Abstract
Bumble bees (Bombus) are vitally important pollinators of wild plants and agricultural crops worldwide. Fragmentary observations, however, have suggested population declines in several North American species. Despite rising concern over these observations in the United States, highlighted in a recent National Academy of Sciences report, a national assessment of the geographic scope and possible causal factors of bumble bee decline is lacking. Here, we report results of a 3-y interdisciplinary study of changing distributions, population genetic structure, and levels of pathogen infection in bumble bee populations across the United States. We compare current and historical distributions of eight species, compiling a database of >73,000 museum records for comparison with data from intensive nationwide surveys of >16,000 specimens. We show that the relative abundances of four species have declined by up to 96% and that their surveyed geographic ranges have contracted by 23–87%, some within the last 20 y. We also show that declining populations have significantly higher infection levels of the microsporidian pathogen Nosema bombi and lower genetic diversity compared with co-occurring populations of the stable (nondeclining) species. Higher pathogen prevalence and reduced genetic diversity are, thus, realistic predictors of these alarming patterns of decline in North America, although cause and effect remain uncertain.
Bumble bees (Bombus) are integral wild pollinators within native plant communities throughout temperate ecosystems (1–5), and recent domestication has boosted their economic importance in crop pollination to a level surpassed only by the honey bee (6). Their robust size, long tongues, and buzz-pollination behavior (high-frequency buzzing to release pollen from flowers) significantly increase the efficiency of pollen transfer in multibillion dollar crops such as tomatoes and berries. Disturbing reports of bumble bee population declines in Europe have recently spilled over into North America, fueling environmental and economic concerns of global decline (7–9). However, the evidence for large-scale range reductions across North America is lacking. Many reports of decline are unpublished, and the few published studies are limited to independent local surveys in northern California/southern Oregon (10), Ontario, Canada (11), and Illinois (12).
Furthermore, causal factors leading to the alleged decline of bumble bee populations in North America remain speculative. One compelling but untested hypothesis for the cause of decline in the United States (10) entails the spread of a putatively introduced pathogen, Nosema bombi, which is an obligate intracellular microsporidian parasite found commonly in bumble bees throughout Europe (13–16) but largely unstudied in North America. Pathogenic effects of N. bombi may vary depending on the host species and reproductive caste and include reductions in colony growth and individual life span and fitness (15, 16). Population genetic factors could also play a role in Bombus population decline (8). For instance, small effective population sizes and reduced gene flow among fragmented habitats can result in losses of genetic diversity with negative consequences (17), and the detrimental impacts of these genetic factors can be especially intensified in bees (18). Population genetic studies of Bombus are rare worldwide. A single study in the United States identified lower genetic diversity and elevated genetic differentiation (FST) among Illinois populations of the putatively declining B. pensylvanicus relative to those of a codistributed stable species (19). Similar patterns have been observed in comparative studies of some European species (8), but most investigations have been geographically restricted and based on limited sampling within and among populations.
Although the investigations to date have provided important information on the increasing rarity of some bumble bee species in local populations, the different survey protocols and limited geographic scope of these studies cannot fully capture the general patterns necessary to evaluate the underlying processes or overall gravity of declines. Furthermore, valid tests of the N. bombi hypothesis and its risk to populations across North America call for data on its geographic distribution and infection prevalence among species. Likewise, testing the general importance of population genetic factors in bumble bee decline requires genetic comparisons derived from sampling of multiple stable and declining populations on a large geographic scale. From such range-wide comparisons, we provide incontrovertible evidence that multiple Bombus species have experienced sharp population declines at the national level. We also show that declining populations are associated with both high N. bombi infection levels and low genetic diversity.
Results
Geographic Range Analysis.
