Abstract
Retinoic acid is a potent differentiation and antiproliferative agent of breast cancer cells, and one of its receptors, retinoic acid receptor β (RARβ), has been proposed to act as a tumor suppressor. In contrast, we report herein that inactivation of Rarb in the mouse results in a protective effect against ErbB2-induced mammary gland tumorigenesis. Strikingly, tissue recombination experiments indicate that the presence of Rarb in the stromal compartment is essential for the growth of mammary carcinoma. Ablation of Rarb leads to a remodeling of the stroma during tumor progression that includes a decrease in angiogenesis, in the recruitment of inflammatory cells, and in the number myofibroblasts. In agreement with this finding, we observed that a markedly reduced expression of chemokine (C-X-C motif) ligand 12 (Cxcl12) in the stroma of Rarb-null mice is accompanied by a decrease in the CXCL12/chemokine C-X-C receptor 4 (CXCR4)/ErbB2 signaling axis in the tumors. Relevance to the human disease is underlined by the finding that gene-expression profiling of the Rarb-deficient mammary stromal compartment identified an ortholog RARβ signature in human microdissected breast tissues that differentiates tumor from normal stroma. Our study thus implicates RARβ in promoting tumorigenesis and suggests that retinoid-based approaches for the prevention and treatment of breast cancer should be redesigned.
Keywords: oncogene, retinoid, stromal derived factor 1
Retinoic acid (RA), a biologically active metabolite generated from dietary vitamin A, acts in a hormone-like manner to regulate cell differentiation and death, and thus plays major roles in embryonic development and tissue remodeling (1–3). RA regulates biological processes by modulating the expression of vast gene networks following activation of several members of the nuclear receptor superfamily, most prominently retinoic acid receptors α (RARα, NR1B1), β (RARβ, NR1B2), and γ (RARγ, NR1B3) (4–7). Another biologically and clinically important attribute of RA and other natural and synthetic derivatives of vitamin A, collectively referred to as “retinoids,” is their potent inhibitory effect on the growth and survival of cancer cells originating from various tissues, including the breast (8). In particular, a large number of studies have shown that RA inhibits the growth of mammary carcinoma cells in culture and in animal models, a process that generally involves the induction of both apoptosis and cell-cycle arrest (9–15). In addition, evidence indicates that RARβ may function as a tumor suppressor in breast cancer (16).
The development and maintenance of the mammary gland requires the permissive and supportive environment provided by the stromal compartment, and, conversely, abnormal stromal components can promote breast tumor growth (17, 18). In particular, myofibroblasts or cancer-associated fibroblasts (CAFs), the main cellular component of tumor stroma (19), have been implicated in promoting tumor growth, angiogenesis, and metastasis (20). The protumorigenic effect of CAFs is believed to result from the production of growth, chemotactic, and angiogenic factors. Among those diffusible molecules, chemokine (C-X-C motif) ligands 12 (CXCL12, stromal derived factor-1, SDF-1) and 14 (CXCL14) appear to play major roles in this process, because the two chemokines have been implicated as regulators of cell proliferation, differentiation, migration, and invasion as well as supporting tumor angiogenesis (20–22). CXCL12 expression also has been shown to be under estrogenic control (23) and to transactivate the oncogenic protein tyrosine kinase ERBB2 via a pathway involving the chemokine C-X-C receptor 4 (CXCR4) and Src kinase activation (24). It thus is believed that CXCL12 secreted by stromal myofibroblasts exerts a considerable influence on the growth of breast cancer cells expressing CXCR4. CXCL14 also is a gene included in a 26-stromal gene predictor that forecasts clinical outcome in breast cancer with better accuracy than signatures derived from whole tumors (25).
Most studies of the mechanisms by which breast cancer cells respond to RA have been done with immortalized cells in culture. It is clear that RA possesses potent antiproliferative activity on breast cancer cells and that the three RARs act cooperatively as a potent tumor-suppressor–signaling pathway. However, RA and other retinoids have not shown effectiveness in preventing and treating breast cancer in women (26, 27), and reports of a beneficial effect of RA treatment on ErbB2-induced mammary tumorigenesis in mice have not been consistent (5, 14, 15). In addition, dietary precursors of RA can promote rather than inhibit cell survival and growth as well as increase the incidence of certain cancers in mouse models and human subjects (28–32). Together, these observations suggest that the role played by RA and its receptors in the initiation and progression of mammary gland carcinomas is more complex than currently envisaged. In the present study, we sought to elucidate the tumor-suppressor activity of RARβ in the context of the whole organism. Our results reveal a role for stromal RARβ in promoting mammary gland tumorigenesis.
Results
RARβ Is Required for Normal Temporal Development of the Mammary Gland.
