Abstract
The genetic basis of variation in fitness of many organisms has been studied in the laboratory, but relatively little is known of fitness variation in natural environments or its causes. Lifetime fitness (recruitment) may be determined solely by producing many offspring, modified by stochastic effects on their subsequent survival up to the point of breeding, or by an additional contribution made by the high quality of the offspring owing to nonrandom mate choice. To investigate the determinants of lifetime fitness, we measured offspring production, longevity, and lifetime number of mates in four cohorts of two long-lived species of socially monogamous Darwin's finch species, Geospiza fortis and G. scandens, on the equatorial Galápagos Island of Daphne Major. Regression analysis showed that the lifetime production of fledglings was predicted by lifetime number of clutches and that recruitment was predicted by lifetime number of fledglings and longevity. There was little support for a hypothesis of selective mating by females. The offspring sired by extrapair mates were no more fit in terms of recruitment than were half-sibs sired by social mates. These findings provide insight into the evolution of life history strategies of tropical birds. Darwin's finches deviate from the standard tropical pattern of a slow pace of life by combining tropical (long lifespan) and temperate (large clutch size) characteristics. Our study of fitness shows why this is so in terms of selective pressures (fledgling production and adult longevity) and ecological opportunities (pulsed food supply and relatively low predation).
Keywords: El Niño, extrapair offspring, heterozygosity, opportunistic breeding
The fitness of an individual refers to its ability to survive and reproduce (1, 2), generally measured as the number of offspring that the individual contributes to the next generation (3). The translation of an individual's potential fitness into realized fitness is governed by the environment. Numerous laboratory experiments on model organisms have revealed the genetic basis of fitness variation, as well as the extent to which it is manifested differently in contrasting environments. Within any one treatment, environmental variation is minimized, so that the resulting measure of fitness approximates the individual's potential in the specified environment. In contrast, for free-living organisms, it is difficult to separate the intrinsic and extrinsic factors affecting fitness. Nonetheless, a full understanding of fitness variation in natural populations requires taking into account salient environmental variation, which can be strong, unpredictable, and of overriding importance to the biological success of individuals (4, 5).
Estimating fitness in natural environments proceeds by seeking statistical associations between fitness variation and properties of the organisms and their environments (6). The first step is to determine which members of the population do best under what circumstances. The second step is to frame hypotheses to explain why fitness varies in terms of identified factors. The third step is to test the hypotheses under experimentally controlled conditions (7–9). We report the results of taking the first two steps in a study of lifetime fitness in two nonmigratory populations of Darwin's finches, Geospiza fortis (medium ground finch) and G. scandens (cactus finch), on the Galápagos island of Daphne Major.
Periodic droughts cause heavy finch mortality through starvation. Although the survival component of fitness is occasionally and strongly influenced by morphology (4, 10), there is no single morphological determinant of fitness over the long-term because selection oscillates in direction according to the particular nature of the food supply at the beginning of droughts (11, 12). The reproductive component of fitness has not been studied as thoroughly. An earlier study (13) found that the longer a bird lived, the more fledglings it produced in its lifetime and the more recruits it contributed to the next generation on average. Morphological variation contributed little to variation in reproductive output and rarely anything to fitness. An outstanding question remaining was whether a breeder's fitness is enhanced by its choice of a particular mate, as argued in many studies of adaptive mate choice (reviewed in refs. 14 and 15).
Mating of Darwin's ground finches is random with respect to morphology (16, 17) and relatedness (18), but may be cryptically nonrandom with respect to other aspects of the quality of the mate, such as genetic compatibility (19–25) and immunocompetence (24, 26, 27). According to the hypothesis of selective mating, in a population with annual turnover of members of monogamous pairs and sequential repairing, the longer-lived an individual, the greater its chance of breeding with a high-quality mate and producing high-quality offspring. The high quality of the mate may be due to inherited characteristics, such as the level of multilocus heterozygosity (15), to noninherited characteristics, such as age and experience (28, 29), or to ecological correlates, such as a territory rich in food resources or nest sites (30). The hypothesis thus predicts that for a given longevity (years lived), individuals with several mates should contribute more offspring to the next generation on average than individuals with just one or two mates (31–33).
