Abstract
The bone loss induced by ovariectomy (ovx) has been linked to increased production of osteoclastogenic cytokines by bone marrow cells, including T cells and stromal cells (SCs). It is presently unknown whether regulatory interactions between these lineages contribute to the effects of ovx in bone, however. Here, we show that the T-cell costimulatory molecule CD40 ligand (CD40L) is required for ovx to expand SCs; promote osteoblast proliferation and differentiation; regulate the SC production of the osteoclastogenic factors macrophage colony-stimulating factor, receptor activator of nuclear factor-κB ligand, and osteoprotegerin; and up-regulate osteoclast formation. CD40L is also required for ovx to activate T cells and stimulate their production of TNF. Accordingly, ovx fails to promote bone loss and increase bone resorption in mice depleted of T cells or lacking CD40L. Therefore, cross-talk between T cells and SCs mediated by CD40L plays a pivotal role in the disregulation of osteoblastogenesis and osteoclastogenesis induced by ovx.
Keywords: estrogen, osteoporosis
Menopause results in decreased production of estrogen (E) and a parallel increase in FSH levels, which together stimulate bone resorption (1) and cause a period of rapid bone loss that is central for the onset of postmenopausal osteoporosis (2). The acute effects of menopause are modeled by ovariectomy (ovx) which, like natural menopause, stimulates bone resorption by increasing osteoclast (OC) formation (3, 4) and lifespan (5, 6). The net bone loss caused by ovx is limited by an increase in bone formation resulting from stimulated osteoblast (OB) formation (7). This compensation is fueled by an expansion of the pool of bone marrow (BM) stromal cells (SCs), increased commitment of SCs to the osteoblastic lineage (7), and enhanced proliferation of early OB precursors (8). The stimulatory effect of ovx on SCs is equally relevant for osteoclastogenesis because one of the consequences of E deprivation is the formation of osteoblastic cells with increased osteoclastogenic activity (9), that is, the capacity to support OC formation.
OC formation occurs when bone marrow macrophages (BMMs) are stimulated by the osteoclastogenic factors receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) (10, 11); however, in conditions of E deficiency, the secretion of the RANKL decoy receptor osteoprotegerin (OPG) decreased (12), whereas RANKL and other cytokines, including TNF-α, IL-1, IL-6, IL-7, and M-CSF, are produced in greater amounts (3, 13, 14). The compensatory increase in bone formation that follows ovx is mitigated by some of these factors, primarily TNF and IL-7, which blunt osteoblastogenesis (15, 16). The pathogenic role of cytokines distinct from RANKL and OPG has been established by studies demonstrating that the silencing of TNF, IL-1, IL-6, IL-7, and M-CSF prevents ovx-induced bone loss (17–23).
OBs are the significant sources of M-CSF, RANKL, OPG, IL-6, and IL-7 (12), whereas T lymphocytes are a critical source of TNF (3). Attesting to the relevance of T cell-produced TNF, T cell-deficient nude mice are protected against the loss of cortical and trabecular bone induced by ovx (24–26). Furthermore, the capacity of ovx to induce bone loss is restored by adoptive transfer into nude mice of T cells from WT mice but not of those from TNF−/− mice (17).
Interactions between T cells and SCs may regulate the response of these lineages to ovx. For example, activated T cells induce SC apoptosis via the Fas/Fas ligand pathway, thus blunting the compensatory increase in bone formation that limits bone loss in ovx mice (26). Another mediator of T-cell–SC cross-talk is the T-cell costimulatory CD40 ligand (CD40L) (27). This surface molecule, also known as CD154, exerts its effects by binding to CD40 (28) and several integrins (29–32). CD40 is expressed on antigen-presenting cells (33), SCs, and OBs (34). The CD40/CD40L system is crucial for T-cell activation and several functions of the immune system. It promotes macrophage activation and differentiation, Ab isotype switching, and the adequate organization of immunological memory in B cells (35). Binding to the integrins αIIbβ3 (29, 30), Mac-1 (31), and α5β1, which are widely expressed in the BM, is known to account for additional inflammatory effects of CD40L (32).
Recently, CD40L has been linked to postnatal skeletal maturation because T cells, through the CD40L/CD40 system, promote production of the antiosteoclastogenic factor OPG by B cells (36). Consequently, CD40L-deficient mice attain a reduced peak bone volume attributable to stimulated bone resorption (36). Low bone density has also been found in children affected by X-linked hyper-IgM syndrome, a condition in which CD40L production is impaired because of a mutation of the CD40L gene (37). Mice lacking T cell-expressed CD40L are protected against parathyroid hormone (PTH)-induced bone loss (38), however, raising the possibility that CD40L may exert antiresorptive activities in unstimulated conditions, although promoting bone resorption under conditions of bone stress.
The present study was designed to evaluate the role of CD40L on ovx-induced bone loss. We show that T cells regulate SC production of osteoclastogenic cytokines through CD40L. As a result, mice lacking T cells or CD40L do not sustain the acute bone loss triggered by the decline of ovarian function.
