Abstract
Selective ion conduction across ion channel pores is central to cellular physiology. To understand the underlying principles of ion selectivity in tetrameric cation channels, we engineered a set of cation channel pores based on the nonselective NaK channel and determined their structures to high resolution. These structures showcase an ensemble of selectivity filters with a various number of contiguous ion binding sites ranging from 2 to 4, with each individual site maintaining a geometry and ligand environment virtually identical to that of equivalent sites in K+ channel selectivity filters. Combined with single channel electrophysiology, we show that only the channel with four ion binding sites is K+ selective, whereas those with two or three are nonselective and permeate Na+ and K+ equally well. These observations strongly suggest that the number of contiguous ion binding sites in a single file is the key determinant of the channel’s selectivity properties and the presence of four sites in K+ channels is essential for highly selective and efficient permeation of K+ ions.
Keywords: nonselective cation channel, potassium channel
The structure determination of several K+ selective and, more recently, bacterial nonselective cation channels has, over the past decade, vastly increased our knowledge of ion selectivity mechanisms (1–8). In K+ channels, the TVGYG signature sequence has emerged as the fundamental element imparting high K+ over Na+ selectivity (9, 10), forming four contiguous and chemically equivalent K+ binding sites composed of backbone carbonyl oxygen atoms from filter residues together with the hydroxyl oxygen atoms from the conserved threonine. Conversely, the TVGDG filter sequence of the NaK channel from Bacillus cereus, although similar in length and amino acid composition to those of K+ channels, forms a nonselective filter preserving the two most intracellular sites of K+ channel filters along with a wide, water-filled vestibular region on the immediately extracellular side (7) (Fig. 1A). Debate lingers in the field about the underlying factors contributing to these selectivity differences. Although available structural studies of K+ channels seem to favor the classical snug-fit model to account for K+ over Na+ selectivity (11, 12), computational studies on K+ channel selectivity, in some cases using an isolated K+ binding site in their calculations, usually invoke one or more of the following concepts: the coordination number of the ion, chemistry of carbonyl oxygen ligands, intrinsic dynamism of the selectivity filter, solvent exposure of the ion binding sites, or the free energy landscapes of ion entrance and translocation in a multiion configuration (13–21). To further understand the underlying principles of ion selectivity in tetrameric cation channels, we engineered a set of cation channel pores whose selectivity filters contain three (equivalent to sites 2–4 or sites 1–3 of a K+ channel) or four (equivalent to sites 1–4 of a K+ channel) ion binding sites and determined their structures to high resolution (1.55–1.75 Å). Combined with single channel electrophysiology, we demonstrated that only the channel with four ion binding sites is K+ selective, whereas those with two (as in wild-type NaK) or three are nonselective and permeate Na+ and K+ equally well.
Fig. 1.
Ion binding and conductance in NaK. (A) The selectivity filter structure of NaK in complex with K+ ions (PDB 3E8H). The 2Fo - Fc map contoured at 1.5σ shows the electron density (blue mesh) of water and K+ represented by red and green spheres, respectively. (B) Superimposition of the NaK filter structure (green) with that of KcsA (magenta, PDB 1K4C). Two diagonally opposite subunits are shown for clarity. (C) Single channel traces of NaK at ± 20 and ± 80 mV and its I-V curve. Currents were recorded using giant liposome patch clamping with 150 mM NaCl and 150 mM KCl in the pipette and bath solutions, respectively. The same method was used in the recordings of all NaK mutants.
Results
The NaK Channel with Two Contiguous Ion Binding Sites Is Nonselective.
The NaK channel shares an overall similar architecture with K+ channel pores, and its two discrete ion binding sites share a seemingly identical chemical environment as sites 3 and 4 in K+ channel filters (Fig. 1A). Superimposition of the NaK and KcsA selectivity filters reveals a virtually identical ligand geometry at sites 3 and 4, formed by the Thr63 and the Val64 residues in NaK (Fig. 1B). However, as shown in single channel recordings using giant liposome patching (Fig. 1C), the channel conducts Na+ and K+ equally well with an I-V curve showing a single channel conductance of about 35 pS and a reversal potential of zero in biionic conditions (150 mM KCl in the bath and 150 mM NaCl in the pipette). Although the single channel openings are noisy and the absolute conductance consequently uncertain, the reversed polarity of channel openings at positive and negative potentials near zero confirm the lack of selectivity between Na+ and K+. Notwithstanding other contributing factors, we hypothesized that number of inline binding sites is likely an important determinant of the drastically different selectivity properties in different tetrameric cation channels. This hypothesis, extended to other members of the family, has direct implications as discussed below.