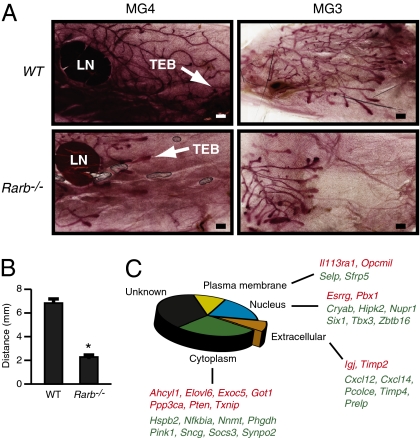
We first investigated whether ablation of RARβ had an influence on the early stages of mammary gland development. Whole-mount staining of mammary gland showed that, 40 d after birth, ablation of Rarb resulted in a delay of gland duct development (Fig. 1 A and B). As could be expected, this delay was transient, because mammary tree outgrowth was found to be normal, and the gland was fully functional at later stages of development, as previously observed (33). Because of the important role played by the stroma in all stages of mammary gland development (34), we next profiled the transcriptome of mammary stroma isolated from 40-d-old wild-type and Rarb−/− females. This analysis identified 72 up-regulated and 96 down-regulated genes in the Rarb−/− mammary gland (greater than log2 0.75 at P < 0.05; Table S1, GSE19925). Functional annotation of the genes responsive to the absence of RARβ using the Ingenuity Pathways Analysis software identified several genes associated with tumor morphology and breast cancer (Fig. 1C and Table S2). Among those genes, we noted down-regulation of Cxcl12 and Cxcl14, which encode potent chemokines that affect breast cancer cell growth.
Fig. 1.
Ablation of Rarb results in a delay of gland duct development and changes in the stromal transcriptome. (A) Mammary whole-mount staining showed that ablation of Rarb results in a delay of mammary tree development in 40-d-old Rarb−/− mice compared with their wild-type siblings. LN, lymph node; MG3 and MG4, mammary glands 3 and 4; TEB, terminal end bud. (Scale bars: 500 μm.) (B) Quantification of the distance between the terminal end buds and the lymph node in wild-type and Rarb−/− mammary gland 4. *P < 0.001. (C) Intracellular localization of genes known to be associated with the cancer phenotype and differentially expressed in Rarb-null stroma. Red, up-regulated genes; green down-regulated genes.
Global Ablation of Rarb in Mice Suppresses ErbB2-Induced Mammary Gland Tumorigenesis.
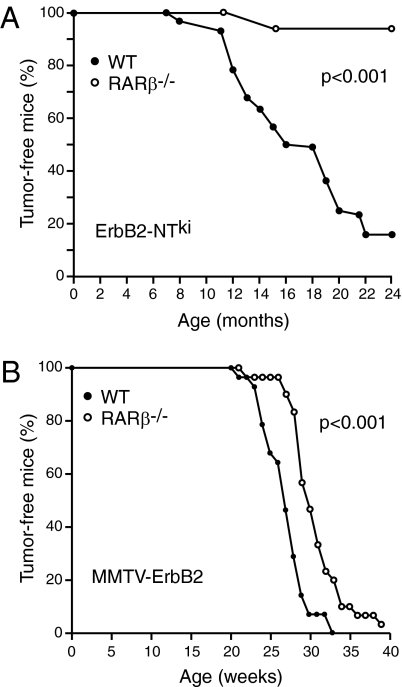
We next used two distinct and well-characterized mouse models of human breast cancer expressing oncogenic forms of ErbB2 in combination with the Rarb-null allele to test the hypothesis that RARβ can act as a tumor suppressor in vivo. In the first model, knocked-in cDNA encoding activated ErbB2 (neuNTki) is expressed under the control of its own promoter following activation by Cre recombinase (35). This model also displays a striking similarity with the human disease with respect to genomic and transcriptional aberrations (36). In the second model, the expression of an activated ErbB2 (neuNDL2-5) is under the control of mouse mammary tumor virus (MMTV) LTR and thus is restricted mainly to the mammary epithelium (37). Unexpectedly, Rarb−/− mice were completely resistant to mammary tumors induced by activated ErbB2 when expressed from its own promoter (ErbB2-NTki; Fig. 2A). In addition, Rarb−/− mice show a significant delay (∼6 wk; P < 0.001) in tumor formation when strong expression of the ErbB2 oncogene was driven by the MMTV promoter (MMTV-ErbB2; Fig. 2B). These observations indicate that, in the context of the whole organism, RARβ is not a tumor suppressor but rather that its presence is required for the full oncogenic potential of ErbB2.
Fig. 2.
Ablation of Rarb suppresses ErbB2-induced mammary tumorigenesis. (A) Rarb−/− mice are resistant to mammary tumors induced by activated ErbB2 when expressed from its own promoter (n = 23 Rarb−/− and 42 wild-type mice). (B) Rarb−/− mice show a significant delay (P < 0.001 log-rank test) in tumor formation when the expression of ErbB2 is driven by the MMTV-LTR promoter (n = 30 Rarb−/− and 27 wild-type mice).
Characteristics of the Tumor Cells and Surrounding Stroma in the Rarb−/− Mice.