Results
Ovx Fails to Cause Bone Loss in T Cell-Depleted and CD40L Null Mice.
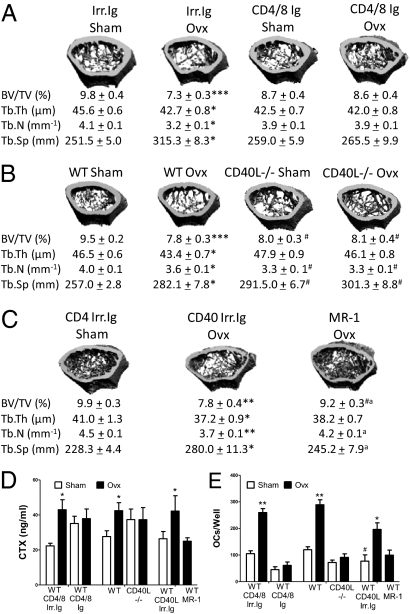
First, mice were depleted of T cells in vivo by injecting anti-CD4/8 Abs (38), a treatment modality referred to hereafter as T-cell depletion. Mice were injected multiple times with CD4/8 or irrelevant Abs from the age of 10–14 wk; underwent sham surgery or ovx at the age of 12 wk; and were killed 2 wk later, a length of time used by others to investigate the acute phase of bone loss induced by ovx (5). Cancellous bone was analyzed by microcomputed tomography (μCT) using femurs harvested at sacrifice. Ovx lowered trabecular bone volume (BV/TV) and deteriorated indices of bone structure in T cell-replete controls (Fig. 1A). In contrast, T cell-depleted mice were completely protected against the loss of BV/TV and the changes in bone structure induced by ovx.
Fig. 1.
Effects (mean ± SEM) of ovx on bone structure, bone resorption, and in vitro OC formation in T cell-depleted mice, CD40L−/− mice, and WT mice treated with the anti-CD40L Ab MR-1. (A) Representative cross-sectional reconstructions of the femur and trabecular structural indices in WT mice treated with irrelevant Ab (Irr. Ig) or anti-CD4 and anti-CD8 Abs (CD4/8 Ig) (n = 10 mice per group). BV/TV, trabecular thickness (Tb.Th), trabecular number (Tb.N), and trabecular space (Tb.Sp) are shown. (B) Representative cross-sectional reconstructions of the femur and trabecular structural indices in WT and CD40L−/− mice (n = 16 mice per group). (C) Representative cross-sectional reconstructions of the femur and trabecular structural indices in WT mice treated with MR-1 or irrelevant Ab (n = 10 mice per group). (D) Serum levels of CTx, a marker of resorption. *P < 0.05. (E) Number of OCs in cultures of whole BM stimulated with RANKL and M-CSF. *P < 0.05, **P < 0.01, and *** = P < 0.001 compared with the corresponding sham-operated group. #P < 0.05 compared with WT sham-operated mice. aP < 0.05 compared with ovx CD40 irrelevant isotope-matched Ab (Irr. Ig).
Bone histomorphometry confirmed that ovx induced a loss of BV/TV and increased in two indices of bone resorption, the number of OCs per bone surface (N.Oc/BS) and the percentage of surfaces covered by OCs (Oc.S/BS), in T cell-replete but not T cell-depleted WT mice (Table 1). Ovx is known to induce an immediate drop in bone formation, which is followed by a long-lasting period of enhanced formation (39). In accordance with this temporal sequence and reports by others (26), measurements of the number of OBs per bone surface and the percentage of surfaces covered by OBs disclosed that neither T cell-replete nor T cell-deficient mice displayed significant changes in trabecular bone formation 2 wk after ovx.
Table 1.
