually “identical” sibling species (36). Our observations of WIPs suggest that species identification in many groups of Hymenoptera and Diptera is enhanced if WIPs are added to the set of taxonomic characters. These two orders are estimated to contain far more than twenty times the number of butterfly species (21, 37). This diversity remains unknown partly due to difficulties in distinguishing morphologically similar species (e.g., ref. 37), also known as “cryptic species” (which often means “not readily distinguishable by a large diurnal mammal with a microscope”). In a recent paper (38) we described cryptic species in the chalcidoid genus Achrysocharoides (wasp family Eulophidae). Five species, three of which were described as new, were initially separated by relying exclusively on distinctive male WIPs (Fig. 3) and subsequently confirmed as distinct species through finding additional differences in morphology and biology. Wings of chalcidoid wasps have long been regarded as poor in features because most species lack pigment patterns. WIPs as morphological characters will aid their identification and species discovery.
The fly family Drosophilidae ranks among the most studied organisms and displays excellent interspecific variation in WIPs (Fig. 4) and low intraspecific variation. When we compared WIPs from closely related Drosophila species, we found the overall pattern to be interspecifically similar but with distinct features for each species (Fig. 4 A–E and L). A superficial visual survey of Diptera (Fig. 5) and Hymenoptera (Fig. 6) wings encounters a diverse colorful array in all small wings (and to some degree in individual wing cells of large wings). There is a wide variety of kinds of WIPs from unicolored to elaborate patterns and spots. The claim that fly (e.g., ref. 2) and wasp wing patterns are no match for the incredible diversity of colorful butterfly wing patterns is obsolete.
WIPs, just as are other traits, are intraspecifically variable and phenotypically plastic. However, our preliminary impression is that they are largely uniform among conspecifics and often appear to be characteristic of a species, at least to the degree encountered in other insect color patterns. An evolutionary or environmentally induced change in wing size may affect the thickness of the membrane, thereby displacing the sequence of colors within the same WIP. The stable pattern may be more relevant to taxonomy and the insect than is the hue or color sequence. For example, the intraspecific variation of WIP in a sample (n = 20) from a laboratory bred Canton-S strain of D. melanogaster is small with a moderate size-dependent color displacement (Fig. 4 A and B). In this case, the wings of males had less variable WIPs than did those of females, despite the larger variation in size of male wings.
We have encountered sexual dimorphism in WIPs in species with completely transparent wings such as parasitic Achrysocharoides wasps and in those with pigment patterns (e.g., Drosophila, Fig. 4L). This dimorphism may either be a result of difference in size (usually large female, small male) affecting the hue but with the same pattern in both sexes or, the more indicative, with different patterns between the sexes (Fig. 3 A–H). The latter case, with species-specific and sexually dimorphic patterns, suggests that sexual selection is one of the driving forces for the evolution of these patterns. When the males and females of the same species have identical WIPs (Fig. 3 I–L), but differ in other external morphological characters, the WIPs can be used to match the sexes.
WIP Perspectives for Biodiversity Studies.
WIPs are an additional and overlooked trait for identifying and discovering (especially cryptic) species, just as have been DNA barcodes (e.g., ref. 36). Two-dimensional patterns on a flat wing are technically straightforward to document and analyze with pattern recognition software tools (39) and couple well with wing morphometrics (40). For phylogenetic classifications, WIPs are promising unexplored traits that can be used to visually map wing topography and measure wing membrane thickness. WIPs may reflect different types of microstructural arrangements in the wing such as nearly flat or strongly corrugated membranes and attendant membrane gradients (Fig. 5 A, S, and L). Alternatively they may independently cross over venation patterns (Fig. 5 M and R). The strong demarcation of the vein system via narrow color transitions along vein margins (e.g., Fig. 5 B and H) has been unrecognized and offers a unique functional and phylogenetic perspective to wing venation; it may even indicate the location and extension of wing veins that have been lost during evolution (Fig. 5E).