Structure and Function of Cyclic Nucleotide-Gated (CNG)-Mimicking NaK Mutant with Three Ion Binding Sites.
CNG channels play a central role in visual and olfactory signal transduction (22–25). They are nonselective tetrameric cation channels that conduct most group 1A cations in addition to permeating Ca2+. From a sequence alignment of several tetrameric cation channel selectivity filter sequences (Fig. S1), CNG channels stand out due to their altered selectivity filter sequences, both in terms of residue composition and signature sequence length, compared to NaK and various K+ channels. These changes likely underlie a different filter architecture. In our accompanying study, we generated a set of NaK mutants by replacing its selectivity filter sequence with those of CNG channels and determined their structures at very high resolutions (see accompanying paper). One such mutant named NaK2CNG-D, in which the NaK selectivity filter sequence of T63VGDGNFS was changed to T63VGDTPP and whose structure was determined to 1.62 Å in complex with K+, will be used as in this discussion.
The NaK2CNG-D structure reveals three contiguous ion binding sites, akin to an intermediate between NaK and KcsA (Fig. 2A). The structural conversion from wild-type NaK to this mutant filter involves a dramatic main-chain conformational change at the acidic residue (Asp66) and the preceding glycine (Gly65) (Fig. S2). Consequently, Gly65 in NaK2CNG-D adopts the same main-chain conformation as the equivalent glycine (Gly77) of the TVGYG filter in KcsA with its backbone carbonyl pointing toward the central axis of the filter (Fig. 2 A and B). This structural change results in a replacement of the water-filled vestibule in NaK with an additional ion binding cage in the mutant, utilizing eight backbone carbonyl oxygen atoms from Val64 and Gly65 as ligands. The position of this site is equivalent to site 2 in the selectivity filter of KcsA and is numbered as such for comparison. Ligand geometry of the three ion binding sites in the NaK2CNG-D mutant is virtually the same as the bottom three sites (sites 2–4) of KcsA with a main-chain rmsd of 0.08 Å between their filter-forming TVG residues (Fig. 2B). However, like the wild-type channel, the NaK2CNG-D mutant is still nonselective. As shown in single channel recordings (Fig. 2C), the I-V curve of the mutant channel has a reversal potential of zero in biionic condition indicating the same permeability for Na+ and K+. The NaK2CNG-D mutant has a higher single channel conductance (∼110 pS) than that of NaK (∼35 pS). This difference could be attributed to the presence of a constriction point at the external entrance of NaK formed by the backbone of Gly67, which is absent in NaK2CNG-D, or the change in the number of ion binding sites. NaK2CNG-D in complex with Na+ has the same structure as that of the K+ complex (accompanying paper), indicating that the channel filter does not undergo structural changes upon Na+ binding.
Fig. 2.
Ion binding and conductance in NaK2CNG-D. (A) The selectivity filter structure of NaK2CNG-D in complex with K+ ions. The Fo - Fc ion omit map (1.62 Å) contoured at 5σ shows the density (blue mesh) of three K+ ions in the filter. (B) Superimposition of the NaK2CNG-D filter structure (yellow) with that of KcsA (magenta). (C) Single channel traces of NaK2CNG-D at ± 80 mV and its I-V curve.
Converting NaK to a K+ Selective Channel.