Effect (mean ± SEM) of ovx and sham surgery on histomorphometric indices of BV/TV and turnover in WT mice depleted of T cells via treatment with anti CD4/8 Abs (CD4/8 Ig) and CD40L−/− mice
| WT Irr. Ig sham | WT Irr. Ig ovx | WT CD4/8 Ig sham | WT CD4/8 Ig ovx | |
| N.Oc/BS, mm−1 | 0.4 ± 0.1 | 1.1 ± 0.2** | 1.0 ± 0.1*** | 1.1 ± 0.1 |
| Oc.S/BS, % | 0.9 ± 0.2 | 2.8 ± 0.5** | 2.7 ± 0.2*** | 2.5 ± 0.2 |
| N.Ob/BS, mm−1 | 14.2 ± 1.4 | 13.4 ± 0.9 | 12.3 ± 0.9 | 14.9 ± 1.1 |
| Ob.S/BS, mm | 17.8 ± 2.9 | 19.9 ± 1.9 | 20.0 ± 1.8 | 22.5 ± 2.4 |
| WT sham | WT ovx | CD40L−/− sham | CD40L−/− ovx | |
| N.Oc/BS, mm−1 | 0.6 ± 0.1 | 1.6 ± 0.1** | 1.5 ± 0.1**** | 1.7 ± 0.2*** |
| Oc.S/BS, % | 1.5 ± 0.2 | 3.9 ± 0.5** | 3.8 ± 0.3**** | 4.0 ± 0.5*** |
| N.Ob/BS, mm−1 | 10.3 ± 0.4*** | 7.2 ± 0.7† | 8.8 ± 0.8 | 8.9 ± 0.7 |
| Ob.S/BS, mm | 13.4 ± 0.7*** | 10.7 ± 1.5† | 11.8 ± 1.1 | 13.2 ± 1.4 |
N.Oc/BS (number of osteoclasts per millimeter of bone surface) and OcS/BS (percentage of bone surface occupied by osteoclasts) are indices of trabecular bone resorption. N.Ob/BS (number of osteoblasts per square millimeter of bone surface) and Ob.S/BS (number of osteoblasts per square millimeter of bone surface) are indices of trabecular bone formation *P < 0.05 and **P < 0.01 compared with the corresponding sham-operated group; ***P < 0.05 compared with irrelevant isotype-matched Ab (Irr. Ig) sham; ****P < 0.05 compared with WT sham (n = 10 mice per group).
†P < 0.05 compared with irrelevant isotype-matched Ab (Irr. Ig) ovx.
Next, WT and congenic CD40L−/− mice were subjected to ovx or sham surgery at the age of 12 wk and killed 2 wk later. Ovx induced bone loss in WT mice, although no changes occurred in CD40L−/− mice (Fig. 1B). Parameters of trabecular structure were also differentially affected by ovx in WT and CD40L null mice, because trabecular bone thickness, trabecular bone number, and trabecular bone separation were more substantially deteriorated by ovx in WT than in CD40L−/− mice. Analysis of cortical bone by μCT revealed that CD40L−/− mice were also protected against cortical bone loss (Fig. S1).
Quantitative bone histomorphometry confirmed that ovx induced a significant loss of BV/TV and increased N.Oc/BS and Oc.S/BS in WT mice but not in CD40L−/− mice (Table 1). Indices of bone formation were not affected by ovx in control and CD40L−/− mice.
To demonstrate a role for CD40L in ovx-induced bone loss in animals with normal bone structure and turnover at baseline, 12-wk-old WT mice were subjected to ovx or sham surgery and treated with the neutralizing anti-CD40L Ab MR-1 (40) or irrelevant isotype-matched Ab (CD40L Irr. Ig) for 3 wk, starting 1 wk before surgery. Neutralization of CD40L by MR-1 treatment decreased the loss of femoral BV/TV significantly as well as the deterioration of indices of bone structure induced by ovx (Fig. 1C).
Measurements of serum levels of C-terminal telopeptide of collagen (CTx), a marker of bone resorption, confirmed that ovx increases bone resorption in control mice but not in T cell-depleted mice, CD40L−/− mice, and mice treated with MR-1 Ab (Fig. 1D).
To determine whether T cells and CD40L promote bone resorption by stimulating osteoclastogenesis, BM was harvested 2 wk after surgery from T cell-depleted mice, CD40L null mice, and their respective controls and cultured with RANKL for 7 d. Ovx increases OC formation in BM from control mice but fails to do so in BM cultures from T cell-depleted, CD40L−/−, and MR-1–treated mice (Fig. 1E). Thus, both in vivo depletion of T cells and silencing of CD40L blunt the capacity of ovx to stimulate osteoclastic bone resorption and induce bone loss.
Ovx Increases the Number and Osteoclastogenic Activity of SCs Through CD40L.
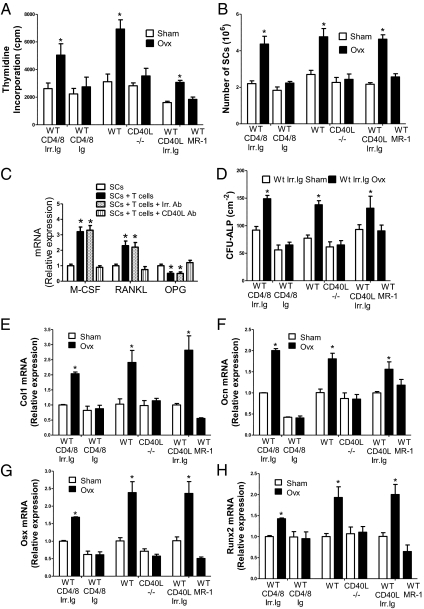
Next, we investigated whether ovx expands SCs and regulates their function through CD40L expressed on T cells. Ovx increased the proliferation of SCs from control mice but not those from T cell-depleted mice, CD40L−/− mice, and MR-1–treated mice (Fig. 2A). As a result, ovx increased the number of SCs harvested from the BM by approximately twofold in samples from control mice. By contrast, ovx had no effects on the number of SCs from T cell-depleted mice and mice lacking CD40L (Fig. 2B).