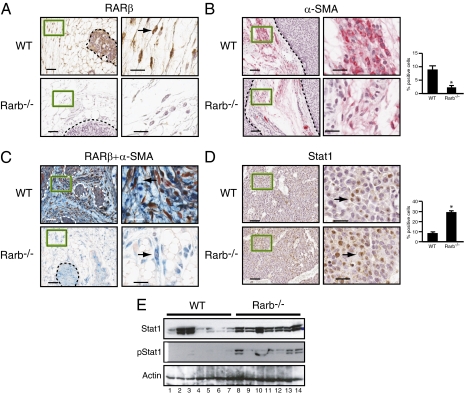
Examination of mammary gland sections taken from MMTV-ErbB2 mice by immunohistochemistry (IHC) showed that RARβ is expressed in both tumor cells and stromal fibroblasts (Fig. 3A). Histological examination also showed that the tumors retain the characteristic cribiform pattern normally observed in MMTV-ErbB–induced tumors. Labeling with an antibody against the myofibroblast marker smooth muscle actin α (α-SMA) showed that the number of α-SMA–positive fibroblasts was significantly decreased in RARβ-null tumors compared with wild-type tumors (Fig. 3B). Double labeling with antibodies against RARβ and α-SMA demonstrated that RARβ (nucleus) is found in α-SMA–positive cells (cytoplasm) but that not all α-SMA–positive fibroblasts express RARβ (Fig. 3C). We excluded the possibility that alteration in the number of mesenchymal stem cells (MSCs) in the bone marrow may be responsible for the observed changes in CAF content, because flow cytometric analysis of the bone marrow revealed equivalent MSC numbers in the bone marrow of RARβ-null mice and of wild-type animals (Fig. S1 A and B). These results indicate that stromal RARβ is expressed in a subpopulation of myofibroblasts and that loss of RARβ dampens their expansion and activity. Further analyses by IHC and Western blotting showed a significant decrease in Ki67 labeling in MMTV-ErbB2/Rarb−/− tumor cells (Fig. S2A), and this decrease was accompanied by a substantial increase in activated signal transducer and activator of transcription 1 (Stat1) levels (Fig. 3 D and E), TUNEL staining, and caspase-3 activation (Fig. S2 B and C). Furthermore, collagen I staining that marks matrix stiffness and is associated with malignant growth and invasion (38) was significantly lower in RARβ-null peritumoral stroma than in wild-type stroma (Fig. S3A). The microenvironment of the MMTV-ErbB2/Rarb−/− tumors also was distinguished by decreased angiogenesis and infiltration of inflammatory cells as assessed by CD31 and CD45 staining, respectively (Fig. S3 B and C). However, these decreases were independent of a homeostatic function of RARβ on the immune system, because RARβ deficiency did not impact the frequencies or absolute numbers of cells of the myeloid and lymphoid compartments (Fig. S4). Taken together, these observations indicate that RARβ expression in a subpopulation of α-SMA–positive CAFs is necessary for their maintenance as well as for their tumor-promoting functions in the tumor microenvironment.
Fig. 3.
Ablation of Rarb affects the composition of the stroma. (A) Paraffin-embedded sections derived from MMTV-ErbB2 tumors in the wild-type and Rarb−/− backgrounds stained for RARβ. (B) Paraffin-embedded sections stained for α-SMA (red, cytoplasmic). Quantification of positive cells is shown on the right (*P < 0.001). (C) Paraffin-embedded sections costained for RARβ (red, nuclear) and α-SMA (blue, cytoplasmic). (D) Paraffin-embedded mammary tumor sections stained for Stat1. Quantification of positive cells is shown on the right (*P < 0.001). Arrows in A, C, and D point to positive cells. [Scale bars (A–D): 50 μm for low magnification (Left) and 20 μm for high magnification (Right).] (E) Western blot analysis of Stat1 and phosphorylated Stat1 (pStat1).
Lack of Tumor Growth in the Inguinal Mammary Cleared Fat Pad of Host Rarb−/− Mice.