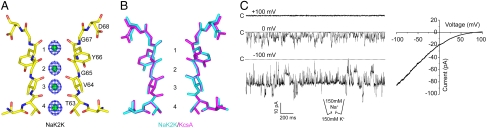
The introduction of an additional discrete ion binding site in the NaK selectivity filter as seen in NaK2CNG-D is clearly insufficient to impart higher K+ selectivity to the channel, suggesting a minimum of four contiguous ion binding sites for high K+/Na+ selectivity. We took our analysis a step further by generating another NaK mutant whose selectivity filter contains four equivalent ion binding sites similar to those of K+ channels. Although there is only a single residue difference in the selectivity filter sequence between NaK and K+ channels (TVGDG versus TVGYG) (Fig. S1), the selectivity filter of an Asp66Tyr mutant of NaK remains largely unchanged and the channel is still nonselective. However, the introduction of a second mutation, Asn68Asp, leads to a significant main-chain conformational change at the GYG residues and results in a mutant channel with selectivity filter similar to that of KcsA (Fig. 3A). Similar to the Asp66Tyr mutant, Asn68Asp alone is not sufficient to convert NaK to a K+ selective channel. In this Asp66Tyr/Asn68Asp double mutant (named NaK2K in the following discussion) whose K+ complex structure was determined at 1.55 Å, the Asp68 side chain folds back toward the channel pore and forms a hydrogen bond with Tyr55 in the pore helix, which likely helps stabilize the selectivity filter in a configuration similar to that of K+ channel filters (Fig. 3A and Fig. S3). In the wild-type NaK channel, on the other hand, the side chain of Asn68 points upward and is exposed to external solution (Fig. 1A). The selectivity filter of NaK2K superimposes well with that of KcsA with main-chain rmsd of 0.17 Å between their filter-forming TVGYG residues (Fig. 3B). However some differences in protein packing surrounding the filter are observed. First, the acidic residue Glu71 on the pore helix of KcsA, which forms a hydrogen bond with Asp80 (equivalent to Asp68 in NaK2K) is replaced by valine in NaK2K. Second, two buried water molecules participate in a hydrogen bonding network surrounding the filter of NaK2K whereas only one water molecule was observed in KcsA (Fig. S3). Interestingly, protein packing surrounding the NaK2K mutant filter is very similar to that of the MthK K+ channel (26) (Fig. S3).
Fig. 3.
Ion binding and conductance in the K+ selective NaK mutant, NaK2K. (A) The selectivity filter structure of NaK2K in complex with K+ ions. The Fo - Fc ion omit map (1.55 Å) contoured at 5σ shows the density (blue mesh) of four K+ ions in the filter. (B) Superimposition of the NaK2K filter structure (green) with that of KcsA (magenta). (C) Single channel traces of NaK2K at 0 and ± 100 mV, and its I-V curve. For a better assessment of reversal potential, the I-V curve was obtained from the average of 20 current traces recorded using a patch containing multiple channels with voltage pulses ramp from -100 to +100 mV over 400 ms duration.
To test whether the selectivity filter change observed in the NaK2K mutant translated into higher K+ selectivity, single channel recordings were obtained in biionic conditions using giant liposome patching. As shown in Figure 3C, the NaK2K mutant is highly K+ selective under biionic conditions with a reversal potential of about 80 mV, equivalent to a permeability ratio (PNa/PK) of about 0.04. The single channel conductance of the mutant is about 120 pS in symmetrical KCl, slightly higher than that of NaK2CNG-D (Fig. S4A). In the absence of K+, NaK2K can also conduct Na+ albeit with a much lower conductance (∼10 pS in symmetrical 150 mM NaCl), suggesting that the filter of NaK2K remains conductive even in the absence of K+ (Fig. S4B). Indeed, the structure of NaK2K in a Na+ only environment is the same as its K+ complex (Fig. S5).
Reduction of Site Number from 4 to 3 in K+ Channel Diminishes K+ Selectivity.
To eliminate the possibility that protein packing differences in the region surrounding the filter rather than number of sites between NaK2CNG-D and NaK2K contribute to the difference in ion selectivity, an additional mutation, NaK2K_T63A, was made, which removes the side-chain hydroxyl group of Thr63 facing the central cavity and eliminates the fourth K+ binding site while maintaining the top three as well as the protein packing surrounding the filter. Consistent with our conclusion, the triple mutant channel loses K+ selectivity, permeating Na+ equally well, similar to the NaK2CNG-D mutant (Fig. 4A). An equivalent mutation in the naturally occurring MthK K+ channel (Thr59Ala) also converts this highly K+ selective channel into a virtually nonselective one (Fig. 4 B and C). Compared to wild-type MthK (26), the structure of MthK pore carrying a Thr59Ala mutation reveals no structure change at the selectivity filter other than the missing threonine side chain. The mutant channel retains strong and equivalent K+ binding at the top three sites, whereas K+ binding at site 4 is significantly diminished (Fig. 4D). These observations are in contrast to studies of the Shaker K+ channel where the equivalent mutant remains K+ selective (9). The discrepancy may be explained by the fact that, unlike the NaK2K mutant or MthK, K+ ions may be required to stabilize the selectivity filter of the Shaker channel in a conductive conformation, much like KcsA. This K+ dependent filter stability adds another layer of K+ selectivity in KcsA and Shaker (4, 27, 28), likely making both channels still K+ selective even after the elimination of site 4. In contrast, K+ is not required to stabilize the filter in MthK and the NaK2K mutant, whose selectivity filters maintain a conductive conformation and are permeable to Na+ in the absence of K+ (26).
