Abstract
The thrombin receptor protease activated receptor-1 (PAR-1) is overexpressed in metastatic melanoma cell lines and tumor specimens. Previously, we demonstrated a significant reduction in tumor growth and experimental lung metastasis after PAR-1 silencing via systemic delivery of siRNA encapsulated into nanoliposomes. Gene expression profiling identified a 40-fold increase in expression of Maspin in PAR-1–silenced metastatic melanoma cell lines. Maspin promoter activity was significantly increased after PAR-1 silencing, suggesting that PAR1 negatively regulates Maspin at the transcriptional level. ChIP analyses revealed that PAR-1 decreases binding of Ets-1 and c-Jun transcription factors to the Maspin promoter, both known to activate Maspin transcription. PAR-1 silencing did not affect Ets-1 or c-Jun expression; rather it resulted in increased expression of the chromatin remodeling complex CBP/p300, as well as decreased activity of the CBP/p300 inhibitor p38, resulting in increased binding of Ets-1 and c-Jun to the Maspin promoter and higher Maspin expression. Functionally, Maspin expression reduced the invasive capability of melanoma cells after PAR-1 silencing, which was abrogated after rescuing with PAR-1. Furthermore, tumor growth and experimental lung metastasis was significantly decreased after expressing Maspin in a metastatic melanoma cell line. Moreover, silencing Maspin in PAR-1–silenced cells reverted the inhibition of tumor growth and experimental lung metastasis. Herein, we demonstrate a mechanism by which PAR-1 negatively regulates the expression of the Maspin tumor-suppressor gene in the acquisition of the metastatic melanoma phenotype, thus attributing an alternative function to PAR-1 other than coagulation.
Keywords: melanoma progression, transcriptional regulation, G protein-coupled receptor signaling, Serpin B5
The thrombin receptor is a seven-transmembrane G protein-coupled receptor also known as protease activated receptor-1 (PAR-1). PAR-1 activation leads to stimulation of G proteins triggering various downstream molecules and signal transduction pathways involved in cell growth, tumor progression, and metastasis of several cancer types including breast, prostate, and melanoma (1–3).
Our laboratory has found that PAR-1 is a key player in the progression of human melanoma, with overexpression of PAR-1 occurring in metastatic melanoma cell lines compared with nonmetastatic and primary melanoma cell lines (4). Furthermore, we and others have shown that melanoma tumors have increased PAR-1 expression compared with nevi (5, 6). Recently, we demonstrated that systemic delivery of PAR-1 siRNA encapsulated into neutral nanoliposomes inhibited tumor growth and experimental lung metastasis of melanoma cells in vivo (7). However, the exact mechanism by which PAR-1 contributes to melanoma growth, angiogenesis, and metastasis is not clear. By using gene expression profiling, we thus identified the serine protease inhibitor Maspin as a PAR-1 downstream target gene.
Maspin was first isolated from human mammary epithelial cells (8, 9). Its expression has been found to be decreased or lost in several malignancies and is associated with decreased aggressiveness by inhibiting tumor cell motility and invasion (10–12). Maspin expression has also been found to increase apoptosis as well as decrease angiogenesis in several cancers (13, 14).
In melanoma, Maspin was found to have a tumor-suppressor function. Denk and colleagues found that metastatic melanoma cells had decreased expression levels of Maspin compared with normal human epidermal melanocytes (15). Moreover, Maspin expression is lost with increased tumor thickness in clinical specimens as the tumor progresses from nonmetastatic to metastatic melanoma (16, 17). Previous reports have shown that early hypermethylation of the Maspin promoter might play a role in silencing Maspin. In addition, loss of Maspin was shown to correlate with increased tumor thickness (15–17).
Herein, we have identified an inverse correlation between PAR-1 and Maspin expression. PAR-1 differentially regulates the binding of c-Jun and Ets-1 transcription factors to the Maspin promoter through inhibition of phosphorylated p38 and activation of the histone acetyltransferase (HAT) CBP/p300, resulting in decreased Maspin expression. To our knowledge, this is the first report to identify PAR-1 as a regulator of the tumor suppressor Maspin, providing another mechanism by which PAR-1 contributes to the malignant phenotype of melanoma, and thus assigning a unique role for PAR-1 in tumor growth and metastasis in addition to its involvement in coagulation.
Results
Validation of Maspin as a Downstream Target Gene of PAR-1.
Our group has previously demonstrated a significant decrease in tumor growth and experimental lung metastasis after silencing PAR-1 in metastatic melanoma cell lines by reducing angiogenic and invasive factors such as VEGF, IL-8, matrix metalloproteinase-2 (MMP-2), and Cx-43 (7, 18). To further investigate how PAR-1 contributes to the malignant phenotype, we sought to identify additional downstream PAR-1 target genes crucial to melanoma progression.