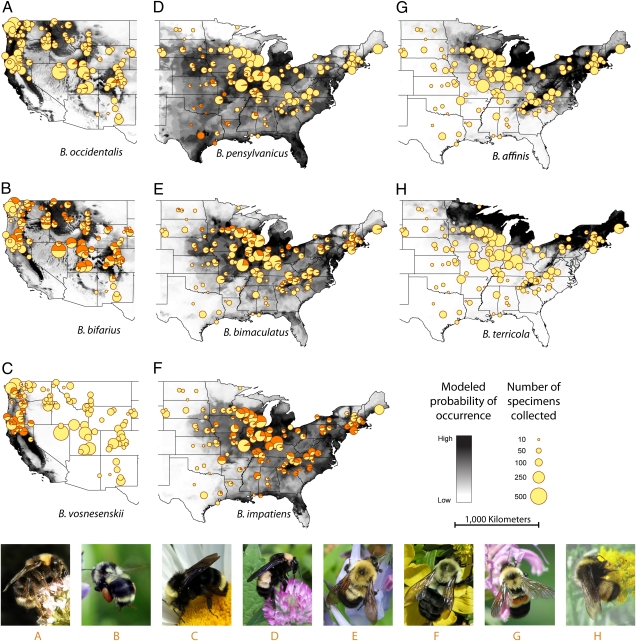
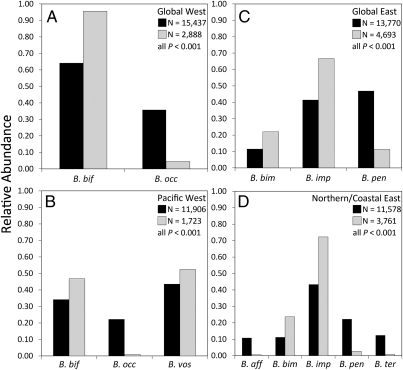
To assess large-scale geographic range reductions and changes in relative abundance (RA), we compared historical collection records with those from current field surveys. Current data are based on surveys (details provided in SI Methods, Contemporary Field Surveys of US Bumble Bees) conducted at 382 sites throughout the United States between 2007 and 2009 (Fig. S1A and Table S1). We netted and identified a total of 16,788 bumble bees, including four focal target species suspected of recent population declines (west: B. occidentalis, N = 129; east: B. affinis, N = 22; B. pensylvanicus, N = 532; B. terricola, N = 31) (10, 12, 20) and four thought to have relatively stable populations (west: B. bifarius, N = 2,760; B. vosnesenskii, N = 902; east: B. bimaculatus, N = 1,033; B. impatiens, N = 3,128) (11, 12, 21). Historical data are based on the assembly of a 73,759-specimen database (SI Methods, US Bumble Bee Natural History Collection Database) of the eight target species recorded from natural history museum collections throughout the United States (Fig. S1B and Table S2). Comparisons of the historical and current data revealed extensive range reductions (Fig. 1 A, D, G, and H) and significant decreases in RA in all four species suspected of population decline (all P < 0.001) (Fig. 2); each was absent from significantly more sites predicted to have high occurrence probabilities than were stable species (Fisher's exact tests; all P < 0.001) (Table S4). Declines in RA appear only within the last 20–30 y, with RA values from current surveys lower than in any decade of the last century (Fig. S1C). The four allegedly stable species showed no clear patterns of range reduction (Fig. 1 B, C, E, and F and Tables S2, S4, and S5) or consistent declines in RA.
Fig. 1.
Summary of Bombus individuals surveyed from 382 collection locations for eight target species, including historical range maps (grayscale shading) with current sightings (pie charts) and associated photographs of hypothesized declining western B. occidentalis (A) and eastern B. pensylvanicus (D), B. affinis (G), and B. terricola (H); stable species are represented by the western B. bifarius (B) and B. vosnesenskii (C), and the eastern B. bimaculatus (E), and B. impatiens (F). Sizes of the pie charts indicate total number of individuals surveyed at each location; size of the orange segment indicates the fraction of the respective target species collected at that site (some locations are pooled across sites for visual clarity; for detailed data, refer to Table S1). Underlying grayscale shading represents the modeled distribution of each target species from unique presence localities obtained from natural history collections (SI Methods, Statistical Niche Models). Photograph A (B. occidentalis) taken by D. Ditchburn, B (B. bifarius) by L. Solter, C (B. vosnesenskii) by M. Layne, D (B. pensylvanicus) by T. Wilson, E (B. bimaculatus) by J. Whitfield, F (B. impatiens) by J. Lucier, G (B. affinis) by J. James-Heinz, and H (B. terricola) by J. Whitfield.
Fig. 2.
Four regional comparisons of pooled historical (1900–1999; black bars) and current relative abundances (2007–2009; gray bars) for six North American bumble bee species using z tests of equal proportions. Methods has a description of the four following geographic regions used in comparisons of relative abundance. (A) Global west, AZ, CA, CO, ID, MT, NM, NV, OR, SD, UT, WA, and WY; B. bifarius: z = −61.71, P < 0.001; B. occidentalis: z = 61.71, P < 0.001. (B) Pacific west, CA, OR, and WA; B. bifarius: z = −15.09, P < 0.001; B. occidentalis: z = 56.26, P < 0.001; B. vosnesenskii: z = 10.40, P < 0.001. (C) Global east, AL, AR, CO, CT, GA, IL, IN, IA, KS, KY, LA, ME, MA, MN, MS, MO, NE, NY, NC, ND, OH, OK, PA, SC, SD, TN, TX, VA, and WI; B. bimaculatus: z = −15.70, P < 0.001; B. impatiens: z = −31.27, P < 0.001; B. pensylvanicus: z = −56.57, P < 0.001. (D) Northern/coastal east, CT, GA, IL, IN, IA, ME, MA, MN, NY, NC, OH, PA, TN, VT, VA, and WI; B. affinis: z = 35.57, P < 0.001; B. bimaculatus: z = −18.40, P < 0.001; B. impatiens: z = −37.19, P < 0.001; B. pensylvanicus: z = 46.01, P < 0.001; B. terricola: z = 38.40, P < 0.001. All have df = 1.