Fig. 2.
Effects (mean ± SEM) of T cells and ovx on SC cytokine mRNA and SC osteoblastic commitment, proliferation, and differentiation in T cell-depleted mice, CD40L−/− mice, and WT mice treated with the anti-CD40L Ab MR-1. (A) SC proliferation as assessed by pulsing with [3H]thymidine for 18 h. (B) Number of BM SCs. (C) Effect of T cells on the expression of SC cytokine mRNA. *P < 0.05 compared with the respective SC-only group. (D) CFU-ALP formation in BM cultures. The average of the colonies counted in six wells is shown. (E–H) SC expression of mRNA of the OB markers type I collagen (Col1), osteocalcin (Ocn), osterix (Osx), and runx2 (Runx2) (n = 4–5 per group). *P < 0.05, compared with the corresponding sham-operated group.
To investigate cytokine production, SCs and T cells purified from intact mice were cocultured for 7 d. The T cells were then removed, and the mRNA levels of M-CSF, RANKL, and OPG were measured by RT-PCR. Incubation with T cells altered the SC mRNA levels of these factors by two- to fourfold (Fig. 2C). Moreover, addition of anti-CD40L Ab but not irrelevant Ab blocked the regulating effects of T cells, suggesting that T cells up-regulate the SC production of osteoblastogenic and osteoclastogenic factors through CD40L.
To investigate the role of CD40L on SC differentiation, BM was harvested 2 wk after ovx or sham surgery and cultured for 1 wk to assess the formation of alkaline phosphatase (ALP)-positive cfu fibroblast, herein defined as CFU-ALP, an index of SC commitment to the osteoblastic lineage. Ovx increased CFU-ALP formation in the BM from T cell-replete mice by approximately twofold, although it had no effect on BM from T cell-depleted mice and CD40L null mice (Fig. 2D), thus indicating that ovx increases the number of SCs with osteogenic potential through T cell-expressed CD40L.
Analysis of the expression levels of osteoblastic genes in SCs revealed that ovx increased the expression of type 1 collagen, osteocalcin, osterix, and runx2 mRNAs by approximately two- to threefold in SCs from control mice (Fig. 2 E–H). In contrast, ovx had no effects on the expression of OB-related genes in SCs from T cell-depleted and CD40L null mice, thus demonstrating that T cells promote the capacity of ovx to expand the osteoblastic pool and cell differentiation along the osteoblastic lineage through CD40L.
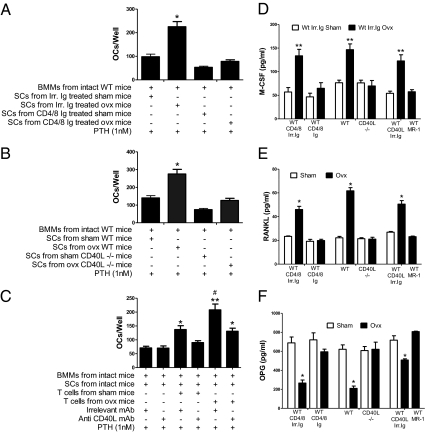
Ovx is also known to increase the osteoclastogenic activity of SCs (9), herein defined as their capacity to support OC formation in vitro. To test the hypothesis that ovx regulates the osteoclastogenic activity of SCs through T cells, control and T cell-depleted WT mice were killed 2 wk after surgery. BM SCs were purified and cocultured with BMMs harvested from intact WT mice for 7 d. The cocultures were stimulated with PTH, an agent that promotes OC formation by targeting SCs. At the end of the culture period, the cultures were stained for tartrate-resistant acid phosphatase and the OCs were counted. Under these conditions, PTH-induced OC formation is a function of SC osteoclastogenic activity. We found that cocultures with SCs from ovx T cell-replete mice produced a larger number of OCs than those with SCs from sham-operated T cell-replete mice (Fig. 3A). By contrast, cocultures with SCs from sham-operated and ovx T cell-depleted mice produced a similar number of OCs, suggesting that T cells are required for ovx to promote the generation of SCs with increased osteoclastogenic activity.
Fig. 3.
Effect (mean ± SEM) of T cells and CD40L on SC osteoclastogenic activity and cytokine secretion. (A) Effect of ovx on the osteoclastogenic activity of SCs from mice treated with irrelevant isotype-matched Ab (Irr. Ig) or anti-CD4 and anti-CD8 Abs (CD4/8 Ig). All cultures were stimulated with PTH (1 nM), an agent that targets SCs. (B) Effect of ovx on the osteoclastogenic activity of SCs from WT and CD40L−/− mice. All cultures were stimulated with PTH (1 nM). (C) Effect of T cells from sham-operated and ovx mice and of CD40L neutralization on the osteoclastogenic activity of SCs. All cultures were stimulated with PTH (1 nM). (D–F) Effect of ovx on the production of M-CSF, RANKL, and OPG by SCs from T cell-depleted mice, CD40L−/− mice, and MR-1–treated mice. *P < 0.05 and **P < 0.01 compared with the corresponding controls. #P < 0.05 compared with the other groups.