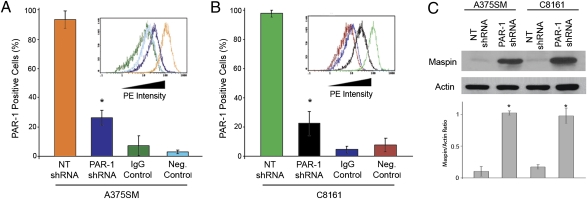
A375SM and C8161 metastatic melanoma cell lines were stably transduced with lentiviral-based PAR-1 shRNA. PAR-1 expression was reduced in both cell lines by more than 75% (Fig. 1 A and B and Fig. S1A). Although cDNA microarray analyses did not reveal a novel genetic program, several genes were regulated by PAR-1. Among them, Maspin expression was increased by more than 40-fold after PAR-1 silencing. Both protein and mRNA levels of Maspin were significantly increased in PAR-1–silenced cell lines (Fig. 1C and Fig. S1B).
Fig. 1.
Maspin expression after PAR-1 silencing in A375SM and C8161 melanoma cell lines. FACS analyses reveal a significant decrease in PAR-1 expression in (A) A375SM and (B) C8161 cells after transduction with PAR-1 shRNA. Mouse IgG was used as an isotype control. As a negative control, secondary phycoerythrin (PE) ab was used without PAR-1 ab. Bar colors in histograms correspond to data from the representative FACS analysis image (Insets). PE intensity indicates PAR-1 expression. (C) Western blot analysis of PAR-1–silenced cells depicts a significant increase in Maspin expression compared with NT-transduced cells. For all panels, data were obtained in triplicate and are expressed as mean values ± SD (*P < 0.001).
Transcriptional Regulation of Maspin After PAR-1 Silencing.
To determine whether PAR-1 is a negative regulator of Maspin, the Maspin promoter was cloned in front of a luciferase reporter construct and transfected into nontargeting (NT) or PAR-1–silenced melanoma cell lines. The promoter activity of Maspin was significantly increased after PAR-1 silencing, suggesting that PAR-1 negatively regulates Maspin at the transcriptional level (Fig. S2).
Differential Binding of Ets-1 and c-Jun Transcription Factors to the Maspin Promoter.
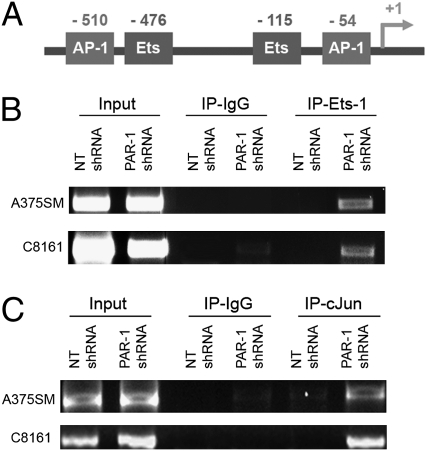
Maspin gene expression is regulated by several transcription factors, including AP-1 (Jun and Fos) and Ets-1 (19). Promoter mapping revealed two Ets-1 binding sites within 550 bp from the translation initiation site (TIS) as well as two AP-1 binding sites (Fig. 2A).
Fig. 2.
Differential binding of Ets-1 and c-Jun transcription factors to the Maspin promoter. (A) Illustration of two AP-1 transcription factor binding sites (−510 and 54 from TIS) and two Ets-1 binding sites (−476 and −115 from TIS) within the first 550 bp of the Maspin promoter. (B) ChIP studies depict increased binding of Ets-1 and (C) c-Jun to the promoter of Maspin in both PAR-1–silenced cell lines compared with NT-transduced cells.
To determine how PAR-1 was regulating Maspin expression, we analyzed whether PAR-1 was affecting transcription factor binding to the Maspin promoter, as no significant differences in expression levels of total or phosphorylated Ets-1 or AP-1 were detected after PAR-1 silencing (Fig. S3 A and B). ChIP studies revealed that silencing PAR-1 increased binding of Ets-1 and c-Jun to the Maspin promoter (Fig. 2 B and C). However, binding of c-Fos was not detected in NT shRNA-transduced cells or PAR-1–silenced cells (Fig. S3C). These results suggest that PAR-1 affects Maspin expression through differential binding of both Ets-1 and c-Jun transcription factors.
Effects of Transcription Factor Binding-Site Mutations on Maspin Promoter Activity.
To further analyze and corroborate that Maspin overexpression in PAR-1–silenced metastatic melanoma cells is a result of differential binding of Ets-1 and c-Jun, point mutations were made in both Ets-1 and AP-1 transcription factor binding sites.
As previously seen, Maspin promoter activity (without mutations) was again increased after PAR-1 silencing in both cell lines (Fig. S4). Mutating the proximal Ets-1 site completely abrogated these effects as Maspin promoter activity in PAR-1–silenced cells was similar to that in NT-transduced cells. Mutating AP-1 at position −54 also reduced Maspin expression in PAR-1–silenced cells but to a lesser degree than with the Ets-1 (−115) mutation in both cell lines. Interestingly, mutating the distal Ets-1 or AP-1 sites did not affect Maspin promoter activity in the C8161 cell line and minimally in A375SM as previously described (Fig. S4) (19). These results suggest that PAR-1 regulates Maspin promoter activity via the proximal Ets-1 site alone or in combination with the proximal c-Jun site.