Behavioural, ecological, morphological, and evolutionary studies of insects with small wings will benefit from the discovery of WIPs in that they probably function in intra- and interspecific signaling. If so, they may be one more of the functionally dependent traits that may block evolutionary changes being driven by quite unrelated selective forces, such as wing aerodynamics, speed of wing hardening following adult eclosion, wing weight and durability. There is a definite possibility that some of the variation in membrane thickness, corrugations, pigmentations and venation reticulations has its adaptive value partly or solely in the WIPs they produce. If WIPs are truly important in the biology of insects, rather than being a byproduct of other physical traits (as is the case with inanimate oil slicks), they may in turn be one of the driving evolutionary processes affecting the nature of wing venation reticulation, with all its seemingly nonsensical variation among insects (which is commonly attributed to need for wing strength).
Wing displays play a central role in visual courtship communications in several families of Diptera (3, 19, 32, 41–44) as well as many other insects, and have been suggested as one of the drivers of the initial evolution of the insect wing (45). However, all research to date on the evolution of Diptera wings and courtship has focused solely on pigment patterns—phrased by the authors as “evolution in black and white” (3, 6, 19). Butterflies, where females may prefer males with bright structural ornamentations, emphasizing intraspecific selection as the driving force (46), reveal one intriguing difference when compared with Hymenoptera and Diptera. Whereas only a few larger species of these two orders are known to have red eye receptors, such receptors are much more common in the Lepidoptera, especially among butterflies (22), as are red butterfly scales produced by multilayer interference or red pigments (9). The Newton series color sequence displayed in single layer WIPs excludes pure red and fits most small insects’ trichromatic UV-blue-green color vision (22), including those with transparent wings. Among flies, attraction to blue and green light in the dark may be stronger than attraction to UV light and red light (47). These observations suggest that the biological significance of WIPs is for visual signaling, including intraspecific recognition by their bearers.
Some peculiar behavior involving wings in small species of Hymenoptera may be explained through WIP display. For instance, why do females of pollinating fig wasps hold their unpigmented wings straight up in the air (48), like billboards, when walking on the fig as they arrive? When the female enters the fig’s fruit-like reproductive structure (syconium) through the very tight opening (ostiole), the wings break off. A drop of liquid is excreted from the end of the abdomen and glues the wings into a protruding and visible position. This may be a species-specific signal that the syconium is now occupied, and the WIPs of these wings may be a part of the signal. Newly eclosed 2–3 mm long tropical microgastrine braconid wasps (37) raise their seemingly pattern-free transparent wings and wave them when encountering a sib while walking in the rearing container. Again, the WIPs may be part of the signal.
WIPs appear to be cheap visual signals, though wing thickness, setae, or other traits that modify a WIP may have strategic as well as materials costs. Unlike moths and butterflies, where color patterns are made with complex scales and pigments, the WIPs of transparent wasp and fly wings appear to be of low cost. For the receiver of the signal, developing and maintaining photoreceptor systems are believed to be very energy consuming and demonstrate clear trade-offs between energy consumption and performance (49, 50). Crepuscular to nocturnal insects use dim light (51) and have evolved attraction to dark or contrasting dark/white swarm markers (e.g., ref. 52). WIPs perceived by insects may be a cheap complement to the unavoidable cost of having a color-sensitive receiving system (49).
WIPs offer opportunities for evo-devo studies that connect wing biophysics and topography to morphogenetics and regulating evolution. Colorful species-specific WIPs are, in contrast with DNA barcodes from highly conserved genes (36), traits that may have major behavioural importance to the insects bearing them, as well as be serendipitous byproducts of other traits. Wasps and flies are very species-rich and small (53), and their extremely thin wings are therefore ideal for displaying WIPs. The WIP is potentially a major contribution to the toolbox for evolution of small insects with transparent wings and thus an important piece of the evolutionary puzzle.