The experiment described above was repeated using SCs from WT and CD40L−/− mice. Cocultures containing SCs from WT ovx mice generated a larger number of OCs, as compared with those with SCs from WT sham-operated mice. Importantly, cocultures with SCs from sham-operated and ovx CD40L−/− mice generated a similar number of OCs (Fig. 3B), thus demonstrating that CD40L is required for ovx to generate SCs with high osteoclastogenic activity.
Next, SCs and BMMs from intact WT mice were cocultured with T cells harvested 2 wk after surgery from WT sham-operated and ovx mice. The cocultures were treated with PTH and either irrelevant Ig or neutralizing anti-CD40L Ab. We found (Fig. 3C) that cocultures with T cells from ovx mice formed a larger number of OCs than those with T cells from sham-operated mice and that neutralization of CD40L eliminated the increased capacity of T cells from ovx mice to stimulate OC formation. These findings confirmed that T cells regulate SC osteoclastogenic activity through CD40L.
One of the mechanisms by which ovx stimulates the osteoclastogenic activity of SCs is by regulating their production of osteoclastogenic cytokines. Thus, we sought to determine the contribution of T cells and CD40L to SC production of osteoclastogenic factors. To achieve this goal, BM was harvested 2 wk after surgery from sham-operated and ovx WT control mice, WT mice immunodepleted of T cells, CD40L−/− mice, and MR-1–treated WT mice. SCs were then purified, and the levels of M-CSF, RANKL, and OPG were measured in the 72-h culture media by ELISA. Ovx increased the SC production of M-CSF and RANKL and lowered that of OPG in T cells from control mice (Fig. 3 D–F), whereas ovx did not alter the production of these three cytokines by SCs from T cell-deficient WT mice and in mice with silent CD40L.
Ovx Disregulates T-Cell TNF Production Through CD40L.
To investigate the contribution of CD40L to the activation of T cells induced by ovx, WT mice were killed 2 wk after ovx or sham surgery, and whole BM was stained for CD4, CD8, and CD40L. Ovx increased the fraction of CD40L+CD4+ cells by approximately threefold, although it had negligible effects on CD8+ cells (Fig. S2A). Moreover, ovx increased the percentage of CD4+ and CD8+ cells expressing the activation marker CD69 by approximately two- to threefold in control mice, although it had no effects in CD40L−/− mice and MR-1–treated WT mice (Fig. S2 B–E). Analysis of T-cell proliferation by in vivo BrdU incorporation disclosed that ovx increased BM T-cell proliferation in WT mice but not in CD40L−/− mice and MR-1–treated WT mice (Fig. S2F). In accordance with the changes in T-cell proliferation, we found that ovx caused an ∼2.5-fold increase in the number of total T cells in WT mice, whereas it had no significant effects in CD40L−/− mice and MR-1–treated mice (Fig. S2G). Together, these findings demonstrate that CD40L plays a pivotal role in the mechanism by which ovx induces the expansion of T cells in the BM.
To investigate whether CD40L expression is required for ovx to increase TNF production in T cells, BM T cells were harvested 2 wk after ovx and sham surgery and cultured for 48 h. Intracellular staining for TNF showed that ovx increased the fraction of T cells producing TNF from 40 ± 0.5% to 48 ± 0.4% in WT mice but not in CD40L−/− mice and MR-1–treated mice. Measurements of TNF mRNA expression by RT-PCR and of TNF protein levels in the culture media by ELISA showed that ovx increased TNF mRNA levels in T cells (Fig. S2H) and TNF protein levels in the T-cell culture media by approximately twofold in samples from WT mice but not in those from CD40L−/− mice and MR-1–treated mice (Fig. S2I). Thus, ovx increases TNF production of T cells through CD40L mainly by increasing the number of BM T cells but also by enhancing the fraction of TNF-positive T cells and the TNF production per T cell.
Discussion
We report that immunodepletion of T cells in vivo and silencing of the T-cell costimulatory molecule CD40L abrogate the capacity of acute E deprivation to stimulate OB and OC formation and induce bone loss. We also show that CD40L is required for ovx to increase the T-cell production of TNF and the SC generation of osteoclastogenic cytokines. This insight supports a role for T-cell/bone-cell cross-talk in the mechanism by which ovx causes bone loss.
Among the mechanisms responsible for ovx-induced bone loss is an expansion in the BM of activated T cells that produce TNF (17, 24). This phenomenon results from enhanced thymic-dependent differentiation of BM-derived progenitors and peripheral expansion of mature T cells (41, 42). Ovx causes the peripheral expansion of T cells by enhancing antigen presentation through increased expression of MHCII (41, 43), which, in turn, is driven by a complex mechanism that involves increased production of IFN-γ and IL-7, blunted generation of TGF-β in the BM (22, 25, 41, 44), and up-regulation of the costimulatory molecule CD80 on dendritic cells secondary to increased oxidative stress (43, 45).