PAR-1 Regulates Binding of Ets-1 and c-Jun to the Maspin Promoter Through CBP/p300.
As no differences in Ets-1 or c-Jun protein levels were found after silencing PAR-1, we determined whether PAR-1 affected HAT activity, which could account for increased binding of Ets-1 and c-Jun to the Maspin promoter after PAR-1 silencing.
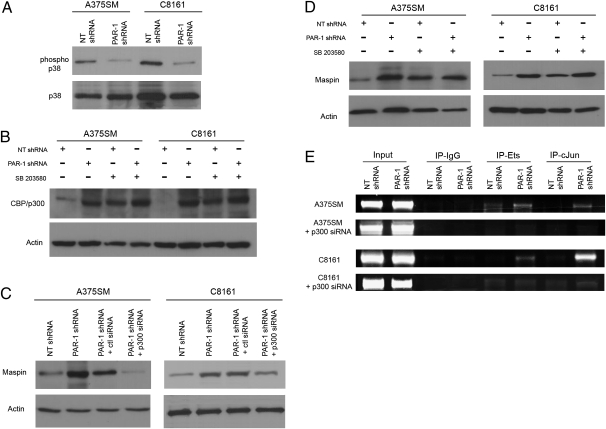
Previous studies have shown that PAR-1 activates the p38 MAPK pathway (20–23). A significant decrease in phospho p38 expression was found in PAR-1–silenced cell lines (Fig. 3A and Fig. S5). Total levels of p38 were not altered. Moreover, p38 activity inhibits the transcriptional coactivator CBP/p300 by decreasing its HAT activity (24, 25). In accordance with these reports, our studies revealed low levels of acetylated CBP/p300 in NT-transduced cells (high PAR-1, high phopsho-p38 expressors) compared with PAR-1–silenced cells (Fig. 3B). By using an inhibitor of phospho-p38 (SB 203580) (26) (Fig. S6A), an increase in CBP/p300 expression is seen in NT-transduced cell lines, demonstrating that p38 inhibits CBP/p300 (Fig. 3B and Fig. S6B). Overall, these results suggest that PAR-1 inhibits CBP/p300 through increased p38 MAPK signaling.
Fig. 3.
PAR-1 regulates Maspin expression by modulating binding of Ets-1 and c-Jun transcription factors via p38 and CBP/p300. (A) Phospho-p38 expression was significantly decreased after silencing PAR-1 in metastatic melanoma cells, whereas total levels of p38 remain unaffected. (B) Phospho-CBP/p300 was increased in PAR-1–silenced cells compared with NT-transduced cells. A decrease in phospho-p38, using 10 μM of the p38 inhibitor (SB203580), results in a significant increase in CBP/p300 expression in NT shRNA-transduced cells (i.e., low CBP/p300 expressors). (C) Silencing CBP/p300 with siRNA in PAR-1–silenced cells, results in decreased Maspin expression similar to levels from NT shRNA-transduced cells. (D) Inhibition of p38 results in increased Maspin expression in NT shRNA-transduced cells. Data are presented as means ± SD from three independent experiments. (E) ChIP shows increased binding of Ets-1 and c-Jun to the Maspin promoter in PAR-1–silenced cells. Silencing CBP/p300 with siRNA in PAR-1–silenced cells decreased binding of Ets-1 and c-Jun to the Maspin promoter.
To determine whether CBP/p300 expression directly affects Maspin expression, CBP/p300 siRNA was transduced in both metastatic cell lines (Fig. S6C). An increase in Maspin expression is seen after PAR-1 silencing, which is abrogated with the use of CBP/p300 siRNA (Fig. 3C and Fig. S6D). Moreover, inhibiting phospho p38 results in increased Maspin expression in NT-transduced cells (Fig. 3D and Fig. S6E), further establishing that PAR-1 regulates Maspin through increased p38 and decreased CBP/p300 expression.
CBP/p300 has been previously shown to bind directly to both c-Jun and Ets-1, thereby affecting their gene transactivation functions (27–29). Thus, we hypothesized that CBP/p300 overexpression, after PAR-1 silencing, increased binding of Ets-1 and c-Jun to the Maspin promoter. To test this hypothesis, ChIP analyses were performed with CBP/p300 siRNA. Silencing melanoma cells with both PAR-1 shRNA and CBP/p300 siRNA resulted in decreased transcription factor binding to the Maspin promoter compared with PAR-1–silenced cells with normal CBP/p300 levels (Fig. 3E). These studies demonstrate that CBP/p300 is crucial for regulating binding of Ets-1 and c-Jun to the Maspin promoter.
Effects of PAR-1 Expression on Melanoma Cell Invasion.