Methods
To acquire precisely comparable data for wing thickness and taxonomy, observations of WIPs were standardised by arranging the wing horizontally against a black background and viewing it at close to perpendicular incident angles under white ring light. Photos of dry and flattened wings were then taken with a 5MP Nikon DS-L1 camera unit on Nikon stereomicroscopes (SMZ1000 and SMZ1500) fitted with ring lights. The camera unit was white-balanced for a white background before the wings were arranged against a black background and captured with the exposure compensation adjusted down by 1–2 stops. Image processing in Photoshop was restricted to cropping, adding up to 10% saturation, shading the background all black and in some cases neutralizing dust from the wing surface with the spot-healing brush tool. Except when indicated otherwise, all wings are of dry specimens from museum collections.
Acknowledgments.
We thank Sven-Axel Bengtson for inspiring discussions on the manuscript; Mary Jane West-Eberhard, Sean Carroll, Heloise Dufor, and Cedric Finet for valuable input; Dietrich Zawischa for providing his source code for the computer-generated Newton series scale; Rita Wallen for help with SEM and TEM imaging; and Stanislav Filin for support and brainstorming. The species-rich insect collections at the Museum of Zoology in Lund have been an invaluable source of identified material for this study. E.S. and J.K. are financially supported by The Swedish Taxonomy Initiative, and D.H.J. by National Science Foundation Grant DEB 0515699.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wittkopp PJ, Beldade P. Development and evolution of insect pigmentation: Genetic mechanisms and the potential consequences of pleiotropy. Semin Cell Dev Biol. 2009;20:65–71. doi: 10.1016/j.semcdb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Parchem RJ, Perry MW, Patel NH. Patterns on the insect wing. Curr Opin Genet Dev. 2007;17:300–308. doi: 10.1016/j.gde.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Wittkopp PJ, Carroll SB, Kopp A. Evolution in black and white: genetic control of pigment patterns in Drosophila. Trends Genet. 2003;19:495–504. doi: 10.1016/S0168-9525(03)00194-X. [DOI] [PubMed] [Google Scholar]
- 4.Werner T, Koshikawa S, Williams TM, Carroll SB. Generation of a novel wing color pattern by the Wingless morphogen. Nature. 2010;464:1143–1148. doi: 10.1038/nature08896. [DOI] [PubMed] [Google Scholar]
- 5.Carroll SB, Prud’homme B, Gompel N. Regulating evolution. Sci Am. 2008;2008:60–67. doi: 10.1038/scientificamerican0508-60. [DOI] [PubMed] [Google Scholar]
- 6.Prud’homme B, Gompel N, Carroll SB. Emerging principles of regulatory evolution. Proc Natl Acad Sci USA. 2007;104(Suppl 1):8605–8612. doi: 10.1073/pnas.0700488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gompel N, Prud’homme B. The causes of repeated genetic evolution. Dev Biol. 2009;332:36–47. doi: 10.1016/j.ydbio.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Prum RO, Quinn T, Torres RH. Anatomically diverse butterfly scales all produce structural colors by coherent scattering. J Exp Biol. 2006;209:748–765. doi: 10.1242/jeb.02051. [DOI] [PubMed] [Google Scholar]