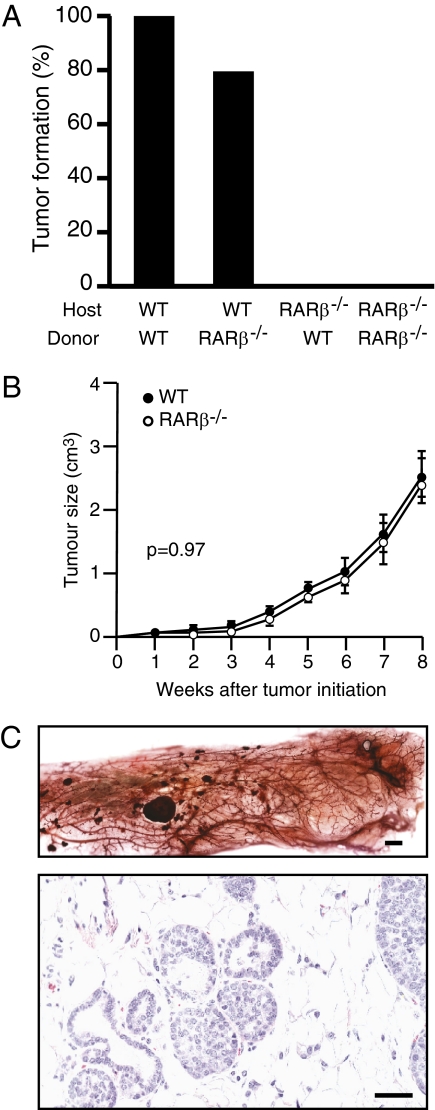
Given the known role played by CAFs in stimulating tumor-cell proliferation, we then investigated whether the protective effect against ErbB2-induced tumorigenesis observed in the absence of Rarb results from an intrinsic lack of action of RARβ in the surrounding stroma. Strikingly, experiments transplanting mammary gland epithelium showed that no tumor discernable by palpation could be found when fat pads of Rarb−/− mice were used as the host tissue, whether the transplanted epithelia came from MMTV-Erbb2 or MMTV-Erbb2/Rarb−/− donors (Fig. 4A). In contrast, mice developed mammary tumors with the same frequency and latency when the fat pads of MMTV-Erbb2 mice were used as host tissue, whether the donor epithelia originated from MMTV-Erbb2 or MMTV-Erbb2/Rarb−/− mice. Donor mice carrying the activated ErbB2 transgene served as a control for the transplantation experiments and confirmed the finding that ablation of Rarb results in a delay in tumor formation. Indeed, the time for 50% of mice developing mammary tumors was 27.7 wk for wild-type animals and 33.1 for Rarb−/− mice (Fig. S5), periods consistent with results of our original experiment (Fig. 2B). The tumors observed in the Rarb−/−-null background did not reach the full size of the tumors growing in the wild-type background (Fig. S5, Inset). On the other hand, we observed no significant difference (P = 0.9673; two-way ANOVA) between the tumor growth rates when tumor cells with either Rarb−/− or wild-type genotypes were transplanted into the inguinal mammary cleared fat pad of wild-type hosts (Fig. 4B). Although tumor formation (as monitored by palpation) did not take place when Rarb−/− host fat pads were used in epithelia transplantation experiments, repopulation of the mammary tree accompanied by increased side-branching of mammary glands was observed in the Rarb−/− host mice (Fig. 3C). Interestingly, small ductal carcinoma in situ (DCIS)-like lesions were present in all host mice (Fig. 3C), suggesting that the absence of RARβ did not impair tumor initiation by ErbB2 in the epithelium but rather affected subsequent tumor growth and progression.
Fig. 4.
Lack of tumor progression and growth in the inguinal mammary cleared fat pad of host Rarb−/− mice. (A) No tumor growth is detected in the Rarb−/− host (n = 10). (B) No significant difference (P = 0.9673, two-way ANOVA) was seen between the tumor growth rates in the tumor cells with Rarb−/− (n = 9) or wild-type (n = 10) genotype when the transformed epithelia were transplanted into the inguinal mammary cleared fat pad of the wild-type hosts. (C) Repopulation of the mammary tree is observed in the host cleared fat pad of Rarb−/− mice transplanted with MMTV-ErbB2 wild-type epithelia together with preneoplastic lesions (Upper). Paraffin-embedded section derived from the host cleared fat pad of Rarb−/− mice transplanted with MMTV-ErbB2 wild-type epithelia showing the presence of DCIS-like tumors (Lower). [Scale bars: 500 μm (Upper) and 50 μm (Lower).]
SDF-1/CXCR4/Src/ErbB2 Signaling Pathway Is Down-Regulated in the MMTV-ErbB2/Rarb−/− Tumors.
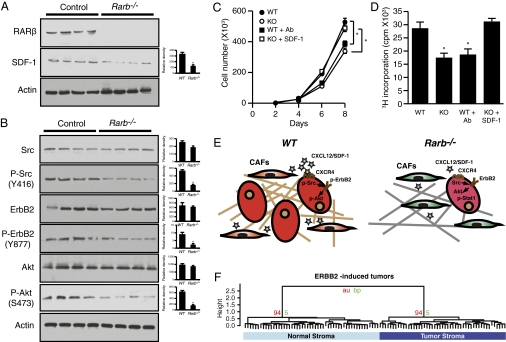
The significant remodeling of the tumor microenvironment was accompanied by a noticeable decrease in the expression of Cxcl12. This observation is consistent with the knowledge that CXCL12/SDF-1 is produced by CAFs (21). Western blot analysis using protein lysates obtained from tumors derived from MMTV-ErbB2/Rarb−/− mice indeed showed a decrease in endogenous CXCL12/SDF-1 (Fig. 5A). Because binding of SDF-1 to CXCR4 can lead to transactivation of ErbB2 via Src kinase activation (24), we next investigated whether the reduced levels of SDF-1 had an impact on CXCR4-dependent signaling in mammary tumors. Indeed, Western blot analysis of protein lysates from tumors obtained from MMTV-ErbB2/Rarb−/− mice demonstrated a decrease in phospho-specific forms of Src (SrcY416), ErbB2 (ErbB2Y877), and Akt (AktS473) (Fig. 5B). These observations were validated further by exposing the ErbB2-transformed TM15 mammary tumor cell line (39) to conditioned media obtained from immortalized fibroblasts derived from the mammary glands of wild-type or RARβ-null mice. As shown in Fig. 5 C and D, TM15 cells cultured in the presence of conditioned media from RARβ-null fibroblasts displayed both a slower growth rate and reduced proliferation as monitored by incorporation of 3H-thymidine. Furthermore, immunoneutralization of SDF-1 in the conditioned medium derived from wild-type fibroblasts reduced cellular growth rate and proliferation, whereas supplementation of SDF-1 to the conditioned medium obtained from RARβ-null fibroblasts reversed the slow growth/proliferation phenotype. A schematic representation of these findings, which are consistent with the known role of the Cxcl12/Cxcr4 signaling pathway in regulating ErbB2 activity (40), is shown in Fig. 5E.