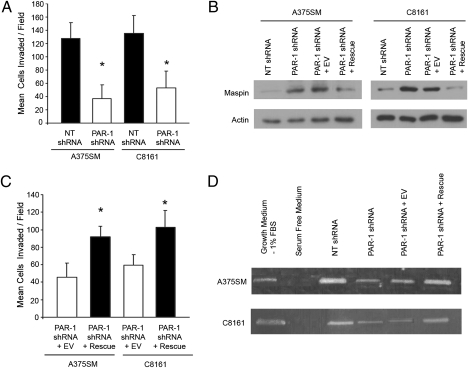
Previously, melanoma cell lines transfected with Maspin were found to have reduced invasive capacity (15). Invasion assays reveal that PAR-1–silenced melanoma cell lines had significantly lower numbers of invading cells (Fig. 4A). To determine that silencing PAR-1 results in decreased cell invasion through up-regulation of Maspin expression, PAR-1 was rescued in PAR-1–silenced cell lines (Fig. S7A). The protein levels of Maspin were significantly decreased after PAR-1 rescue, similar to Maspin protein levels from NT-transduced cells (Fig. 4B and Fig S7B). To ascertain the inverse correlation between PAR-1 and Maspin, we used the SB-2 nonmetastatic melanoma cell line (i.e., low PAR-1 expressor) transduced with a PAR1 expression vector (30). Overexpression of PAR-1 in SB-2 cells also resulted in decreased expression of Maspin (Fig. S7C).
Fig. 4.
Effects of altering PAR-1 levels on Maspin expression and invasion. (A) Invasion assays demonstrate a significant decrease in the number of invasive PAR-1–silenced cells. Data are presented as means ± SD (*P < 0.001). (B) Western blots revealed that Maspin is significantly reduced in PAR-1–rescued cells similar to Maspin protein levels from NT-transduced cells. (C) Rescuing PAR-1 significantly increased cell invasiveness compared with PAR-1–silenced cells transduced with EV (*P < 0.001). Data are presented as means ± SD. (D) Zymography assay depicts decreased MMP-2 activity in PAR-1–silenced cells. Rescuing PAR-1 results in increased MMP-2 activity compared with PAR-1-silenced cells transduced with EV. FBS 1% is used as a positive control. Serum-free medium served as a negative control.
To corroborate that silencing PAR-1 results in decreased invasion through up-regulation of Maspin, PAR-1–rescued cells were subsequently used in invasion assays. Fig. 4C demonstrates that rescuing PAR-1 results in a significant increase in invasion. Furthermore, to determine whether the mechanism by which Maspin decreases the invasive capability of cells is a result of decreased MMP-2 activity, zymography assays were performed. In both A375SM and C8161 cell lines, PAR-1 silencing (i.e., high Maspin expression) resulted in decreased MMP-2 activity compared with NT-transduced cells (i.e., low Maspin expression; Fig. 4D). These effects were reversed when PAR-1 was rescued, showing that MMP-2 activity was higher in the PAR-1–rescued cells compared with PAR-1–silenced cells transduced with an empty vector (EV) construct (Fig. 4D).
To further demonstrate that Maspin was indeed affecting invasion, Maspin was overexpressed in both parental cell lines (i.e., low Maspin expression; Fig. S8A). These cells now showed decreased invasiveness (Fig. S8B), as previously reported (15). Moreover, a zymography assay was performed to determine whether Maspin was decreasing invasion by directly inhibiting MMP-2 activity. Fig. S8C demonstrates a decrease in MMP-2 activity in cells transduced with a Maspin expression vector, thereby suggesting that Maspin directly affects MMP-2 activity.
Effects of Maspin Expression on Tumor Growth and Experimental Lung Metastasis.
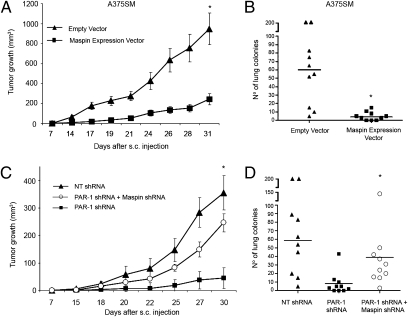
To determine the significance of Maspin in our in vivo model, we injected metastatic melanoma cell lines transduced with a Maspin expression vector, and found that overexpression of Maspin in A375SM significantly reduced tumor growth and experimental lung metastasis (Fig. 5 A and B). IHC analyses on tumors arising from cells transduced with a Maspin expression vector showed increased Maspin expression as well as decreased MMP-2 expression. Moreover, these Maspin-overexpressing tumors have decreased VEGF expression (Fig. S9). We have previously shown that PAR-1 can regulate expression of VEGF and MMP-2 in vivo (18). However, we now show that this can occur through regulation of Maspin by PAR-1.
Fig. 5.