Historically, B. occidentalis and B. pensylvanicus had among the broadest geographic ranges of any bumble bee species in North America (Fig. 1 and Table S5). However, the current surveys detected B. occidentalis only throughout the intermountain west and Rocky Mountains; it was largely absent from the western portion of its range (Figs. 1A and 2) (detected range-area reduction = 28%). B. pensylvanicus (Figs. 1D and 2) was not observed across most of its historical northern and eastern range (estimated reduction = 23%) and was abundant only in the south across the Gulf states and in the western portion of the Midwest. Similarly, B. affinis (Figs. 1G and 2), which was once found throughout the eastern United States and northern Midwest, was detected only in small numbers (N = 22) at three locations in Illinois and one in Indiana (estimated reduction = 87%). B. terricola (sister species to B. occidentalis) (22), which formerly occupied northern and upland regions of the east and Midwest (Figs. 1H and 2), was less abundant relative to the historical data (Fig. 2) but still detectable at a number of northeastern and high-elevation Appalachian Mountain sites (Fig. 1H) (estimated reduction = 31%) (Table S5).
Host Pathogen Infection.
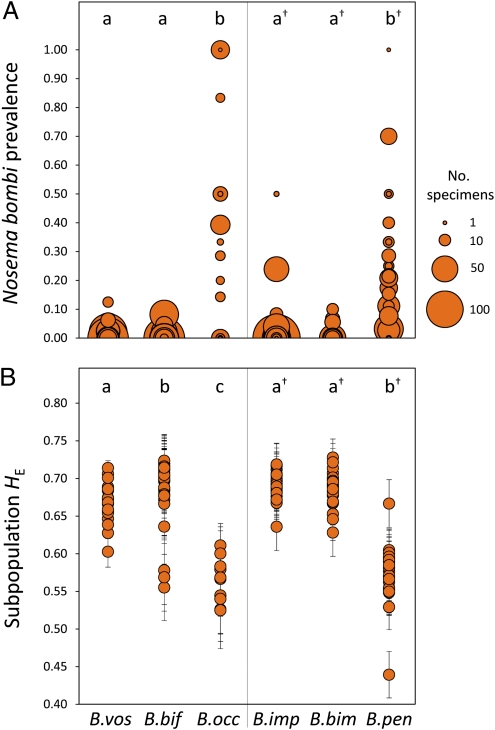
We also investigated the relationship between patterns of decline and levels of pathogen infection. To quantify the prevalence of N. bombi in the target species (SI Methods, Pathogen Screening), we examined midgut tissues from 6,708 specimens for presence of the microsporidian spores using phase-contrast microscopy. We confirmed the identity of N. bombi by sequencing a ∼600-bp fragment, including the internal transcribed spacer and parts of the large and small rRNA genes (13). We found significantly higher prevalence of N. bombi in declining B. occidentalis (37% of individuals surveyed) and B. pensylvanicus (15.2%) than in the stable species [binomial generalized linear models (GLM); P < 0.001] (Fig. 3A and Table S6). B. affinis and B. terricola were excluded from statistical analyses because of small sample sizes, but the available data show that B. affinis followed the infection trend of the other declining species with infected individuals collected at four of five sites (7 of 14 total individuals infected). The trend for B. terricola was less strong, although the proportion of infected individuals was nonetheless greater than that of any stable species (two of nine sites and 3 of 32 individuals infected). The infection intensities were also highest within B. occidentalis and B. pensylvanicus individuals (SI Methods, Pathogen Screening). All sequenced North American N. bombi isolates were genetically identical to European isolates (Table S7).
Fig. 3.
Nosema bombi infection prevalence (A) and microsatellite gene diversity (B). Average N. bombi prevalence (A) for B. vosnesenskii was 1.33% across all sites (n = 903, detected at 10 of 28 sites); B. bifarius was 0.57% (n = 2096, 7 of 88 sites); B. occidentalis was 37.2% (n = 172, 18 of 39 sites); B. impatiens was 0.73% (n = 2864; 10 of 131 sites); B. bimaculatus was 0.28% (n = 1070, three of 95 sites); and B. pensylvanicus was 15.2% (n = 545; 29 of 64 sites). Each circle represents a collecting site; its size indicates the number of individuals screened. Letters above each species plot indicate pairs with significantly different prevalence (P < 0.001) assessed by binomial GLMs (Table S6). (B) Average HE (± SE) per subpopulation. Letters indicate species pairs with significantly different HE (P = 0.001) as determined by 1,000 subpopulation permutations. In both A and B, statistical comparisons were conducted separately for western (no †) and eastern (†) species.