In support of a role of T cells in the bone loss induced by E deprivation are reports from our laboratory that ovx fails to induce trabecular and cortical bone loss in nude mice (17, 24, 25, 44) and in WT mice treated with abatacept (43), an agent that blocks T-cell costimulation and induces T-cell anergy and apoptosis (46, 47). An independent confirmation of the role of T cells was provided by Yamaza et al. (26), who reported that ovx fails to induce bone loss in nude mice and WT mice in which T-cell activation was blocked by aspirin. By contrast, Lee et al. (48) showed that nude mice are protected against the loss of cortical but not trabecular bone induced by ovx. In the same study, other strains of T-cell and T- and B-cell deficient mice were found to lose either trabecular or cortical bone after ovx (48). The discrepancy between our reports (17, 24, 25, 43, 44) and that of Lee et al. (48) is likely explained by differences in the experimental design, the lack of B cells in some models, and compensation mechanisms.
To avoid the confounding effect of compensation mechanisms, we investigated the effects of ovx in WT mice that were depleted of T cells in vivo by treatment with anti-CD4 and anti-CD8 Abs. We also determined the effects of ovx in CD40L−/− mice, a strain in which T-cell activation is impaired. Because T cell-deficient mice and CD40L−/− mice have reduced bone mass and increased bone turnover at baseline (36), additional studies were conducted in WT mice treated with MR-1, an Ab that neutralizes CD40L in vivo (40). T-cell immunodepletion was achieved by initiating anti-CD4/8 Ab treatment 2 wk before surgery. This design was required because the conditioning effect of T cells on SCs persists for ∼2 wk (38). Postsurgical follow-up was limited to 2 wk because partial T-cell recovery was observed in a preliminary study after longer CD4/8 Ab treatment. The length of the study was sufficient to evaluate the effects of gonadectomy on bone, because the rate of bone loss is highest in the first 2 wk after ovx. The changes in bone resorption and bone volume induced by ovx parallel those in lymphopoiesis and T-cell activation, which also peak early after ovx (41–43). Long-term studies are required to determine the role of CD40L during the chronic adaptation to E deficiency, however.
We found that both in vivo immunodepletion of T cells and silencing of CD40L confer complete protection against the increase in bone resorption and the bone loss induced by ovx. We also found that ovx up-regulates the expression of CD40L in CD4+ T cells and that silencing of CD40L abrogates the increase in T-cell TNF production that follows E withdrawal. Together, these data confirm that T cells and their costimulatory molecule CD40L play a pivotal role in ovx-induced bone loss.
A key finding of this study is that the deletion of CD40L blunts the stimulatory effects of ovx on SCs. Specifically, we found CD40L to be required for ovx to increase the capacity of SCs to support OC formation through enhanced production of M-CSF and RANKL and diminished secretion of OPG. Ovx was found to up-regulate the proliferation and differentiation of SCs, a finding likely to suggest that differentiation is restricted to SCs that are distinct from those undergoing proliferation. Moreover, SCs from T cell-depleted mice and CD40L−/− mice failed to respond to ovx with an increase in proliferation and enhanced differentiation into OBs. Thus, a critical additional mechanism by which T cells disregulate bone homeostasis in ovx mice is through CD40L-mediated cross-talk between T cells and SCs, which results in enhanced osteoclastogenesis and osteoblastogenesis. We thus propose a model (Fig. S3) whereby ovx increases the number of activated CD40L-expressing T cells that promote the expression of M-CSF and RANKL by SCs and down-regulates the SC production of OPG. The net result is a significant increase in the rate of osteoclastogenesis. The interaction of CD40L with CD40 on SCs in the context of E deficiency appears to override the protective effects of CD40/CD40L costimulation on basal B-cell OPG production (36), distorting the balance of OC formation in favor of bone loss.
The CD40L-mediated T-cell/SC cross-talk has been previously shown to play a critical role in the bone loss induced by continuous infusion of PTH, which is a model of primary hyperparathyroidism (38). Therefore, it will be important to determine if the conditioning effect of CD40L on SCs contributes to bone loss in other skeletal diseases.
Strikingly, although silencing of CD40L impairs the response to bone catabolic stimuli, such as ovx and PTH, CD40L is required for normal bone modeling and baseline remodeling. In fact, the current and previous reports from our laboratory (36, 38) have disclosed that CD40L−/− mice have a lower bone volume than WT controls, primarily because stimulation of CD40 signaling in B cells by T cell-expressed CD40L induces OPG production, thus reducing bone resorption (36). These findings are concordant with studies in humans demonstrating that osteopenia is a frequent clinical feature of subjects with X-linked hyper-IgM syndrome, an inherited disorder caused by mutations in the gene encoding CD40L (37).
T cell-deficient mice are also known to have low bone mass and increased bone resorption attributable to impaired B-cell production of OPG (36). In this study, however, indices of bone volume and structure were not significantly lower in sham-operated T cell-depleted mice as compared with T cell-replete controls because of the short duration of the T-cell depletion.