Fig. 5.
The SDF-1/CXCR4/ErbB2 signaling pathway is down-regulated in MMTV-ErbB2/Rarb−/− tumors, and the Rarb-null signature is represented in breast cancer stroma. (A) (Left) Western blot analysis of RARβ and SDF-1 expression in lysates derived from MMTV-ErbB2 tumors in the wild-type and Rarb−/− backgrounds. (Right) Quantification of relative expression of each protein. *P < 0.01. (B) (Left) Western blot analysis of total and phosphorylated SRC, ErbB2, and Akt in lysates derived from MMTV-ErbB2 tumors in the wild-type and Rarb−/− backgrounds. (Right) Quantification of relative expression of each protein. *P < 0.01. (C) Growth curves of TM15 cells cultured in the presence of conditioned media derived from wild-type or RARβ-null mammary fibroblasts and immunoneutralized with an antibody against SDF-1 or supplemented with SDF-1. *P < 0.01. (D) Proliferation of TM15 cells cultured as in panel C. *P < 0.01 compared with wild type. (E) Schematic representation of perturbed signaling pathways in ErbB2-induced tumors and their microenvironments in wild-type and Rarb−/− mice. Stars represent relative amount of CXCL12/SDF-1. (F) Dendrogram for the 106 ortholog RARβ gene set in the human microdissected stroma samples showing a perfect split between tumor and normal stroma. The stability of the clustering was assessed with an Approximately Unbiased (au) value of 0.94 after 10,000 bootstrap (bp, bootstrapping probability) resamples. *P < 0.001.
Rarb-Null Stromal Gene Set Separates Tumor from Normal Stroma in Human Breast Tumors.
To establish the relevance of these findings to human breast cancer, we compared the mouse mammary stromal Rarb-null expression signature (Table S1) with the expression signatures derived from laser-captured tumor stroma (49 samples) and adjacent normal stroma (52 samples) obtained from patients with breast cancer (25). As shown in Fig. 5F, the dendrogram for the 106-ortholog Rarb gene set represented in the expression platform used (Agilent) for the analysis of the human samples indicates a perfect split between human normal and tumor stroma; moreover, this split is reproducible at 94% by bootstrapping resampling. Similarly, the heat map generated from the human stroma data set showed that this 106-gene subset derived from the mouse Rarb-null signature was sufficient to distinguish normal from tumor stroma in all patients. In particular, we could show that this list has surprisingly many genes that distinguish normal stroma from tumor (Fig. S6 A and B). Finally, the expression of RARB itself, but not of RARA, has the ability to distinguish human normal from tumor stroma [false-discovery rate (FDR) adjusted two-sided Wilcox test P values: RARB = 7.447 × 10−6 and RARA = 0.2312].
Discussion
The antiproliferative effects of retinoids and their receptors have been studied extensively and validated in isolated breast tumor cells in culture. In particular, previous studies with breast cancer cells have shown that RARβ possesses many of the functional characteristics of a tumor suppressor. However, it now is recognized that the growth of malignant tumors is dependent on complex cell–cell interactions between tumor cells and the neighboring stroma. Whether RA signaling mediates some form of communication between the stroma and tumor cells remains essentially unresolved. Using well-defined mouse models of human breast cancer and the RARβ-null mice in combination with experiments in transplanting mammary gland epithelium, we show that RARβ in the stroma has a dominant role in promoting the growth of epithelial mammary tumors and that this role, in large part, is mediated through the CXCL12/CXCR4/ErbB2 signaling pathway.