Genetic Diversity.
We tested whether population genetic diversity and structure are related to the observed patterns of population decline and stability by genotyping 8–11 microsatellite loci in six of the target species (insufficient samples were available for B. affinis and B. terricola) (Table S8). Declining populations had significantly reduced gene diversity (HE) relative to species with stable populations (Fig. 3B and Table 1). Also, foragers of the declining B. pensylvanicus and to a lesser extent, B. occidentalis (relative to B. bifarius but not B. vosnesenskii) originated from significantly fewer colonies at survey sites than foragers of stable species (Tables S8 and S9). Contrary to expectations from an earlier local study of B. pensylvanicus in Illinois (19), there was no evidence that declining populations had significantly elevated range-wide population structure relative to stable species. Estimates of genetic differentiation (FST and D) were low for all taxa (Table 1). FST ranged from 0.004 to 0.007, and D ranged from 0.026 to 0.042 for most species (Table 1); however, both were slightly higher in B. bifarius (FST = 0.026; D = 0.140) and B. occidentalis (declining; FST = 0.032; D = 0.124). Only B. bifarius exhibited intraspecific clustering (Fig. S1D) when species were analyzed with the Bayesian genotype clustering algorithm STRUCTURE (23). Overall, these species seem genetically cohesive, and it seems probable that populations experience substantial gene flow, even at large geographic scales.
Table 1.
Gene diversity (total HE) and measures of among-subpopulation genetic structure (FST and D) for target Bombus species
| Species | N | Loci | Total HE (interlocus SE)* | FST (95% CI) | D |
| B. bimaculatus | 472 | 11 | 0.693 (0.027) | 0.005 (0.002–0.007)† | 0.026 |
| B. impatiens | 622 | 10 | 0.692 (0.029) | 0.004 (0.002–0.007)† | 0.034 |
| B. pensylvanicus | 342 | 11 | 0.577 (0.030) | 0.007 (0.003–0.011)† | 0.036 |
| B. vosnesenskii | 364 | 8 | 0.676 (0.013) | 0.005 (0.000–0.010)† | 0.042 |
| B. bifarius | 587 | 8 | 0.700 (0.043) | 0.026 (0.019–0.034)† | 0.140 |
| B. occidentalis | 93 | 8 | 0.584 (0.037) | 0.032 (0.014–0.053)† | 0.124 |
CI, confidence interval.
*Total HE calculated by pooling all individuals in a species.
†FST > 0 at P < 0.01.
Discussion
From a large-scale interdisciplinary study of Bombus species across the United States, we have quantified dramatic range-wide population declines in B. occidentalis, B. pensylvanicus, B. affinis, and B. terricola that have occurred over the last few decades. Our data show that these species are significantly less abundant and absent from many more localities than would be predicted from natural history collections, providing a broad-scale geographic perspective of decline (Fig. 1). Although these species have become rare or absent throughout large areas of their historical ranges, co-occurring species, such as B. bifarius, B. vosnesenskii, B. impatiens, and B. bimaculatus, remain relatively abundant and widespread.
The wide-scale reductions in range and abundance of North American species, which also confirm earlier studies of decline at local levels, are striking and cause for concern. However, it is unlikely that species have become fully extirpated from regions where we did not detect them. Although we surveyed the majority of geographic regions multiple times over multiple years, establishing local extinction would require more intensive sampling than was possible within the constraints of a 3-y nationwide study. Our conservative interpretation of the data is that, based on historical information and the large number of sites and specimens surveyed, declining species have become sufficiently rare in parts of their ranges to be difficult to detect. The persistence of residual populations beyond the ranges detected in our surveys is fully expected under the emerging pattern of changing bumble bee diversity in both North America and Europe, where global extinction of species has been rare to date. Rather, both continents are witnessing major reductions in the range and abundance of multiple species. In Europe, accumulating evidence suggests that narrow climatic niche breadth combined with reductions in food and nesting resources are responsible for the gradual declines observed in many Bombus since the 1950s. These declines seem to occur more rapidly near range margins (9), which may also be the case in the United States (e.g., greater losses of B. occidentalis west of the Cascade–Sierra crest and declines of B. pensylvanicus in the north and northeast). However, contrary to a developing consensus in Europe that bumble bees with narrower climatic ranges are most susceptible to decline (9), population declines in the United States can occur in some of the most previously abundant species that formerly occupied broad climatic ranges. Additional causes of decline, thus, seem to be at play in North America.