In summary, the present investigation demonstrates that acute E deprivation disregulates osteoblastogenesis, and osteoclastogenesis through the T-cell costimulatory receptor CD40L. Understanding the cross-talk between T cells and mesenchymal stem cells may yield previously undescribed therapeutical strategies for postmenopausal bone loss.
Materials and Methods
Animals, in Vivo T-Cell Depletion, and Treatment with Anti-CD40L Ab.
All the animal procedures were approved by the Institutional Animal Care and Use Committee of Emory University. WT mice were depleted of T cells in vivo as previously described (38). Effective depletion was confirmed by flow cytometry (Fig. S4). Mice were injected i.p. with 250 μg of the anti-mouse CD40L Ab MR-1 (40) (a gift of C. Larsen, Emory University) or isotype-matched irrelevant Ab at days −6, −3, 0, 7, and 14 relative to surgery.
μCT Measurements and Quantitative Bone Histomorphometry.
μCT scanning and analysis of the distal femur were performed as reported previously (38), using a Scanco μCT-40 scanner (Scanco Medical). Histomorphometric analysis of the distal metaphysis of the femur was performed as described (38) by the Histomorphometry Core Laboratory of the University of Alabama at Birmingham. Additional information is provided in SI Materials and Methods.
In Vitro OC Formation.
Whole BM or a mixture of BMMs, SCs, and T cells was cultured for 7 d in the presence of either 15 ng/mL RANKL (kindly provided by X. Feng, University of Alabama at Birmingham) and 10 ng/mL M-CSF or human PTH 1–34 (1 nM) to induce OC formation as described (38). Additional information is provided in SI Materials and Methods.
SC Purification and SC and T-Cell Coculture.
SCs were purified and cocultured with T cells as described (38). Additional information is provided in SI Materials and Methods.
Serum CTx and Cytokine Assays.
Serum CTx was measured by ELISA as described (38). TNF, RANKL, OPG, and M-CSF were measured in 72-h T-cell and SC culture medium by ELISA as described (38).
CFU-ALP Assay and Thymidine Incorporation Assay.
Colony-forming assays and a [3H]thymidine incorporation assay were carried out as described (38). Additional information is provided in SI Materials and Methods.
Flow Cytometry, Real-Time RT-PCR, and Primers.
Assays were carried out as previously described (38). Additional information is provided in SI Materials and Methods.
Statistical Analysis.
Information is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to the University of Alabama at Birmingham, Center for Metabolic Bone Disease-Histomorphometry Core Laboratory (National Institutes of Health Grant P30-AR46031) for the histomorphometric analysis presented herein and to Dr. Xu Feng (University of Alabama at Birmingham, Alabama) for the generous gift of RANKL. This study was supported by the National Institutes of Health (Grants AR49659 and AG28278). M.N.W. is supported, in part, by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grants AR053607 and AR059364) and by a Veterans Affairs Merit Grant (5I01BX000105).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013492108/-/DCSupplemental.
References
- 1.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 2.Riggs BL, Khosla S, Melton LJ., III Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 3.Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: An inflammatory tale. J Clin Invest. 2006;116:1186–1194. doi: 10.1172/JCI28550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Krum SA, et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27:535–545. doi: 10.1038/sj.emboj.7601984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jilka RL, et al. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow. Evidence for autonomy from factors released during bone resorption. J Clin Invest. 1998;101:1942–1950. doi: 10.1172/JCI1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Gregorio GB, et al. Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17 beta-estradiol. J Clin Invest. 2001;107:803–812. doi: 10.1172/JCI11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1 and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem. 1996;271:28890–28897. doi: 10.1074/jbc.271.46.28890. [DOI] [PubMed] [Google Scholar]
- 10.Kong YY, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 11.Hofbauer LC, et al. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commun. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 13.Manolagas SC, Kousteni S, Jilka RL. Sex steroids and bone. Recent Prog Horm Res. 2002;57:385–409. doi: 10.1210/rp.57.1.385. [DOI] [PubMed] [Google Scholar]
- 14.Pfeilschifter J, Köditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert LC, Rubin J, Nanes MS. The p55 TNF receptor mediates TNF inhibition of osteoblast differentiation independently of apoptosis. Am J Physiol Endocrinol Metab. 2005;288:E1011–E1018. doi: 10.1152/ajpendo.00534.2004. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, et al. Endogenous TNFalpha lowers maximum peak bone mass and inhibits osteoblastic Smad activation through NF-kappaB. J Bone Miner Res. 2007;22:646–655. doi: 10.1359/jbmr.070121. [DOI] [PubMed] [Google Scholar]