Although they are socially monogamous, Darwin's finches engage in extrapair mating (EPM). Thus, a related issue is whether EPM reflects a choice by females that is not expressed or is only weakly expressed in the pattern of pairing with social mates (14, 34, 35). The frequency of extrapair young (EPY) in the nests of Darwin's finches is 10–20% (36), which is typical of small passerines (34, 35, 37). The offspring sired by extrapair fathers have enhanced fitness in some populations of socially monogamous passerine birds (20, 23), but not in others (38, 39). If extrapair mates are chosen because of their intrinsic quality, then a combination of the numbers of extrapair and social mates should predict recruitment of the offspring better than the numbers of the social mates alone. This forms a second prediction of the selective mating hypothesis.
Thus, lifetime fitness may be determined solely by the number of offspring that a breeder is able to produce, modified by factors affecting their subsequent survival, or possibly with an additional contribution made by the high quality of the offspring owing to nonrandom mate choice. We present the results of a study conducted in 1978–1998 designed to investigate fitness variation in light of these considerations; the study period did not include years of strong selective mortality (4, 10–12). We first used multiple linear regression to predict fledgling production and recruitment by members of four cohorts of each of the two species raised under contrasting conditions. Members of two cohorts hatched in relatively dry years (1978 and 1981), and members of the other two cohorts hatched in wet years (1983 and 1987) with much longer breeding seasons. We then used the same method to predict offspring recruitment by the number of social and extrapair mates for the 1987 cohorts; extrapair paternity (EPP) was not determined in the other years. Third, we conducted two tests of mate quality of extrapair males by comparing them with the social males of the same females. In the first, a direct test, we compared multilocus heterozygosity and age between the extrapair mates and social mates. In the second, an indirect test, we compared fitnesses of extrapair offspring and their half-sib nest-mates sired by the social mate of the mother (39). These tests were supplemented by additional information on the characteristics of extrapair mates (SI Results).
Darwin's finches on Daphne Major are well suited for this investigation for several reasons. One advantage is that the system is entirely natural. A second advantage is the extreme variation in climate, and hence food supply, from extreme wet and productive conditions caused by prolonged and abundant rain during El Niño events to droughts (La Niña events) and food scarcity. This makes it possible to measure fitness variation across a spectrum of environmental conditions. A third advantage is the long-term nature of the study. It has been ongoing since 1973 and has encompassed a broad range of climatic conditions. Fourth, the island is small and isolated, making it ideal for use in determining the fitness of known individuals. Cross-generational monitoring of offspring survival and reproduction in extensively pedigreed populations is necessary to relate their fitness to the timing and location of breeding and to the location, identity, and origin of biological parents (13, 40–42). For natural populations in general, it is rarely possible to follow known individuals from birth to reproduction to death, estimate their contributions to the next generation, and identify the important factors determining their success (43).
Results
Reproductive Output.
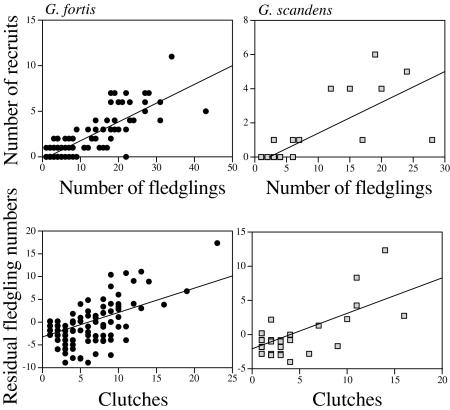
The number of successful clutches (Methods) produced in a lifetime is the strongest and most consistent predictor of reproductive output as measured by lifetime number of offspring fledged by a breeder (Table 1). A breeder must live a long time to produce many clutches; longevity and number of clutches are strongly and positively correlated (r = 0.60–0.85). Nevertheless, with the effect of number of clutches held constant statistically, longevity of the breeder adds significantly to the prediction in a few cases. The number of mates over the lifetime is a significant additional factor in predicting the number of fledglings for females of the 1983 cohorts of both G. fortis and G. scandens; the more mates these females had, the more fledglings that they produced (Fig. 1). This is consistent with the selective mating hypothesis.
Table 1.