Before this study, circumstantial evidence linking the timing of Bombus population declines in the Pacific west to the collapse of commercial bumble bee production in California after N. bombi infection (24) led to the hypothesis that N. bombi had escaped into wild populations and was responsible for the declines (10). This temporal correlation was not verified by collection of N. bombi infection data in wild bees. Nevertheless, the hypothesis became widely reported (7, 9, 25, 26). The significantly elevated N. bombi prevalence in declining Bombus populations detected in our study is consistent with the hypothesis that this pathogen could be adversely affecting some species. These observations are reminiscent of reports of other introduced fungal pathogens that pose widespread threats to some taxa, including frogs (Batrachochytrium dendrobatidis) and bats (Geomyces destructans) (27, 28), but confirming a direct link between N. bombi and North American bumble bee decline will require further research. Comparative studies of susceptibility in declining and stable species will reveal whether the increased prevalence in declining species is the result of higher susceptibility to the pathogen or if N. bombi is simply more common in declining species for other reasons. Regarding the geographic origin of N. bombi, the identical ribosomal RNA (rRNA) sequence in North American and European isolates is consistent with the hypothesis of a recent introduction, but in-depth sampling and genetic screening are needed to determine whether N. bombi is invasive or a distinct North American strain. There is additional need to study other known bumble bee pathogens, such as Crithidia bombi (29, 30), and possible viruses that could contribute to the observed species declines.
Estimates of lower range-wide genetic diversity suggest that B. occidentalis and B. pensylvanicus may also have smaller effective population sizes than stable co-occurring Bombus species, and this may play a role in bumble bee decline. The increased potential for inbreeding and genetic drift in small effective populations could lead to increased susceptibility to environmental pressures (17, 18, 31), including N. bombi. On the positive side, high rates of gene flow, inferred from the low levels of genetic structure in both declining and stable species, suggest that diversity lost through drift in small effective populations could be replenished by dispersal. However, high dispersal rates could also facilitate the spread of infectious agents like N. bombi. Bumble bees are known to pick up certain pathogens while foraging on flowers (32), although there is no empirical evidence to indicate that N. bombi is transmitted in this fashion. Nonetheless, if infected reproductives disperse relatively long distances for mating or colony-founding, this could facilitate N. bombi transmission among populations. Our inference of high dispersal could, however, be reflecting past gene flow if habitat fragmentation has been too recent for migration and drift to reach equilibrium at the broad geographic scale presented here. Intensive genetic analyses of individuals and populations at a local level across a fragmented landscape could provide information about barriers to dispersal at a finer scale. Behavioral studies of dispersal distances of reproductives would further elucidate the potential for gene flow.
Understanding the link between pathogen infection levels and population genetic parameters is a promising avenue for future research, and exploring species- and population-specific genetic differences in susceptibility to N. bombi infection would provide an important test of the pathogen hypothesis of decline. In this context, phylogenetic relationships may also be important in susceptibility to N. bombi or more generally, to population decline. Three of four seriously declining species in the United States are close relatives (B. affinis, B. terricola, and B. occidentalis) within the subgenus Bombus sensu stricto (22). Only two other Bombus s. s. species occur in North America. One of these is critically imperiled or possibly extinct (B. franklini) (33) and therefore, could not be included in this study. The other occurs in Alaska (B. moderatus) and has yet to be fully assayed. B. pensylvanicus (subgenus Thoracobombus) is not closely related to Bombus s. s. species, but given the pattern of decline among North American Bombus s. s. relatives, we suspect other Thoracobombus (B. sonorus, B. californicus, and B. fervidus) may be at risk and deserve future monitoring.
Pollinator decline has become a worldwide issue (9, 34), raising increasing concerns over impacts on global food production (35), stability of pollination services (36), and disruption of plant–pollinator networks (2, 3). The loss of pollinator diversity may have wide-ranging effects on both natural (e.g., wildflower pollination) and agricultural systems, where a heterogeneous community of native species can help buffer against the decline of managed species (5). Large-scale coordinated efforts to address the status of native pollinators in North America are, however, in their infancy, and bumble bee research is at the forefront. Future research on the complex interactions of habitat fragmentation, loss of floral and nesting resources, disease, and climate is needed to identify the major factors that lead to decline in bumble bee biodiversity. In accordance with the goals of the United Nations Convention on Biological Diversity to reduce the rate of species loss by 2010 (37), such efforts to elucidate the causes and ecological impacts of bumble bee decline, in coordination with informed conservation strategies, will go a long way to mitigating further losses.