- 17.Roggia C, et al. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci USA. 2001;98:13960–13965. doi: 10.1073/pnas.251534698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ammann P, et al. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest. 1997;99:1699–1703. doi: 10.1172/JCI119333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimble RB, Bain S, Pacifici R. The functional block of TNF but not of IL-6 prevents bone loss in ovariectomized mice. J Bone Miner Res. 1997;12:935–941. doi: 10.1359/jbmr.1997.12.6.935. [DOI] [PubMed] [Google Scholar]
- 20.Lorenzo JA, et al. Mice lacking the type I interleukin-1 receptor do not lose bone mass after ovariectomy. Endocrinology. 1998;139:3022–3025. doi: 10.1210/endo.139.6.6128. [DOI] [PubMed] [Google Scholar]
- 21.Jilka RL, et al. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 22.Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110:1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cenci S, Weitzmann MN, Gentile MA, Aisa MC, Pacifici R. M-CSF neutralization and egr-1 deficiency prevent ovariectomy-induced bone loss. J Clin Invest. 2000;105:1279–1287. doi: 10.1172/JCI8672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cenci S, et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106:1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y, et al. Estrogen prevents bone loss through transforming growth factor beta signaling in T cells. Proc Natl Acad Sci USA. 2004;101:16618–16623. doi: 10.1073/pnas.0404888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaza T, et al. Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE. 2008;3:e2615. doi: 10.1371/journal.pone.0002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 28.Quezada SA, Jarvinen LZ, Lind EF, Noelle RJ. CD40/CD154 interactions at the interface of tolerance and immunity. Annu Rev Immunol. 2004;22:307–328. doi: 10.1146/annurev.immunol.22.012703.104533. [DOI] [PubMed] [Google Scholar]
- 29.André P, et al. CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- 30.Prasad KS, et al. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci USA. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zirlik A, et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007;115:1571–1580. doi: 10.1161/CIRCULATIONAHA.106.683201. [DOI] [PubMed] [Google Scholar]
- 32.Léveillé C, et al. CD40 ligand binds to alpha5beta1 integrin and triggers cell signaling. J Biol Chem. 2007;282:5143–5151. doi: 10.1074/jbc.M608342200. [DOI] [PubMed] [Google Scholar]
- 33.Grammer AC, Lipsky PE. CD40-mediated regulation of immune responses by TRAF-dependent and TRAF-independent signaling mechanisms. Adv Immunol. 2000;76:61–178. doi: 10.1016/s0065-2776(01)76019-1. [DOI] [PubMed] [Google Scholar]
- 34.Ahuja SS, et al. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology. 2003;144:1761–1769. doi: 10.1210/en.2002-221136. [DOI] [PubMed] [Google Scholar]
- 35.Grewal IS, Flavell RA. The role of CD40 ligand in costimulation and T-cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, et al. B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood. 2007;109:3839–3848. doi: 10.1182/blood-2006-07-037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Granados E, et al. Osteopenia in X-linked hyper-IgM syndrome reveals a regulatory role for CD40 ligand in osteoclastogenesis. Proc Natl Acad Sci USA. 2007;104:5056–5061. doi: 10.1073/pnas.0605715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Y, et al. T cells potentiate PTH-induced cortical bone loss through CD40L signaling. Cell Metab. 2008;8:132–145. doi: 10.1016/j.cmet.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lean JM, Chow JW, Chambers TJ. The rate of cancellous bone formation falls immediately after ovariectomy in the rat. J Endocrinol. 1994;142:119–125. doi: 10.1677/joe.0.1420119. [DOI] [PubMed] [Google Scholar]
- 40.Fernández FG, McKane B, Marshbank S, Patterson GA, Mohanakumar T. Inhibition of obliterative airway disease development following heterotopic murine tracheal transplantation by costimulatory molecule blockade using anti-CD40 ligand alone or in combination with donor bone marrow. J Heart Lung Transplant. 2005;24(Suppl):S232–S238. doi: 10.1016/j.healun.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Cenci S, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan MR, et al. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci USA. 2005;102:16735–16740. doi: 10.1073/pnas.0505168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grassi F, et al. Oxidative stress causes bone loss in estrogen-deficient mice through enhanced bone marrow dendritic cell activation. Proc Natl Acad Sci USA. 2007;104:15087–15092. doi: 10.1073/pnas.0703610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y, et al. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacifici R. T cells: Critical bone regulators in health and disease. Bone. 2010;47:461–471. doi: 10.1016/j.bone.2010.04.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreland L, Bate G, Kirkpatrick P. Abatacept. Nat Rev Drug Discov. 2006;5:185–186. doi: 10.1038/nrd1989. [DOI] [PubMed] [Google Scholar]
- 47.Ruderman EM, Pope RM. The evolving clinical profile of abatacept (CTLA4-Ig): A novel co-stimulatory modulator for the treatment of rheumatoid arthritis. Arthritis Res Ther. 2005;7(Suppl 2):S21–S25. doi: 10.1186/ar1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee SK, et al. T lymphocyte-deficient mice lose trabecular bone mass with ovariectomy. J Bone Miner Res. 2006;21:1704–1712. doi: 10.1359/jbmr.060726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.