We first demonstrate that global loss of RARβ in mice results in a transient delay in postnatal mammary gland development and changes the transcriptome profile of the stroma. Unexpectedly, a complete or substantial decrease in mammary gland tumorigenesis is observed when the RARβ-null mice are exposed to the oncogenic insult of activated ErbB2 in two well-characterized mouse models of human breast cancer (35, 37). Tissue recombination experiments convincingly demonstrate that the presence of Rarb in the stroma is essential for the growth and progression of mammary carcinoma. Indeed, ErbB2-induced tumors in the Rarb−/− background display decreased cell proliferation, angiogenesis, collagen levels, recruitment of inflammatory cells, and chemokine expression. Furthermore, they exhibit enhanced apoptosis and elevated levels of activated Stat1, a suppressor of the neoplastic behavior (41, 42). Taken together, our results indicate that RARβ expression in the stroma shapes the tumor microenvironment favoring tumor growth and invasion. Accordingly, the composition of the RARβ-null stroma lacks such a microenvironment and associated factors conducive to tumor promotion and progression. This assessment is validated further by the finding of small DCIS-like tumors in the mammary fat pads of RARβ-null mice acting as hosts for ErbB2-transformed epithelium originating from both wild-type and RARβ-null mammary glands. These observations support the concept that loss of RARβ does not affect tumor initiation by ErbB2 significantly but lessens the capacity of the surrounding stroma to provide the microenvironment required for the growth and invasion of tumor cells.
The mechanism through which stromal RARβ achieves its tumor-promoting effect probably involves distinct cell types, transcriptional programs, and signaling pathways. However, our analysis shows that the reduction in the number of α-SMA/RARβ–positive myofibroblasts and the lower expression of Cxcl12 by these cells sharply reduces CXCL12/SDF-1 levels in RARβ-null stroma. The decrease in stromal CXCL12/SDF-1 production is accompanied by a reduction in the activity of the Src/ErbB2/Akt signaling pathway in the RARβ-null tumors, a pathway that previously had been shown to contribute to the survival, invasion, and growth of breast cancer cells (24). The physiological relevance of the involvement of the CXCL12/SDF-1 in this process was demonstrated further by the observation that addition of the chemokine to conditioned medium obtained from Rarb−/− mammary fibroblasts restored the growth rate of mammary tumor cells to that generated with conditioned medium obtained from wild-type mammary fibroblasts. The CXCL12/SDF-1 chemokine also is known to act as a chemotactic agent for inflammatory cells and bone marrow-derived endothelial cells. Thus, the reduction in CXCL12/SDF-1 probably contributes to the decrease in infiltration of inflammatory cells and angiogenesis observed in the RARβ-null tumors.
The relevance of our findings to the human disease is emphasized by the observation that the mouse Rarb−/− stromal signature is sufficient to distinguish normal from tumor stroma in all breast cancer patients surveyed in our analysis. Moreover, expression of RARB by itself in laser-dissected stromal cells also has the ability to distinguish human normal stroma from tumor stroma. The finding that RARβ has a dominant role as a facilitator of tumor proliferation, rather than a more restricted role as a tumor suppressor in breast carcinoma cells, was clearly unexpected. It would be of interest to investigate whether this tumor-promoting attribute is specific to stromal RARβ or is shared with other RAR isoforms likely to be present in the tumor microenvironment. Given the poor clinical record of RA agonists exhibited so far in the prevention and treatment of breast cancer, this work suggests that the development of retinoids with distinct activity (agonist versus antagonist) as well as cell type-specific biological effects could provide improved retinoid-based therapeutic approaches to manage breast cancer and other solid tumors.
Materials and Methods
Mice.
Mouse strains used in this study and the protocols to genotype them have been described previously for Rarb−/− (33), MMTV-Cre and neuNTki (35), and MMTV-neuNDL2-5 mice (37). All strains were maintained in the FVB genetic background. Further details are given in SI Materials and Methods.
Cell Culture.
TM15 cells were maintained in DMEM supplemented with 10% FBS as previously described (39). Mouse mammary gland fibroblast cell lines were generated from neuNDL2-5 mice on either Rarb-null or wild-type backgrounds as detailed in SI Materials and Methods. The fibroblasts were treated with RA (Sigma) at a concentration of 0.5 μM for 2 d to generate conditioned media. Conditioned media from Rarb-null or wild-type mouse mammary gland fibroblasts also were supplemented with either CXCL12/SDF-1 protein or anti-CXCL12 antibody (R&D Systems) at final concentrations of 10 ng/mL and 50 μg/mL, respectively.
Immunohistochemical Analysis.
Immunohistochemical analyses were performed on 4-μm formalin-fixed paraffin-embedded sections of tumors from mice killed 2 mo after tumor initiation. Immunostaining was done using the VECTASTAIN avidin-biotin complex kit (Vector Laboratories) as previously described (43).
Apoptosis Analysis.
Apoptosis was detected in paraffin wax-embedded sections by TUNEL analysis with terminal deoxynucleotidyl transferase (Roche Diagnostics) and IHC with anti-caspase 3 antibody. Ten randomly selected fields per section were documented by photomicroscopy.
Transplantation Studies.