Methods
Study Species.
We selected eight historically abundant North American Bombus as focal taxa, because preliminary observations suggested that these species have experienced recent demographic trajectories ranging from population declines to possible expansions. In the western United States, we focused on B. occidentalis (declining), B. vosnesenskii (stable), and B. bifarius (stable); target species in the east were B. pensylvanicus (declining), B. affinis (declining), B. terricola (declining), B. bimaculatus (stable), and B. impatiens (stable). All statistical analyses are presented separately for western and eastern taxa.
Distribution and Relative Abundance Comparisons (SI Methods).
To determine contemporary distributions and relative abundances, between 2007 and 2009, we surveyed all bumble bee species present at 382 sites in 40 US states for a period of ∼1 ± 0.5 SD person-h. Only target species were killed; other sampled species were released at the end of each survey. To determine historical distributions and relative abundances, we compiled a 73,759-specimen natural history collection database. The current iteration of the Bombus database is available on request (from S.A.C.) and on completion, will be hosted on the Global Biodiversity Information Facility (GBIF). We predicted potential historical ranges of each species with the statistical niche modeling algorithm MaxEnt v3.3 (38). We used z tests of equal proportions (Eq. 1) to compare relative abundances of target species between contemporary and historical collections (1900–1999) across four geographic categories: global west, B. bifarius and B. occidentalis; Pacific west, B. bifarius, B. occidentalis, and B. vosnesenskii; global east, B. bimaculatus, B. impatiens, and B. pensylvanicus; and northern/coastal east, B. affinis, B. bimaculatus, B. impatiens, B. pensylvanicus, and B. terricola (Fig. 2 has the states included) (Eq. 1).
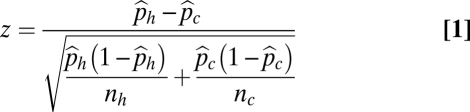 |
where 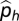 = estimated historic relative abundance,
= estimated historic relative abundance, 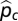 = estimated current relative abundance, nh = total historic abundance across all target bumble bee species, and nc = total current abundance of all target bumble bee species. A similar approach to determine changes in relative abundance of bumble bee communities has been applied previously (11). Nonstatistical comparisons of relative abundance were also made for each decade (Fig. S1C). We partitioned the relative abundance analysis into these four regional categories, because B. vosnesenskii, B. affinis, and B. terricola are more restricted in geographic range than the other target species. The more restricted regional categories, Pacific west and northern/coastal east, allowed a more direct geographic comparison of these species.
= estimated current relative abundance, nh = total historic abundance across all target bumble bee species, and nc = total current abundance of all target bumble bee species. A similar approach to determine changes in relative abundance of bumble bee communities has been applied previously (11). Nonstatistical comparisons of relative abundance were also made for each decade (Fig. S1C). We partitioned the relative abundance analysis into these four regional categories, because B. vosnesenskii, B. affinis, and B. terricola are more restricted in geographic range than the other target species. The more restricted regional categories, Pacific west and northern/coastal east, allowed a more direct geographic comparison of these species.
We used predictions from our statistical niche models (Fig. 1) in two additional assessments of decline patterns. We created binary presence–absence rasters from the continuous MaxEnt models (logistic threshold = 0.20), which produced conservative (i.e., omitted several actual survey observations) but reasonably realistic distribution maps for the eight target species. For each species, survey sites within the presence distribution were scored as an expected occurrence (any omitted actual occurrences caused by the conservative threshold were added to this presence class), and we calculated the fraction of expected sites where the species was observed; differences among species were tested with Fisher's exact tests (Table S4). To obtain estimates of range-area losses for declining species, we then calculated the areas of minimum convex polygons, constructed in ArcView 9.2, for species occurrences in historical records and contemporary surveys, constraining areas to environments classified as suitable in the binary MaxEnt rasters (Table S5) and adjusting estimates downward to compensate for range loss overprediction caused by sampling error (SI Methods, Comparisons of Historical and Contemporary Collections). These niche model-based approaches are only approximations of range loss for the declining Bombus species, because they do not account for differences in abundance across the species’ ranges and assume occupancy of all environmentally suitable sites; however, given the broad distributions of North American Bombus and presently available data, they provide a useful initial approximation to be refined with future survey efforts.
Pathogen Analyses (SI Methods, Pathogen Screening).
We determined the prevalence (individuals per species per site) and intensity (spores per microliter) of infection with N. bombi by phase-contrast microscopy. Differences in prevalence were tested using binomial GLMs. Species identity of N. bombi was confirmed by DNA sequencing of small and large rRNA subunits and internal transcribed spacer (ITS) region (GenBank accession nos. HM142724–HM142729 and HM173334–HM173341) (Table S7).