Prediction of reproductive success (fledglings) and overall fitness (recruits) in four cohorts produced in years of contrasting conditions
| Species | Sex | F | N | adj R2 | Clutches | Longevity | Mates |
| 1978: Average rainfall (137 mm), low breeding density (2–3 broods) | |||||||
| Fledglings | |||||||
| G. fortis | F | 28.77 | 26 | 0.7144 | 0.0034 | 0.0042 | — |
| G. fortis | M | 39.21 | 36 | 0.7038 | 0.0011 | 0.0156 | — |
| G. scandens | F | 21.84 | 17 | 0.5928 | 0.0003 | — | — |
| G. scandens | M | 4.15* | 15 | 0.2418 | — | — | — |
| Recruits | |||||||
| G. fortis | F | 14.78 | 26 | 0.3811 | 0.0008 | — | — |
| G. fortis | M | 17.76 | 36 | 0.3431 | 0.0002 | — | — |
| G. scandens | F | 3.47* | 17 | 0.3316 | 0.0382 | 0.0227† | — |
| G. scandens | M | 5.25 | 15 | 0.2877 | — | 0.0393† | — |
| 1981: Low rainfall (73 mm), medium breeding density (1–2 broods) | |||||||
| Fledglings | |||||||
| G. fortis | F | 38.79 | 18 | 0.7080 | <0.0001 | — | — |
| G. fortis | M | 40.88 | 28 | 0.6112 | <0.0001 | — | — |
| G. scandens | F | — | — | — | — | — | — |
| G. scandens | M | 27.13 | 17 | 0.6439 | 0.0001 | — | — |
| Recruits | |||||||
| G. fortis | F | 15.78 | 18 | 0.4965 | 0.0011 | — | — |
| G. fortis | M | 39.32 | 28 | 0.6020 | <0.0001 | — | — |
| G. scandens | F | — | — | — | — | — | — |
| G. scandens | M | 16.91 | 17 | 0.5299 | 0.0009 | — | — |
| 1983: High rainfall (1,359 mm), high breeding density (<8 broods) | |||||||
| Fledglings | |||||||
| G. fortis | F | 114.40 | 155 | 0.6944 | <0.0001 | <0.0001 | 0.0251 |
| G. fortis | M | 158.05 | 94 | 0.7765 | <0.0001 | 0.0076 | — |
| G. scandens | F | 72.74 | 69 | 0.7705 | 0.0028 | 0.0002 | 0.0043 |
| G. scandens | M | 51.34 | 38 | 0.5878 | <0.0001 | — | — |
| Recruits | |||||||
| G. fortis | F | 232.99 | 155 | 0.6036 | <0.0001 | — | — |
| G. fortis | M | 52.82 | 94 | 0.5372 | 0.0001 | 0.0109 | — |
| G. scandens | F | 44.87 | 69 | 0.5762 | <0.0001 | — | 0.0039 |
| G. scandens | M | 23.19 | 38 | 0.3918 | <0.0001 | — | — |
| 1987: High rainfall (622 mm), medium breeding density (<7 broods) | |||||||
| Fledglings | |||||||
| G. fortis | F | 36.96 | 88 | 0.4651 | <0.0001 | 0.0347 | — |
| G. fortis | M | 22.80 | 82 | 0.4672 | <0.0001 | — | — |
| G. scandens | F | 22.57 | 13 | 0.6723 | 0.0006 | — | — |
| G. scandens | M | 34.71 | 17 | 0.6982 | <0.0001 | — | — |
| Recruits | |||||||
| G. fortis | F | 22.68 | 88 | 0.3479 | <0.0001 | — | 0.0072† |
| G. fortis | M | 29.84 | 82 | 0.2716 | <0.0001 | — | — |
| G. scandens | F | 3.59* | 13 | 0.2458 | — | — | — |
| G. scandens | M | 7.68 | 17 | 0.3386 | 0.0143 | — | — |
All F ratios are significant at P < 0.05 (most at P < 0.0001), except where indicated by an asterisk (*P > 0.05). Significance of partial regression coefficients of the predictor variables is given in the body of the table. All coefficients but three (indicated by †) are positive.
Fig. 1.