Fig. 4.
Ion selectivity in NaK2K_T63A and MthK_T59A mutants. Single channel traces at ± 80 mV and their I-V curves of (A) NaK2K_T63A and (B) MthK_T59A recorded using giant liposome patch clamping in biionic conditions (150 mM KCl/150 mM NaCl). (C) Single channel traces of wild-type MthK and I-V curve of wild-type MthK recorded in planar lipid bilayers with 150 mM KCl on one side and 150 mM NaCl on the other. (D) Structure of the MthK ion conduction pore with a Thr59Ala mutation. The 2Fo - Fc ion omit map (1.75 Å) contoured at 2σ shows strong density (blue mesh) of K+ ions at the top three sites but much weaker density at site 4.
Discussion
The structures of wild-type NaK and its mutants, NaK2CNG-D and NaK2K, showcase a series of selectivity filters comprising two, three, or four contiguous ion binding sites, respectively, with similar chemical environments. Although the ligand geometry of each individual site in these channels is virtually identical to equivalent sites in the KcsA K+ channel, with ligand-to-K distances in the range of 2.6–3.1 Å (Fig. S6), only the selectivity filter containing four ion binding sites exhibits high K+ selectivity. Furthermore, the higher K+ selectivity resulting from four sites does not sacrifice, and may actually enhance, the rate of K+ permeation, as NaK2K has a higher single channel conductance for K+ than NaK2CNG-D or NaK2K_T63A. Similar results were also obtained for MthK and its Thr59Ala mutant. Having four equivalent and contiguous ion binding sites in the filter, therefore, seems to be an essential feature in K+ channels that allows selective and efficient permeation of K+ ions. This necessity has not been adequately explained or taken into account by the classical snug-fit model or current theoretical calculations. The findings herein clearly demonstrate that the underlying mechanisms of ion selectivity in K+ channels are far from being fully grasped and warrant further investigation.
To reveal the structural mechanism of ion selectivity in a tetrameric cation channel, a detailed analysis of how K+ and Na+ competing for the ion binding sites in a multiion pore is necessary. Whether in a nonselective channel pore with two or three ion binding sites or in a K+ selective pore with four ion binding sites, K+ ions always reside at the center of each site surrounded by eight oxygen ligands in a square antiprism configuration. Conversely, Na+ binding seems to preferably occur toward the edges of ion binding sites almost in plane with the ligand groups (7, 21, 26) (also see accompanying paper). It is interesting to note that, in the recent high-resolution structural study of the K+ selective MthK pore, one K+ ion is seen to remain bound in the filter even at low-K+/high-Na+ concentrations, preferably at site 1 or 3. This observation indicates that K+ binding at the four seemingly identical sites is not equivalent at low concentration with sites 1 or 3 being more favorable for K+ over Na+ (26). This preference for K+ at specific sites does not seem to be the case for nonselective NaK2CNG-D, in which K+ ion at all three sites can be replaced by Na+ in an equivalent low-K+/high-Na+ environment. However, even with high-resolution structures in our studies, an accurate determination of the position and occupancy of K+ or Na+ ions in the filter is not trivial, complicated by the fact that water molecules, which have the same number of electrons as Na+, can also bind. Further studies are therefore needed to determine unambiguously the difference in ion binding of two competing ions (Na+ and K+) within selective and nonselective cation channel pores.
Materials and Methods
All NaK mutants used in structural and functional studies were generated on the background of NaKN∆19, a truncated form of the NaK channel from Bacillus cereus lacking the N-terminal 19 residues. Protein expression, purification, and crystallization were performed following the same procedures as those for NaKN∆19 (29). Crystals were of space group I4 with unit cell dimensions around a = b = 68 Å, c = 89 Å, α = β = γ = 90°, and contained two molecules per asymmetric unit. The fourfold axis of the channel tetramer coincided with the crystallographic tetrad. The structures were determined by molecular replacement using the NaKNΔ19 structure with selectivity filter region omitted as an initial search model. Detailed data collection and refinement statistics are listed in Table 1.