In vivo effects of Maspin in melanoma. Maspin was stably transduced into A375SM cells (i.e., low Maspin expression) and injected s.c. and i.v. into nude mice. Significant decreases in (A) tumor growth and (B) experimental lung metastasis were seen in mice injected with A375SM expressing Maspin. (C) Silencing Maspin in PAR-1–silenced cells significantly increased tumor growth similar to levels from NT-transduced cells. Data are presented as means ± SEM. (D) Silencing Maspin in PAR-1–silenced cells significantly increased lung colony formation compared with PAR1-silenced cells alone. (*P < 0.01; n = 10 per group.)
To fully establish that PAR-1 inhibits the Maspin tumor-suppressor gene to promote the metastatic melanoma phenotype, Maspin was stably silenced in PAR-1–silenced cells (i.e., high Maspin expression; Fig. S10A). As seen in Fig. 5 C and D, silencing Maspin expression in PAR-1–silenced cells significantly increased melanoma tumor growth and experimental lung metastasis, although not to levels of NT-transduced cells. Furthermore, IHC analyses revealed that silencing Maspin in PAR-1–silenced cells increased expression of MMP-2 and VEGF (Fig. S10B).
Taken together, our data reveal a mechanism by which overexpression of PAR-1 contributes to the acquisition of the malignant phenotype of human melanoma by down-regulating the Maspin tumor-suppressor gene.
Discussion
Maspin has been previously described as having antiangiogenic and antimetastatic functions in several cancers (31, 32). In fact, receptor activator of NF-κB ligand-activated IκB kinase-α has been shown to translocate to the nucleus of prostate epithelial cells, resulting in inhibition of Maspin, thereby inducing prostate cancer metastasis to bone (33). Receptor activator of NF-κB ligand is known to regulate bone metastasis in prostate and breast cancers but does not seem to play a major role in melanoma metastasis (34–36). Although PAR-1 is known to activate the NF-κB pathway (37, 38), IκB kinase-α does not seem to function as a Maspin inhibitor in melanoma cells, again pointing to the tissue-specific regulation and function of Maspin.
Recently, the expression level of Maspin was found to be decreased in metastatic melanoma cells, which resulted in a more invasive phenotype (15). Increased tumor thickness also correlated with decreased Maspin expression, as only tumor samples less than 1 mm thick had positive Maspin expression (16, 17). Although Maspin promoter hypermethylation has been suggested to decrease Maspin expression (15, 17), we have found a link between PAR-1 and Maspin regulation through CBP/p300 HAT activity.
Metastatic melanoma cell lines were stably transduced with PAR-1 shRNA or NT shRNA and subjected to cDNA microarray analyses. Through such analyses, we have recently identified connexin 43 as a downstream target of PAR-1 (18). Herein, we describe PAR-1 regulation of Maspin in melanoma, which was found to be increased by more than 40-fold after PAR-1 silencing. These two regulatory events are not mutually exclusive, suggesting that PAR-1 is involved in several steps in the metastatic cascade including attachment to endothelial cells (Cx-43) and invasion (Maspin and MMP-2). Thus, the changes in the metastatic capacity of melanoma cells after PAR-1 silencing can occur through the regulation of several genes including, but not limited to, Cx-43, Maspin, MMP-2, VEGF, IL-8, and PAFR.
To further elucidate the role of Maspin in the metastatic phenotype of melanoma, we determined whether PAR-1 was regulating Maspin transcription. The Maspin promoter was thus cloned in front of a luciferase reporter vector. Our results indicate that Maspin promoter activity was increased by almost threefold in both A375SM and C8161 PAR-1–silenced melanoma cell lines. The significant increase in Maspin promoter luciferase activity demonstrates that PAR-1 regulates Maspin at the transcriptional level.
Although we did not find a difference in the protein expression of Ets-1 or AP-1 (Jun and Fos) after PAR-1 silencing, our ChIP analyses demonstrate an increase in both Ets-1 and c-Jun binding to the Maspin promoter in PAR-1–silenced cells. Interestingly, ChIP analyses failed to detect c-Fos binding. There are several possibilities that can explain the apparent lack of c-Fos binding, including Jun binding to another Fos family member or the AP-1 site on the Maspin promoter having a higher affinity for Jun homodimers than Jun/Fos heterodimers. Nevertheless, inhibition of c-Jun alone has been found to inhibit Maspin promoter activity (39). Mutational analyses for each AP-1 and Ets-1 sites in PAR-1–silenced cell lines further revealed that the proximal AP-1 and Ets-1 sites were essential for regulation of Maspin promoter activity. These results confirm previous reports by Zhang et al. showing that the distal Ets-1 and AP-1 sites were inefficient in causing Maspin promoter activation (19).
As PAR-1 did not affect the expression of either Ets-1 or c-Jun, we sought to elucidate the mechanism by which silencing PAR-1 resulted in increased binding of these transcription factors to the Maspin promoter. Previous reports demonstrated that p38 is activated by PAR-1 and that CBP/p300 HAT activity can be decreased by p38 (24, 25). Indeed, our results revealed a decrease in phospho-p38 along with an increase in CBP/p300 in PAR-1–silenced cells with a concomitant increase in Maspin expression. ChIP analyses show that inhibiting CBP/p300 decreased binding of Ets-1 and c-Jun to the Maspin promoter even after PAR-1 silencing, thereby corroborating previous work demonstrating that CBP/p300 affected their transactivation capability (27, 28, 40, 41). It is important to note that PAR-1 does not affect expression of Ets-1 or AP-1. Rather, PAR-1 signaling increases binding of these transcriptional cofactors to the Maspin promoter. These data therefore suggest that silencing PAR-1 increases Ets-1 and c-Jun binding to the Maspin promoter as a result of decreased phospho-p38 levels and increased CBP/p300 expression.