- 9.Berthier S. Iridescences. The Physical Colors of Insects. New York: Springer; 2007. [Google Scholar]
- 10.Kinoshita S. Structural Colors in the Realm of Nature. Singapore: World Scientific Publishing; 2008. [Google Scholar]
- 11.Parker AR. 515 million years of structural color. J Opt A-Pure Appl Op. 2000;2:R15–R28. [Google Scholar]
- 12.Vukusic P, Sambles JR. Photonic structures in biology. Nature. 2003;424:852–855. doi: 10.1038/nature01941. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita S, Yoshioka S, Miyazaki J. Physics of structural colors. Rep Prog Phs. 2008;71:076401. doi: 10.1088/0034-4885/71/7/076401. [DOI] [Google Scholar]
- 14.Stanislav NG, editor. Functional Surfaces in Biology. Little Structures with Big Effects. Vol 1. The Netherlands: Springer; 2009. p. 384. [Google Scholar]
- 15.Ghiradella H. Light and color on the wing: structural colors in butterflies and moths. Appl Optics. 1991;30:3492–3500. doi: 10.1364/AO.30.003492. [DOI] [PubMed] [Google Scholar]
- 16.Beldade P, Brakefield PM. The genetics and evo-devo of butterfly wing patterns. Nat Rev Genet. 2002;3:442–452. doi: 10.1038/nrg818. [DOI] [PubMed] [Google Scholar]
- 17.Seago AE, Brady P, Vigneron J-P, Schultz TD. Gold bugs and beyond: A review of iridescence and structural colour mechanisms in beetles (Coleoptera) J R Soc Interface. 2008;6(Suppl 2):S165–S184. doi: 10.1098/rsif.2008.0354.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vukusic P, Wootton RJ, Sambles JR. Remarkable iridescence in the hindwings of the damselfly Neurobasis chinensis chinensis(Linnaeus) (Zygoptera: Calopterygidae) P Roy Soc B-Biol Sci. 2004;271:595–601. doi: 10.1098/rspb.2003.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards KA, Doescher LT, Kaneshiro KY, Yamamoto D. A database of wing diversity in the Hawaiian Drosophila. PLoS One. 2007;2:1–12. doi: 10.1371/journal.pone.0000487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley R. The Biomechanics of Insect Flight: Form, Function, Evolution. Princeton: Princeton University Press; 2000. [Google Scholar]
- 21.Gaston KJ. The magnitude of global insect species richness. Conserv Biol. 1991;5:283–296. [Google Scholar]
- 22.Briscoe AD, Chittka L. The evolution of color vision in insects. Ann Rev Entomol. 2001;46:471–510. doi: 10.1146/annurev.ento.46.1.471. [DOI] [PubMed] [Google Scholar]
- 23.North G, French V. Insect wings: Patterns upon patterns. Curr Biol. 1994;4:611–614. doi: 10.1016/s0960-9822(00)00133-0. [DOI] [PubMed] [Google Scholar]
- 24.Goureau M. On the iridescence of the wings of insects (Translated from French) Ann Soc Entomol Fr, 2nd series. 1843;1:201–215. [Google Scholar]
- 25.Mason CW. Structural colors in insects. II. J Phys Chem. 1926;31:321–354. [Google Scholar]
- 26.Gauld I, Bolton B. The Hymenoptera. Oxford: Oxford University Press; 1988. [Google Scholar]
- 27.Bachli G, Vilela CR, Andersson Escher S, Saura A. The Drosophilidae (Diptera) of Fennoscandia and Denmark. Fauna ent Scand. 2004;39:1–362. [Google Scholar]
- 28.Zawischa D. What are the causes of colour? Institut für Theoretische Physik, Universität Hannover. Available at http://www.itp.uni-hannover.de/~zawischa/ITP/origins.html.