Table 1.
Data collection and refinement statistics for NaK mutants and MthK pore (T59A) in complex with K+
| Protein (in K+) | NaK2CNG-D | NaK2K | MthK pore (T59A) |
| Data collection | |||
| Space group | I4 | I4 | P4212 |
| Cell dimensions a = b, c, Å | 67.747, 89.444 | 67.939, 89.638 | 63.600, 44.047 |
| Resolution, Å | 50-1.62 | 50-1.55 | 50-1.75 |
| Rsym, % | 4.8 (82.3) | 4.8 (97.6) | 7.2 (100) |
| I/σI | 39.2 (1.85) | 35.0 (1.0) | 27.6 (1.5) |
| No. reflections- total (unique) | 187,040 (25,607) | 191,495 (28,703) | 86,388 (9,484) |
| Completeness | 99.9 (100) | 97.2 (77.0) | 98.9 (99.8) |
| Redundancy | 7.3 (7.2) | 6.7 (2.8) | 9.1 (7.9) |
| Refinement | |||
| Resolution, Å | 1.62 | 1.55 | 1.75 |
| Rwork/Rfree | 20.7/22.2 | 20.0/21.5 | 21.2/23.6 |
| No. atoms | |||
| Protein | 1,446 | 1,487 | 639 |
| MPD/ion | 5/9 | 5/9 | -/5 |
| Water | 132 | 72 | 67 |
| rmsd | |||
| Bond angles, Å | 1.074 | 1.088 | 1.072 |
| Bond lengths, Å | 0.0051 | 0.0051 | 0.0054 |
Values in parenthesis are for highest resolution shell. Five percent of the data were used in the Rfree calculation. MPD, 2-methyl-2,4-pentanediol.
Ion selectivity studies of NaK, its mutants, and MthK_T59A were performed using giant liposome patch clamping. NaK and its mutants for functional assays contained an extra Phe92-to-Ala mutation to enhance the single channel conductance. The proteins were purified in the detergent decyl maltoside and reconstituted in lipid vesicles composed of 3∶1 ratio of 1-palmitoyl-2-oleoyl-phosphatidylethanolamine and 1-palmitoyl-2-oleoyl-phosphatidylglycerol (Avanti Polar Lipids) as described (30, 31). The wild-type MthK was analyzed in lipid bilayers as described (32). Giant liposomes were obtained by air drying followed by rehydration. Detailed methods of giant liposome patching as well as protein purification and structure determination of the NaK mutants are the same as described in Materials and Methods of the accompanying paper as well as in our SI Text. Purification, crystallization, and structure determination of the ion conduction pore of the MthK_T59A mutant follow the same procedures as described (26).
Supplementary Material
Acknowledgments.
Use of the Advanced Photon Source and the Advanced Light Source was supported by the US Department of Energy, Office of Energy Research. We thank the beamline staff for assistance in data collection. This work was supported in part by The Howard Hughes Medical Institute and by grants from the National Institute of Health (NIH) (GM079179 to Y.J.), The David and Lucile Packard Foundation, and the Welch Foundation (Grant I-1578). M.G.D. was supported by Ruth L. Kirschstein National Research Service Award Predoctoral Fellowship (5 F31 GM07703) from NIH, and D.B.S. was supported by NIH Training Grant (T32 GM008297).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 3K03, 3OUF, and 3OUS for the K+ complexes of NaK2CNG-D, NaK2K, and MthK pore (Thr59Ala), respectively].
See companion article on page 592.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013636108/-/DCSupplemental.