Functionally, Maspin has been found to decrease the invasive capability of various cancer cell types, including melanoma. Our results also show a significant decrease in invasion of PAR-1–silenced cells as well as in Maspin-overexpressing melanoma cell lines. Furthermore, the decrease in invasion was abrogated when PAR-1 was rescued in PAR-1–silenced cells, which reverted to having low levels of Maspin similar to levels from NT-transduced cells.
This decrease in invasion in PAR-1–silenced, Maspin-expressing cells can be explained by a decrease in MMP-2 activity. We have previously demonstrated, through IHC analyses, that MMP-2 levels are decreased in tumors from mice treated with PAR-1 siRNA–dioleoyl-phosphatidylcholine (7). Previous studies have also shown that re-expressing Maspin reduces the expression of MMP-2 and the invasive capability of cells (15). Thus, silencing PAR-1 results in increased Maspin expression, ultimately leading to decreased cell invasiveness as a result of decreased MMP-2 activity.
We further tested the significance of Maspin expression in our in vivo mouse model. Low Maspin-expressing cells were stably transduced with a Maspin expression vector and used for in vivo studies. These studies showed decreased tumor growth and experimental lung metastasis, thereby demonstrating that Maspin was indeed acting as a tumor-suppressor gene in human melanoma. We further observed that MMP-2 expression was decreased in tumors from Maspin-expressing melanoma cells, thus corroborating our in vitro data.
To further demonstrate that inhibition of Maspin by PAR-1 promotes the metastatic melanoma phenotype, PAR-1–silenced cells (i.e., high Maspin expression) were transduced with Maspin shRNA. In vivo, these cells now have increased tumor growth and increased experimental lung metastasis as compared with cells transduced with only PAR-1 shRNA. Although a significant increase in tumor growth and experimental lung metastasis was seen when Maspin was silenced in A375SM PAR-1–silenced cells, it did not completely reach the levels of NT shRNA-transduced cells. Thus, Maspin is an essential gene (yet not the sole gene) involved in PAR-1 regulation of melanoma metastasis.
Moreover, previous studies have shown that Maspin expression inversely correlates with angiogenesis in melanoma and breast cancer (14, 16, 42). When VEGF was analyzed by IHC from in vivo tumors, overexpression of Maspin resulted in decreased VEGF expression. This correlation was also seen when Maspin was silenced in PAR-1–silenced cells whereby these tumors now show increased levels of VEGF. Thus, PAR-1 affects melanoma invasion and metastasis through Maspin as well as regulation of MMP-2 and VEGF.
Overall, our data show that PAR-1 regulates the expression of the tumor-suppressor Maspin through differential binding of Ets-1 and c-Jun to the Maspin promoter via regulation of CBP/p300 activity, thus demonstrating a unique mechanism by which PAR-1 contributes to the malignant phenotype of melanoma.
Materials and Methods
ChIP.
ChIP assays were performed by using the ChIP-IT Express kit from Active Motif according to the manufacturer's protocol. The PCR for Maspin transcription factors (Ets-1, c-Fos, and c-Jun) was performed using the REDAccuTaq LA DNA Polymerase (Sigma) in a 25-μL reaction mixture containing 5% DMSO. A 662-bp fragment spanning −579 to +83 region of the Maspin promoter was amplified by PCR by using 10 μM of primers 5-GGCCTGAGTAATCCTAATCACAG-3′ and 5′CCGCTCGAGGCAGCGGTGGCTCACCTGGGCAGC-3′.
PAR-1 Rescue Expression Vector.
PAR-1 constructs with an N-terminal prolactin signal peptide and flag tag (provided by Shaun R. Coughlin, University of California, San Francisco, CA) were combined with a nontargetable PAR-1 coding region and ligated (into the pLVX-DsRed-Monomer-C1 vector; Clontech) as previously described (18).
Invasion Assays.
Invasion assays were performed using Biocoat Matrigel invasion chambers (Becton-Dickinson) primed according to the manufacturer's instructions. FBS 20% in normal growth medium was placed in the lower chamber as a chemoattractant. Melanoma cells (2.5 × 103) in 500 μL of growth medium containing 0.2% FBS were added to the upper chamber of the Matrigel plate and incubated at 37 °C overnight. Cells on the lower surface of the filter were stained with Hema 3 staining kit (Fisher) and counted. Data were expressed as average number of cells from four fields per sample that migrated to the lower chamber. Triplicates were run per cell condition.
Zymography.