- 29.Guild GM, Connelly PS, Ruggiero L, Vranich KA, Tilney LG. Actin filament bundles in Drosophila wing hairs: Hairs and bristles use different strategies for assembly. Mol Biol Cell. 2005;16:3620–3631. doi: 10.1091/mbc.E05-03-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll S, et al. Pattern formation and eyespot determination in butterfly wings. Science. 1994;265:109–114. doi: 10.1126/science.7912449. [DOI] [PubMed] [Google Scholar]
- 31.Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Bi. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- 32.Prud’homme B, et al. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature. 2006;440:1050–1053. doi: 10.1038/nature04597. [DOI] [PubMed] [Google Scholar]
- 33.Gompel N, Prud’homme B, Wittkopp PJ, Kassner VA, Carroll SB. Chance caught on the wing: cis-regulatory evolution and the origin of pigment patterns in Drosophila. Nature. 2005;433:481–487. doi: 10.1038/nature03235. [DOI] [PubMed] [Google Scholar]
- 34.Janzen DH, Hallwachs W, Burns JM. A tropical horde of counterfeit predator eyes. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.0912122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver JC, Robertson KA, Monteiro A. Accommodating natural and sexual selection in butterfly wing pattern evolution. P Roy Soc B-Biol Sci. 2009;276:2369–2375. doi: 10.1098/rspb.2009.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Janzen DH, et al. Integration of DNA barcoding into an ongoing inventory of complex tropical biodiversity. Mol Ecol Resour. 2009;9:1–26. doi: 10.1111/j.1755-0998.2009.02628.x. [DOI] [PubMed] [Google Scholar]
- 37.Smith MA, et al. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc Natl Acad Sci USA. 2008;105:12359–12364. doi: 10.1073/pnas.0805319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansson C, Shevtsova E. Three new species of Achrysocharoides Girault (Hymenoptera: Eulophidae) parasitoids of Phyllonorycter spp. (Lepidoptera: Gracillariidae) on Acer platanoides and Robinia pseudoacacia. Zootaxa. 2010;2388:23–43. [Google Scholar]
- 39.Bhanu B, Li R, Heraty J, Murray E. Automated classification of skippers based on parts representation. Am Entomol. 2008;54:228–231. [Google Scholar]
- 40.Favret C. Wing morphometry helps diagnose cryptic species and resurrect Mindarus pinicolus (Hemiptera: Aphididae) Ann Entomol Soc Am. 2009;102:970–981. [Google Scholar]
- 41.Arthur WE. Functional aspects of Drosophila courtship. Biol Rev. 1983;58:275–292. [Google Scholar]
- 42.Briceno RD, Eberhard WG. Decisions during courtship by male and female medflies (Diptera, Tephritidae): Correlated changes in male behavior and female acceptance criteria in mass-reared flies. Fla Entomol. 2002;85:14–31. [Google Scholar]
- 43.Buschbeck EK, Hoy RR. Visual system of the stalk-eyed fly, Cyrtodiopsis quinqueguttata (Diopsidae, Diptera): an anatomical investigation of unusual eyes. J Neurobiol. 1998;37:449–468. doi: 10.1002/(sici)1097-4695(19981115)37:3<449::aid-neu10>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 44.Zimmer M, Diestelhorst O, Lunau K. Courtship in long-legged flies (Diptera: Dolichopodidae): Function and evolution of signals. Behav Ecol. 2003;14:526–530. [Google Scholar]
- 45.Alexander RD, Brown WL., Jr Mating behavior and the origin of insect wings. Occasional Papers of the Museum of Zoology, University of Michigan. 1963;628:1–19. [Google Scholar]
- 46.Kemp DJ. Female butterflies prefer males bearing bright iridescent ornamentation. P Roy Soc B-Biol Sci. 2007;274:1043–1047. doi: 10.1098/rspb.2006.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stringer IAN, Meyer-Rochow VB. Attraction of flying insects to light of different wavelengths in a Jamaican cave. Mémoires de Biospéologie. 1994;21:133–139. [Google Scholar]
- 48.Michaloud G, Devez AR. Vanves, France: SRFS; 1982. Pollination ecology in tropical figs—A case of mutualism. 26′ DVD film. [Google Scholar]
- 49.Niven JE, Anderson JC, Laughlin SB. Fly photoreceptors demonstrate energy-information trade-offs in neural coding. PLoS Biol. 2007;5:e116. doi: 10.1371/journal.pbio.0050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J Exp Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- 51.Kelber A, Roth LSV. Nocturnal colour vision—Not as rare as we might think. J Exp Biol. 2006;209:781–788. doi: 10.1242/jeb.02060. [DOI] [PubMed] [Google Scholar]
- 52.Diabaté A, et al. Spatial swarm segregation and reproductive isolation between the molecular forms of Anopheles gambiae. P Roy Soc B-Biol Sci. 2009;276:4215–4222. doi: 10.1098/rspb.2009.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siemann E, Tilman D, Haarstad J. Insect species diversity, abundance, and body size relationships. Nature. 1996;380:704–706. [Google Scholar]