The no. 4 inguinal glands from of 21-d-old Rarb−/− or wild-type host females were cleared of mammary epithelium as previously described (44). Fragments (3 mm3) from matched mammary tissue of the donor mice with ErbB2 transgene in either Rarb−/− or wild-type background were transplanted into the cleared mammary fat pad of host mice.
Flow Cytometry.
Single-cell suspensions of mammary glands for flow cytometry were prepared as described (45) with minor modifications.
Microarray Analysis.
RNA from Rarb−/− and wild-type stroma was hybridized to Affymetrix GeneChip Mouse Genome 430 2.0 Arrays. Normalization, gene filtering, and comparative gene-expression analysis were done with the FlexArray software developed by the Genome Québec Centre at McGill University (GSE19925).
Finding Orthologs in Human Data.
We used gene-expression data from the McGill Cancer Centre Breast Cancer dataset comprising 49 tumor stroma and 52 normal (tumor adjacent and breast reduction) human samples derived by Laser Capture Microdissection (GSE9014 and GSE4823). Probes with the highest interquartile range across all the human stroma samples were used to select the best probe for genes with more than one representative probe. Using the Biomart package in R, as well as the Mouse Genome Informatics database, we found 147 human orthologs of the 159 differentially expressed mouse genes of the gene list. These orthologs then were mapped to the good probe set of the human stroma data, leaving us with 106 corresponding human orthologs.
Clustering.
Unsupervised hierarchical clustering and subsequent assessment of cluster stability was performed using the orthologs of the differentially expressed genes via PVClust package version 1.2–1 in R version 2.8.1. To cluster the data, correlation distance and Ward's algorithm were applied. Stability was assessed using 10,000 bootstrap resampling.
Statistical Testing.
The ability of each gene within the human ortholog RARβ gene list to differentiate between tumor and normal stroma was determined using a two-sided Wilcoxon test. A one-sided Wilcoxon test was used to determine whether the human orthologs of the RARβ gene list were enriched for genes that differentiate between tumor and normal stroma compared with the general distribution of genes in the microarray. The Global Test package version 4.12.0 in R version 2.8.1 was used to check the ability of the 106 human ortholog RARβ gene list to distinguish tissue type (tumor or normal). We used a logistic regression based on an asymptotic distribution (P < 0.05).
Supplementary Material
Acknowledgments
We thank Carlo Ouellet and Céline Champigny for technical help with handling of the animals and Catherine R. Dufour for analysis of microarray expression datasets. This work was funded by a Terry Fox New Frontiers Group grant from the Canadian Cancer Society Research Institute (to V.G., M.P., M.T.H., and W.J.M.) and by Canadian Institutes for Health Research Operating Grants MOP-64275 (to V.G.) and MOP-82801 (to M.S.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE19925).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011845108/-/DCSupplemental.
References
- 1.Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Dev Dyn. 2003;226:237–244. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- 2.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noy N. Between death and survival: Retinoic acid in regulation of apoptosis. Annu Rev Nutr. 2010;30:201–217. doi: 10.1146/annurev.nutr.28.061807.155509. [DOI] [PubMed] [Google Scholar]
- 4.Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: Lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- 5.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hua S, Kittler R, White KP. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell. 2009;137:1259–1271. doi: 10.1016/j.cell.2009.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross-Innes CS, et al. Cooperative interaction between retinoic acid receptor-alpha and estrogen receptor in breast cancer. Genes Dev. 2010;24:171–182. doi: 10.1101/gad.552910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 9.Rosenauer A, et al. Estrogen receptor expression activates the transcriptional and growth-inhibitory response to retinoids without enhanced retinoic acid receptor alpha expression. Cancer Res. 1998;58:5110–5116. [PubMed] [Google Scholar]
- 10.Wang Q, Yang W, Uytingco MS, Christakos S, Wieder R. 1,25-Dihydroxyvitamin D3 and all-trans-retinoic acid sensitize breast cancer cells to chemotherapy-induced cell death. Cancer Res. 2000;60:2040–2048. [PubMed] [Google Scholar]
- 11.Moon RC, Constantinou AI. Dietary retinoids and carotenoids in rodent models of mammary tumorigenesis. Breast Cancer Res Treat. 1997;46:181–189. doi: 10.1023/a:1005995925246. [DOI] [PubMed] [Google Scholar]
- 12.Donato LJ, Noy N. Suppression of mammary carcinoma growth by retinoic acid: Proapoptotic genes are targets for retinoic acid receptor and cellular retinoic acid-binding protein II signaling. Cancer Res. 2005;65:8193–8199. doi: 10.1158/0008-5472.CAN-05-1177. [DOI] [PubMed] [Google Scholar]
- 13.Donato LJ, Suh JH, Noy N. Suppression of mammary carcinoma cell growth by retinoic acid: The cell cycle control gene Btg2 is a direct target for retinoic acid receptor signaling. Cancer Res. 2007;67:609–615. doi: 10.1158/0008-5472.CAN-06-0989. [DOI] [PubMed] [Google Scholar]