MMP-2 activity was determined on substrate-impregnated gels as previously described (43).
In Vivo Experiments.
In vivo experiments were performed as previously described (7) in accordance with an animal protocol approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee. Briefly, 5 × 105 A375SM cells transduced with a Maspin expression vector or EV or 2.5 × 105 A375SM cells transduced with NT shRNA alone or PAR-1 shRNA alone or in combination with Maspin shRNA were injected s.c. into nude mice. Levels were measured twice weekly and mice were killed at 5 wk. To analyze for experimental lung metastasis, 1 × 106 cells were injected i.v. into the tail vein of mice, which were killed after 5 wk, at which time the lungs were fixed in Bouin solution and the metastatic colonies counted (n = 10 per group).
Statistics.
The Student t test was used to evaluate the data. P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Didier Trono (École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland) for providing the lentiviral backbone vectors used to incorporate PAR-1 shRNA as well as Rachel Bar-Shavit (Hadassah-University Hospital, Jerusalem, Israel) for providing PAR-1–expressing SB-2 cells. We also thank Shaun R. Coughlin (Cardiovascular Research Institute, University of California, San Francisco, CA) for kindly providing the PAR-1 expression constructs. This work was supported by National Institutes of Health (NIH) Grant R01 CA76098, NIH Specialized Programs of Research Excellence in Skin Cancer Grant P50-CA093459 (to M.B.E.), and a McCarthy Fellowship in Skin Cancer Research (to G.J.V.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSM596624).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006886108/-/DCSupplemental.
References
- 1.Even-Ram S, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–914. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 2.Tellez C, Bar-Eli M. Role and regulation of the thrombin receptor (PAR-1) in human melanoma. Oncogene. 2003;22:3130–3137. doi: 10.1038/sj.onc.1206453. [DOI] [PubMed] [Google Scholar]
- 3.Traynelis SF, Trejo J. Protease-activated receptor signaling: New roles and regulatory mechanisms. Curr Opin Hematol. 2007;14:230–235. doi: 10.1097/MOH.0b013e3280dce568. [DOI] [PubMed] [Google Scholar]
- 4.Tellez C, McCarty M, Ruiz M, Bar-Eli M. Loss of activator protein-2alpha results in overexpression of protease-activated receptor-1 and correlates with the malignant phenotype of human melanoma. J Biol Chem. 2003;278:46632–46642. doi: 10.1074/jbc.M309159200. [DOI] [PubMed] [Google Scholar]
- 5.Massi D, et al. Expression of protease-activated receptors 1 and 2 in melanocytic nevi and malignant melanoma. Hum Pathol. 2005;36:676–685. doi: 10.1016/j.humpath.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Tellez CS, et al. Quantitative analysis of melanocytic tissue array reveals inverse correlation between activator protein-2alpha and protease-activated receptor-1 expression during melanoma progression. J Invest Dermatol. 2007;127:387–393. doi: 10.1038/sj.jid.5700539. [DOI] [PubMed] [Google Scholar]
- 7.Villares GJ, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68:9078–9086. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou Z, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 9.Sheng S, et al. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci USA. 1996;93:11669–11674. doi: 10.1073/pnas.93.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sager R, Sheng S, Pemberton P, Hendrix MJ. Maspin: A tumor suppressing serpin. Curr Top Microbiol Immunol. 1996;213:51–64. doi: 10.1007/978-3-642-61107-0_4. [DOI] [PubMed] [Google Scholar]
- 11.Maass N, et al. Down regulation of the tumor suppressor gene Maspin in breast carcinoma is associated with a higher risk of distant metastasis. Clin Biochem. 2001;34:303–307. doi: 10.1016/s0009-9120(01)00220-x. [DOI] [PubMed] [Google Scholar]
- 12.Zou Z, et al. Maspin expression profile in human prostate cancer (CaP) and in vitro induction of Maspin expression by androgen ablation. Clin Cancer Res. 2002;8:1172–1177. [PubMed] [Google Scholar]
- 13.Jiang N, Meng Y, Zhang S, Mensah-Osman E, Sheng S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene. 2002;21:4089–4098. doi: 10.1038/sj.onc.1205507. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med. 2000;6:196–199. doi: 10.1038/72303. [DOI] [PubMed] [Google Scholar]
- 15.Denk AE, et al. Loss of maspin expression contributes to a more invasive potential in malignant melanoma. Pigment Cell Res. 2007;20:112–119. doi: 10.1111/j.1600-0749.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 16.Chua R, et al. Maspin expression, angiogenesis, prognostic parameters, and outcome in malignant melanoma. J Am Acad Dermatol. 2009;60:758–766. doi: 10.1016/j.jaad.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Wada K, Maesawa C, Akasaka T, Masuda T. Aberrant expression of the maspin gene associated with epigenetic modification in melanoma cells. J Invest Dermatol. 2004;122:805–811. doi: 10.1111/j.0022-202X.2004.22308.x. [DOI] [PubMed] [Google Scholar]