Genetic Analyses (SI Methods, Genetic Analysis).
Six species were genotyped at 8–11 microsatellite loci. Full sibs collected at each site were determined using COLONY 2.0 (39), and a single genotype per colony was retained for analysis. Differences among species in the proportion of unique colonies per site were tested using GLMs with quasibinomial errors. We calculated Nei's measure of gene diversity (HE) and interlocus SE (40), and differences among species were tested by 1,000 randomizations of subpopulation estimates of HE (using only loci successfully genotyped in all species within each region). Intraspecific genetic differentiation was estimated using FST (41), actual differentiation (D) (42), and the computer program STRUCTURE v.2.3.3 (23).
Supplementary Material
Acknowledgments
We especially acknowledge R. Thorp for his early-warning observations of the declining status of B. franklini, which galvanized the bee community into action. We thank our associates and student assistants J. Knoblett, H. Ikerd, J. Grixti, I. Stewart, W. Stewart, P. Karnstedt, M. Behle, D. Bonnie, S. Czarnik, L. Lewis, D. Young, J. Cech, J. Whitfield, H. Hines, C. Rasmussen, C. Russell, and G. Lamba. We are indebted to the curators from the museums and institutions listed in Table S2 who loaned specimens or supplied electronic specimen data. We are grateful to D. Ditchburn, J. James-Heinz, M. Layne, J. Lucier, J. Whitfield, and T. Wilson for use of their photographs in Fig. 1 and L. Chittka, D. Janzen, D. Steinkraus, and J. Whitfield for critical reading of earlier versions of the manuscript. This research was supported by the United States Department of Agriculture (CSREES-NRI 2007-02274) and the United States Fish and Wildlife Service.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. HM142724–HM142729 and HM173334–HM173341).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014743108/-/DCSupplemental.
References
- 1.Hegland SJ, Totland O. Is the magnitude of pollen limitation in a plant community affected by pollinator visitation and plant species specialization levels? Oikos. 2008;117:883–891. [Google Scholar]
- 2.Fontaine C, Dajoz I, Meriguet J, Loreau M. Functional diversity of plant-pollinator interaction webs enhances the persistence of plant communities. PLoS Biol. 2006;4:e1. doi: 10.1371/journal.pbio.0040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proc Biol Sci. 2004;271:2605–2611. doi: 10.1098/rspb.2004.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbet SA, Williams IH, Osborne JL. Bees and the pollination of crops and flowers in the European Community. Bee World. 1991;72:47–59. [Google Scholar]
- 5.Kremen C, Williams NM, Thorp RW. Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci USA. 2002;99:16812–16816. doi: 10.1073/pnas.262413599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delaplane KS, Mayer DF. Crop Pollination by Bees. Wallingford, United Kingdom: CABI; 2000. [Google Scholar]
- 7.Committee on the Status of Pollinators in North America, National Research Council . Status of Pollinators in North America. Washington, DC: National Academy Press; 2007. [Google Scholar]
- 8.Goulson D, Lye GC, Darvill B. Decline and conservation of bumble bees. Annu Rev Entomol. 2008;53:191–208. doi: 10.1146/annurev.ento.53.103106.093454. [DOI] [PubMed] [Google Scholar]
- 9.Williams PH, Osborne JL. Bumblebee vulnerability and conservation world-wide. Apidologie (Celle) 2009;40:367–387. [Google Scholar]
- 10.Thorp RW, Shepherd MD. Subgenus Bombus. Latreille, 1802 (Apidae: Apinae: Bombini) 2005. Available at www.xerces.org/Pollinator_Red_List/Bees/Bombus_Bombus.pdf. Accessed December 14, 2010.