- 14.Rao GN, Ney E, Herbert RA. Effect of retinoid analogues on mammary cancer in transgenic mice with c-neu breast cancer oncogene. Breast Cancer Res Treat. 1998;48:265–271. doi: 10.1023/a:1005957620881. [DOI] [PubMed] [Google Scholar]
- 15.Rao GN, Ney E, Herbert RA. Changes associated with delay of mammary cancer by retinoid analogues in transgenic mice bearing c-neu oncogene. Breast Cancer Res Treat. 1999;58:241–254. doi: 10.1023/a:1006315716713. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez S, et al. Structure, function and modulation of retinoic acid receptor beta, a tumor suppressor. Int J Biochem Cell Biol. 2007;39:1406–1415. doi: 10.1016/j.biocel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Bissell MJ, Labarge MA. Context, tissue plasticity, and cancer: Are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sappino AP, Skalli O, Jackson B, Schürch W, Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988;41:707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Fan X, Houghton J. Tumor microenvironment: The role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 21.Allinen M, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17:792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 24.Cabioglu N, et al. CXCL-12/stromal cell-derived factor-1alpha transactivates HER2-neu in breast cancer cells by a novel pathway involving Src kinase activation. Cancer Res. 2005;65:6493–6497. doi: 10.1158/0008-5472.CAN-04-1303. [DOI] [PubMed] [Google Scholar]
- 25.Finak G, et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 26.Chiesa MD, et al. Italian Oncology Group for Clinical Research. Tamoxifen vs tamoxifen plus 13-cis-retinoic acid vs tamoxifen plus interferon alpha-2a as first-line endocrine treatments in advanced breast cancer: Updated results of a phase II, prospective, randomised multicentre trial. Acta Biomed. 2007;78:204–209. [PubMed] [Google Scholar]
- 27.Singletary SE, et al. Phase II clinical trial of N-(4-hydroxyphenyl)retinamide and tamoxifen administration before definitive surgery for breast neoplasia. Clin Cancer Res. 2002;8:2835–2842. [PubMed] [Google Scholar]
- 28.The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 29.Mikkelsen S, Berne B, Staberg B, Vahlquist A. Potentiating effect of dietary vitamin A on photocarcinogenesis in hairless mice. Carcinogenesis. 1998;19:663–666. doi: 10.1093/carcin/19.4.663. [DOI] [PubMed] [Google Scholar]
- 30.Albright CD, Salganik RI, Van Dyke T. Dietary depletion of vitamin E and vitamin A inhibits mammary tumor growth and metastasis in transgenic mice. J Nutr. 2004;134:1139–1144. doi: 10.1093/jn/134.5.1139. [DOI] [PubMed] [Google Scholar]
- 31.Møllersen L, Paulsen JE, Olstørn HB, Knutsen HK, Alexander J. Dietary retinoic acid supplementation stimulates intestinal tumour formation and growth in multiple intestinal neoplasia (Min)/+ mice. Carcinogenesis. 2004;25:149–153. doi: 10.1093/carcin/bgg176. [DOI] [PubMed] [Google Scholar]
- 32.Omenn GS, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 33.Luo J, Pasceri P, Conlon RA, Rossant J, Giguère V. Mice lacking all isoforms of retinoic acid receptor β develop normally and are susceptible to the teratogenic effects of retinoic acid. Mech Dev. 1995;53:61–71. doi: 10.1016/0925-4773(95)00424-6. [DOI] [PubMed] [Google Scholar]
- 34.Watson CJ, Khaled WT. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development. 2008;135:995–1003. doi: 10.1242/dev.005439. [DOI] [PubMed] [Google Scholar]
- 35.Andrechek ER, et al. Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc Natl Acad Sci USA. 2000;97:3444–3449. doi: 10.1073/pnas.050408497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodgson JG, et al. Copy number aberrations in mouse breast tumors reveal loci and genes important in tumorigenic receptor tyrosine kinase signaling. Cancer Res. 2005;65:9695–9704. doi: 10.1158/0008-5472.CAN-05-0755. [DOI] [PubMed] [Google Scholar]
- 37.Guy CT, et al. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levental KR, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillon RL, et al. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li YM, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 41.Lynch RA, Etchin J, Battle TE, Frank DA. A small-molecule enhancer of signal transducer and activator of transcription 1 transcriptional activity accentuates the antiproliferative effects of IFN-gamma in human cancer cells. Cancer Res. 2007;67:1254–1261. doi: 10.1158/0008-5472.CAN-06-2439. [DOI] [PubMed] [Google Scholar]
- 42.Widschwendter A, et al. Prognostic significance of signal transducer and activator of transcription 1 activation in breast cancer. Clin Cancer Res. 2002;8:3065–3074. [PubMed] [Google Scholar]
- 43.Liu X, et al. Evolutionary conservation of the clk-1-dependent mechanism of longevity: Loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev. 2005;19:2424–2434. doi: 10.1101/gad.1352905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deome KB, Faulkin LJ, Jr., Bern HA, Blair PB. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 45.Diehn M, et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.