- 18.Villares GJ, et al. Overexpression of protease-activated receptor-1 contributes to melanoma metastasis via regulation of connexin 43. Cancer Res. 2009;69:6730–6737. doi: 10.1158/0008-5472.CAN-09-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Maass N, Magit D, Sager R. Transactivation through Ets and Ap1 transcription sites determines the expression of the tumor-suppressing gene maspin. Cell Growth Differ. 1997;8:179–186. [PubMed] [Google Scholar]
- 20.Mitsui H, Maruyama T, Kimura S, Takuwa Y. Thrombin activates two stress-activated protein kinases, c-Jun N-terminal kinase and p38, in HepG2 cells. Hepatology. 1998;27:1362–1367. doi: 10.1002/hep.510270524. [DOI] [PubMed] [Google Scholar]
- 21.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- 22.Kanda Y, Mizuno K, Kuroki Y, Watanabe Y. Thrombin-induced p38 mitogen-activated protein kinase activation is mediated by epidermal growth factor receptor transactivation pathway. Br J Pharmacol. 2001;132:1657–1664. doi: 10.1038/sj.bjp.0703952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Luo J, Fu Y, He S. Induction of interleukin-8 secretion and activation of ERK1/2, p38 MAPK signaling pathways by thrombin in dermal fibroblasts. Int J Biochem Cell Biol. 2006;38:1571–1583. doi: 10.1016/j.biocel.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Poizat C, Puri PL, Bai Y, Kedes L. Phosphorylation-dependent degradation of p300 by doxorubicin-activated p38 mitogen-activated protein kinase in cardiac cells. Mol Cell Biol. 2005;25:2673–2687. doi: 10.1128/MCB.25.7.2673-2687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sánchez-Molina S, et al. The histone acetyltransferases CBP/p300 are degraded in NIH 3T3 cells by activation of Ras signalling pathway. Biochem J. 2006;398:215–224. doi: 10.1042/BJ20060052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trevino JG, et al. Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: Implications for angiogenesis. Cancer Res. 2005;65:7214–7222. doi: 10.1158/0008-5472.CAN-04-3858. [DOI] [PubMed] [Google Scholar]
- 27.Bannister AJ, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 28.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 29.Jayaraman G, et al. p300/cAMP-responsive element-binding protein interactions with ets-1 and ets-2 in the transcriptional activation of the human stromelysin promoter. J Biol Chem. 1999;274:17342–17352. doi: 10.1074/jbc.274.24.17342. [DOI] [PubMed] [Google Scholar]
- 30.Even-Ram SC, et al. Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the alpha vbeta 5 integrin. J Biol Chem. 2001;276:10952–10962. doi: 10.1074/jbc.M007027200. [DOI] [PubMed] [Google Scholar]
- 31.Bailey CM, Khalkhali-Ellis Z, Seftor EA, Hendrix MJ. Biological functions of maspin. J Cell Physiol. 2006;209:617–624. doi: 10.1002/jcp.20782. [DOI] [PubMed] [Google Scholar]
- 32.Chen EI, Yates JR. Maspin and tumor metastasis. IUBMB Life. 2006;58:25–29. doi: 10.1080/15216540500531721. [DOI] [PubMed] [Google Scholar]
- 33.Luo JL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 34.Antonarakis ES, Carducci MA, Eisenberger MA. Novel targeted therapeutics for metastatic castration-resistant prostate cancer. Cancer Lett. 2010;291:1–13. doi: 10.1016/j.canlet.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fizazi K, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 36.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 37.Lin CH, et al. c-Src mediates thrombin-induced NF-kappaB activation and IL-8/CXCL8 expression in lung epithelial cells. J Immunol. 2006;177:3427–3438. doi: 10.4049/jimmunol.177.5.3427. [DOI] [PubMed] [Google Scholar]
- 38.Rahman A, et al. Galpha(q) and Gbetagamma regulate PAR-1 signaling of thrombin-induced NF-kappaB activation and ICAM-1 transcription in endothelial cells. Circ Res. 2002;91:398–405. doi: 10.1161/01.res.0000033520.95242.a2. [DOI] [PubMed] [Google Scholar]
- 39.O'Donnell JD, Linger RJ, Kruk PA. BRCA1 185delAG mutant protein, BRAt, up-regulates maspin in ovarian epithelial cells. Gynecol Oncol. 2010;116:262–268. doi: 10.1016/j.ygyno.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 40.Arias J, et al. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 41.Yang C, Shapiro LH, Rivera M, Kumar A, Brindle PK. A role for CREB binding protein and p300 transcriptional coactivators in Ets-1 transactivation functions. Mol Cell Biol. 1998;18:2218–2229. doi: 10.1128/mcb.18.4.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaefer JS, Zhang M. Role of maspin in tumor metastasis and angiogenesis. Curr Mol Med. 2003;3:653–658. doi: 10.2174/1566524033479519. [DOI] [PubMed] [Google Scholar]
- 43.Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281:2911–2922. doi: 10.1074/jbc.M508683200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.