Cataract is the leading cause of blindness in the world, and most cataracts are age-related (1). The proteins in the lens degrade with time, and the subsequent aggregation of the modified proteins produces an increase in light scattering, and hence a loss of vision (2). There are cases, however, in which cataract is an inherited single-gene disorder. Currently, around 60 such cataracts have been identified (3). In these cases, the cause of the disease is a mutation in the genes that code for the lens proteins. Unlike age-related cataracts, inherited cataracts occur in children as well as adults (4). In PNAS, Banerjee et al. (5) report a previously undescribed mechanism for an inherited congenital cataract. They carefully examine the delicately balanced interactions among the lens proteins that maintain the proper function of the lens.
The human lens consists of three main types of proteins: α-, β-, and γ-crystallins, which together account for almost 90% of the total lens proteins (6). These proteins are packed at high concentrations (>300 mg/mL) in the lens fiber cells (6). The short-range spatial order that results from this dense protein packing helps to minimize fluctuations in the refractive index of the lens so that light scattering is reduced and the lens is transparent (7, 8). If the short-range order is disrupted, there is an increase in light scattering, which often leads to cataract.
Genetic association studies have revealed the first step in the disruption in short-range order of inherited congenital cataracts: a point mutation in one of the lens proteins. Over the past 10 y, the full mechanism for cataract formation (i.e., how the point mutation produces an increase in light scattering) has been worked out for specific mutations. The most detailed studies are those by J. Pande, A. Pande, G. B. Benedek, and their collaborators on the mutations that occur in γD-crystallin. For example, these investigators have shown that the second reactive cysteine introduced by the R14C mutation (C110 is already present in the WT protein) produces disulfide cross-linked oligomers that eventually precipitate from solution (9, 10); the loss of a charged residue in the R58H and R36S mutations reduces the protein solubility, and protein crystals form (11), and the increase in hydrophobicity attributable to the P23T mutation produces aggregates with low and retrograde solubility (12–14).
In all these cases, there is no significant change in protein structure. The cataract occurs because a change in the protein–protein interactions leads to the formation of a condensed phase—consisting solely of the mutant protein—that scatters light. This scheme (mutation → loss of protein solubility → condensed phase → increase in light scattering → cataract) has become the predominant explanation for inherited congenital cataracts connected with γD-crystallin. Indeed, Messina-Bass et al. (15), who described the nuclear cataract associated with the E107A mutation (Fig. 1), suggested that “this mutation results in a reduction in the protein solubility, which in turn causes this type of nuclear cataract.”
Fig. 1.
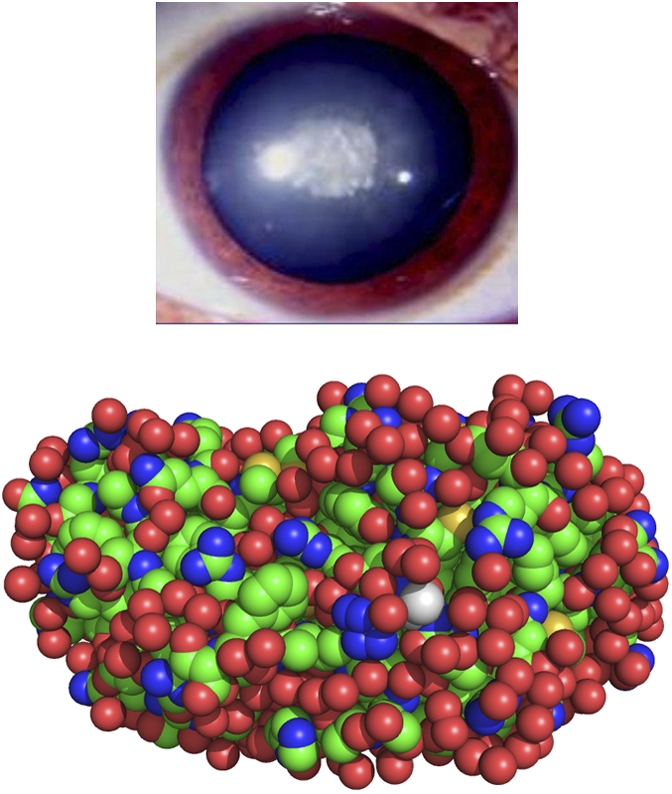
Cataract associated with the E107A mutation in human γD-crystallin. (Upper) Morphology of the cataract. Reprinted with permission from Molecular Vision (15). (Lower) Replacement of glutamic acid 107 by alanine. The alanine residue (shown in white) was introduced into the WT structure [Protein Data Bank ID code 1hk0 (11)] using PyMOL (18).
The unique aspect of the results described by Banerjee et al. (5) for the E107A mutation is that their observations do not follow the scheme outlined above. No condensed phase is produced in vitro. In fact, the solubility of the mutant protein does not change at all; like the WT, it remains soluble to over 325 mg/mL. As with the other γD-crystallin mutations, there is no significant change in protein structure. The only substantial change attributable to the loss of the negatively charged glutamic acid residue is an increase in the overall positive charge of the protein.