- 11.Colla SR, Packer L. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers Conserv. 2008;17:1379–1391. [Google Scholar]
- 12.Grixti JC, Wong LT, Cameron SA, Favret C. Decline of bumble bees (Bombus) in the North American Midwest. Biol Conserv. 2009;142:75–84. [Google Scholar]
- 13.Tay WT, O'Mahony EM, Paxton RJ. Complete rRNA gene sequences reveal that the microsporidium Nosema bombi infects diverse bumblebee (Bombus spp.) hosts and contains multiple polymorphic sites. J Eukaryot Microbiol. 2005;52:505–513. doi: 10.1111/j.1550-7408.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 14.Larsson R. Cytological variation and pathogenicity of the bumble bee parasite Nosema bombi (Microspora, Nosematidae) J Invertebr Pathol. 2007;94:1–11. doi: 10.1016/j.jip.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Otti O, Schmid-Hempel P. A field experiment on the effect of Nosema bombi in colonies of the bumblebee Bombus terrestris. Ecol Entomol. 2008;35:577–582. [Google Scholar]
- 16.Rutrecht ST, Brown MJF. Differential virulence in a multiple-host parasite of bumble bees: Resolving the paradox of parasite survival? Oikos. 2009;118:941–949. [Google Scholar]
- 17.Allendorf FW, Luikart GH. Conservation and the Genetics of Populations. Oxfors: Blackwell; 2007. [Google Scholar]
- 18.Zayed A. Bee genetics and conservation. Apidologie (Celle) 2009;40:237–262. [Google Scholar]
- 19.Lozier JD, Cameron SA. Comparative genetic analyses of historical and contemporary collections highlight contrasting demographic histories for the bumble bees Bombus pensylvanicus and B. impatiens in Illinois. Mol Ecol. 2009;18:1875–1886. doi: 10.1111/j.1365-294X.2009.04160.x. [DOI] [PubMed] [Google Scholar]
- 20.Xerces Society Red List of Bees. 2010. Available at http://www.xerces.org/pollinator-redlist/. Accessed December 14, 2010.
- 21.McIver J, Thorp R, Erickson K. Pollinators of the invasive plant, yellow starthistle (Centaurea solstitialiss) in north-eastern Oregon, USA. Weed Biol Manage. 2009;9:137–145. [Google Scholar]
- 22.Cameron SA, Hines HM, Williams PH. A comprehensive phylogeny of the bumble bees (Bombus) Biol J Linn Soc. 2007;91:161–188. [Google Scholar]
- 23.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flanders RV, Wehling WF, Craghead AL. Laws and regulations on the import, movement and release of bees in the United States. In: Strickler K, Cane JH, editors. For Nonnative Crops, Whence Pollinators of the Future? Lanham, MD: Thomas Say Publications in Entomology; 2003. pp. 99–111. [Google Scholar]
- 25.Watanabe ME. Colony collapse disorder: Many suspects, no smoking gun. Bioscience. 2008;58:384–388. [Google Scholar]
- 26.Federman A. Plight of the bumble bee. Earth Island Journal. 2009;24:34–39. [Google Scholar]
- 27.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frick WF, et al. An emerging disease causes regional population collapse of a common North American bat species. Science. 2010;329:679–682. doi: 10.1126/science.1188594. [DOI] [PubMed] [Google Scholar]
- 29.Otterstatter MC, Thomson JD. Within-host dynamics of an intestinal pathogen of bumble bees. Parasitology. 2006;133:749–761. doi: 10.1017/S003118200600120X. [DOI] [PubMed] [Google Scholar]
- 30.Otterstatter MC, Thomson JD. Does pathogen spillover from commercially reared bumble bees threaten wild pollinators? PLoS One. 2008;3:e2771. doi: 10.1371/journal.pone.0002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankham R. Genetics and extinction. Biol Conserv. 2005;126:131–140. [Google Scholar]
- 32.Durrer S, Schmid-Hempel P. Shared use of flowers leads to horizontal pathogen transmission. Proc Roy Soc Lond B Bio. 1994;258:299–302. [Google Scholar]
- 33.International Union for Conservation of Nature. The IUCN Red List of Threatened Species 2010.3. 2010. Available at http://www.iucnredlist.org/apps/redlist/details/135295/0. Accessed December 14, 2010.
- 34.Potts SG, et al. Global pollinator declines: Trends, impacts and drivers. Trends Ecol Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Klein AM, et al. Importance of pollinators in changing landscapes for world crops. Proc Biol Sci. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biesmeijer JC, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- 37.Marton-Lefèvre J. Biodiversity is our life. Science. 2010;327:1179. doi: 10.1126/science.1188424. [DOI] [PubMed] [Google Scholar]
- 38.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190:231–259. [Google Scholar]
- 39.Jones O, Wang J. COLONY: A program for parentage and sibship inference from multilocus genotype data. Mol Ecol Res. 2009;10:551–555. doi: 10.1111/j.1755-0998.2009.02787.x. [DOI] [PubMed] [Google Scholar]
- 40.Nei M, Kumar S. Molecular Evolution and Phylogenetics. New York: Oxford University Press; 2000. [Google Scholar]
- 41.Weir BS, Cockerham CC. Estimating F-statistics for the analysis of population structure. Evolution. 1984;38:1358–1370. doi: 10.1111/j.1558-5646.1984.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 42.Jost L. G(ST) and its relatives do not measure differentiation. Mol Ecol. 2008;17:4015–4026. doi: 10.1111/j.1365-294x.2008.03887.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.