References
- 1.Doyle DA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 3.Nishida M, Cadene M, Chait BT, MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007;26:4005–4015. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valiyaveetil FI, Leonetti M, Muir TW, Mackinnon R. Ion selectivity in a semisynthetic K+ channel locked in the conductive conformation. Science. 2006;314:1004–1007. doi: 10.1126/science.1133415. [DOI] [PubMed] [Google Scholar]
- 5.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 6.Tao X, Avalos JL, Chen J, MacKinnon R. Crystal structure of the eukaryotic strong inward-rectifier K+ channel Kir2.2 at 3.1 A resolution. Science. 2009;326:1668–1674. doi: 10.1126/science.1180310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam A, Jiang Y. Structural analysis of ion selectivity in the NaK channel. Nat Struct Mol Biol. 2009;16:35–41. doi: 10.1038/nsmb.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi N, Ye S, Alam A, Chen L, Jiang Y. Atomic structure of a Na+ and K+ conducting channel. Nature. 2006;440:570–574. doi: 10.1038/nature04508. [DOI] [PubMed] [Google Scholar]
- 9.Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heginbotham L, Abramson T, MacKinnon R. A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science. 1992;258:1152–1155. doi: 10.1126/science.1279807. [DOI] [PubMed] [Google Scholar]
- 11.Bezanilla F, Armstrong CM. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972;60:588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mullins LJ. An analysis of pore size in excitable membranes. J Gen Physiol. 1960;43:105–117. doi: 10.1085/jgp.43.5.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noskov SY, Berneche S, Roux B. Control of ion selectivity in potassium channels by electrostatic and dynamic properties of carbonyl ligands. Nature. 2004;431:830–834. doi: 10.1038/nature02943. [DOI] [PubMed] [Google Scholar]
- 14.Noskov SY, Roux B. Importance of hydration and dynamics on the selectivity of the KcsA and NaK channels. J Gen Physiol. 2007;129:135–143. doi: 10.1085/jgp.200609633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bostick DL, Brooks CL., 3rd Selectivity in K+ channels is due to topological control of the permeant ion’s coordinated state. Proc Natl Acad Sci USA. 2007;104:9260–9265. doi: 10.1073/pnas.0700554104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas M, Jayatilaka D, Corry B. The predominant role of coordination number in potassium channel selectivity. Biophys J. 2007;93:2635–2643. doi: 10.1529/biophysj.107.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varma S, Sabo D, Rempe SB. K+/Na+ selectivity in K channels and valinomycin: Over-coordination versus cavity-size constraints. J Mol Biol. 2008;376:13–22. doi: 10.1016/j.jmb.2007.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varma S, Rempe SB. Tuning ion coordination architectures to enable selective partitioning. Biophys J. 2007;93:1093–1099. doi: 10.1529/biophysj.107.107482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dudev T, Lim C. Determinants of K+ vs Na+ selectivity in potassium channels. J Am Chem Soc. 2009;131:8092–8101. doi: 10.1021/ja900168k. [DOI] [PubMed] [Google Scholar]
- 20.Egwolf B, Roux B. Ion selectivity of the KcsA channel: A perspective from multi-ion free energy landscapes. J Mol Biol. 2010;401:831–842. doi: 10.1016/j.jmb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson AN, et al. Mechanism of potassium-channel selectivity revealed by Na(+) and Li(+) binding sites within the KcsA pore. Nat Struct Mol Biol. 2009;16:1317–1324. doi: 10.1038/nsmb.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 23.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 24.Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 25.Finn JT, Grunwald ME, Yau KW. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- 26.Ye S, Li Y, Jiang Y. Novel insights into K+ selectivity from high-resolution structures of an open K+ channel pore. Nat Struct Mol Biol. 2010;17:1019–1023. doi: 10.1038/nsmb.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starkus JG, Kuschel L, Rayner MD, Heinemann SH. Ion conduction through C-type inactivated Shaker channels. J Gen Physiol. 1997;110:539–550. doi: 10.1085/jgp.110.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogielska EM, Aldrich RW. A mutation in S6 of Shaker potassium channels decreases the K+ affinity of an ion binding site revealing ion-ion interactions in the pore. J Gen Physiol. 1998;112:243–257. doi: 10.1085/jgp.112.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alam A, Jiang Y. High-resolution structure of the open NaK channel. Nat Struct Mol Biol. 2009;16:30–34. doi: 10.1038/nsmb.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alam A, Shi N, Jiang Y. Structural insight into Ca2+ specificity in tetrameric cation channels. Proc Natl Acad Sci USA. 2007;104:15334–15339. doi: 10.1073/pnas.0707324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heginbotham L, LeMasurier M, Kolmakova-Partensky L, Miller C. Single streptomyces lividans K(+) channels: Functional asymmetries and sidedness of proton activation. J Gen Physiol. 1999;114:551–560. doi: 10.1085/jgp.114.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Berke I, Chen L, Jiang Y. Gating and inward rectifying properties of the MthK K+ channel with and without the gating ring. J Gen Physiol. 2007;129:109–120. doi: 10.1085/jgp.200609655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.