Banerjee et al. (5) demonstrate that even though no condensed phase forms, the increase in charge produces an increase in light scattering. To do this, they move beyond the established scheme for inherited congenital cataracts, in which it is assumed that the interactions between the mutant proteins dominate the behavior of the system. Their key insight is to consider the attraction between the positively charged E107A mutant and the negatively charged α-crystallins. They show that the association between E107A and α-crystallin increases the amount of light scattering (compared with the light scattering caused by a WT γD-crystallin/α-crystallin mixture) at protein compositions and solution temperatures comparable to those of the lens in vivo. This finding confirms the recent prediction of Thurston and his colleagues (16, 17) that the strength of the interaction between native γ- and α-crystallins is essentially optimal for lens transparency and that small changes in this interaction increase light scattering and lead to cataract.
The work of Banerjee et al. (5) is an important step toward a more complete understanding of inherited congenital cataracts. Their combination of experimental measurements and computational modeling may also prove useful in the study of age-related cataracts.
Footnotes
The author declares no conflict of interest.
See companion article on page 574.
References
- 1.Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290:2057–2060. doi: 10.1001/jama.290.15.2057. [DOI] [PubMed] [Google Scholar]
- 2.Sharma KK, Santhoshkumar P. Lens aging: Effects of crystallins. Biochim Biophys Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiels A, Bennett TM, Hejtmancik JF. Cat-Map: Putting cataract on the map. Mol Vis. 2010;16:2007–2015. [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy MA, Francis PJ, Berry V, Bhattacharya SS, Moore AT. Molecular genetic basis of inherited cataract and associated phenotypes. Surv Ophthalmol. 2004;49:300–315. doi: 10.1016/j.survophthal.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee PR, Pande A, Patrosz J, Thurston GM, Pande J. Cataract-associated mutant E107A of human γD-crystallin shows increased attraction to α-crystallin and enhanced light scattering. Proc Natl Acad Sci USA. 2011;108:574–579. doi: 10.1073/pnas.1014653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloemendal H, et al. Ageing and vision: Structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Benedek GB. Theory of transparency of the eye. Appl Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 8.Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- 9.Pande A, Gillot D, Pande J. The cataract-associated R14C mutant of human γ D-crystallin shows a variety of intermolecular disulfide cross-links: a Raman spectroscopic study. Biochemistry. 2009;48:4937–4945. doi: 10.1021/bi9004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pande A, et al. Molecular basis of a progressive juvenile-onset hereditary cataract. Proc Natl Acad Sci USA. 2000;97:1993–1998. doi: 10.1073/pnas.040554397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pande A, et al. Crystal cataracts: Human genetic cataract caused by protein crystallization. Proc Natl Acad Sci USA. 2001;98:6116–6120. doi: 10.1073/pnas.101124798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pande A, et al. Decrease in protein solubility and cataract formation caused by the Pro23 to Thr mutation in human γ D-crystallin. Biochemistry. 2005;44:2491–2500. doi: 10.1021/bi0479611. [DOI] [PubMed] [Google Scholar]
- 13.Pande A, Ghosh KS, Banerjee PR, Pande J. Increase in surface hydrophobicity of the cataract-associated P23T mutant of human gammaD-crystallin is responsible for its dramatically lower, retrograde solubility. Biochemistry. 2010;49:6122–6129. doi: 10.1021/bi100664s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pande A, et al. NMR study of the cataract-linked P23T mutant of human gammaD-crystallin shows minor changes in hydrophobic patches that reflect its retrograde solubility. Biochem Biophys Res Commun. 2009;382:196–199. doi: 10.1016/j.bbrc.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messina-Baas OM, Gonzalez-Huerta LM, Cuevas-Covarrubias SA. Two affected siblings with nuclear cataract associated with a novel missense mutation in the CRYGD gene. Mol Vis. 2006;12:995–1000. [PubMed] [Google Scholar]
- 16.Dorsaz N, Thurston GM, Stradner A, Schurtenberger P, Foffi G. Colloidal characterization and thermodynamic stability of binary eye lens protein mixtures. J Phys Chem B. 2009;113:1693–1709. doi: 10.1021/jp807103f. [DOI] [PubMed] [Google Scholar]
- 17.Stradner A, Foffi G, Dorsaz N, Thurston G, Schurtenberger P. New insight into cataract formation: Enhanced stability through mutual attraction. Phys Rev Lett. 2007;99:198103. doi: 10.1103/PhysRevLett.99.198103. [DOI] [PubMed] [Google Scholar]
- 18.DeLano WL. The PyMOL Molecular Graphics System. San Carlos, CA: Delano Scientific; 2006. [Google Scholar]