Predictions of recruits and fledglings of the 1983 cohorts of females. Recruits (Upper) are predicted by production of fledglings, and residual fledgling numbers (Lower) are predicted by the number of clutches after the effects of number of mates and longevity are statistically controlled for (Table 1). All quantities are lifetime estimates. The regression slopes of the recruit relationships for G. fortis (b = 0.21 ± 0.01 SEM) and G. scandens (b = 0.18 ± 0.02 SEM) are almost identical.
Fitness.
Fitness (recruitment) is best predicted by lifetime number of fledglings in all cohorts. Longevity and number of mates make minor and inconsistent additional contributions. The selective mating hypothesis is supported only by the results of the 1983 cohort of G. scandens females (n = 69). Predictions for males and females are generally concordant, due in part because some males and females of the same cohort bred with each other; nonetheless, note the differences in sample sizes of males and females listed in Table 1.
Environmental variation among years has no clear effect on the performance of the major predictors (clutches and fledglings), nor is there a difference between species (Table 1). The minor predictors (longevity and mates) are most evident in the 1983 cohorts, possibly reflecting an effect of the unusual conditions when they were produced, although an effect of large sample sizes on detectability confounds this interpretation.
EPP.
The overall frequency of EPP is estimated to be 0.171 in G. fortis (n = 1,248) and 0.103 in G. scandens (n = 368 offspring) (Table S1). A correspondingly higher proportion of G. fortis fathers are cuckolded (0.453; n = 77) compared with G. scandens fathers (0.317; n = 60). Taking EPP into account does not change the predictions of fitness and fledglings for the 1987 cohort of either species (compare Tables 1 and 2) and provides no support for the selective mating hypothesis. We conclude that analyses of other cohorts are not likely to be distorted by ignoring EPP, providing that the (unknown) occurrence of EPP is similar in different years (39).
Table 2.
Prediction of reproductive success (fledglings) and overall fitness (recruits) of males of the 1987 cohort, taking into account EPP
| Species | F | n | Adjusted R2 | Clutches | Longevity | Mates |
| Fledglings | ||||||
| G. fortis | 47.13 | 82 | 0.3707 | <0.0001 | — | — |
| G. scandens | 30.16 | 17 | 0.6678 | <0.0001 | — | — |
| Recruits | ||||||
| G. fortis | 27.41 | 82 | 0.2552 | <0.0001 | — | — |
| G. scandens | 7.16* | 17 | 0.3230 | 0.0173 | — | — |
All F ratios are significant at P < 0.0001, except where indicated by an asterisk (*P < 0.05). Significance of partial regression coefficients of the predictor variables is given in the body of the table. All coefficients are positive.
Genetic Effects of Extrapair Mates.
Table 3 compares the genetic characteristics of social and extrapair mates and their offspring. None of the results supports the hypothesis of selective mating. Females do not choose extrapair mates that are more different from them genetically compared with their social mates, and thus the offspring sired by extrapair mates are not more heterozygous than the offspring of the social mates on average. Moreover, females do not choose extrapair mates that are more heterozygous than their social mates. The frequency of G. fortis EPY that became recruits (0.235; n = 68) is not higher, but in fact is somewhat lower than the frequency of within-pair siblings from the same nest that became recruits (0.320; n = 97; χ21 = 1.01; P = 0.314). Either the microsatellite loci are the wrong ones to use for such tests (because they are selectively neutral and not linked to salient loci) or there really is no choice on the basis of genetic characteristics that females are able to detect. Social and extrapair mates do not differ in terms of genetically inherited morphological traits or culturally inherited song traits (SI Results, Tables S2 and S3).
Table 3.
Genetic characteristics of social and extrapair mates and their offspring
| Species | Social mate,mean ± SD | Extrapair mate,mean ± SD | Paired-t | Df | P |
| Nei's D between mates | |||||
| G. fortis | 0.819 ± 0.250 | 0.838 ± 0.267 | 0.62 | 49 | 0.5349 |
| G. scandens | 0.730 ± 0.128 | 0.767 ± 0.327 | 0.25 | 8 | 0.8071 |
| Heterozygosity of mates | |||||
| G. fortis | 0.643 ± 0.142 | 0.669 ± 0.121 | 1.07 | 48 | 0.2888 |
| G. scandens | 0.736 ± 0.089 | 0.691 ± 0.117 | 0.74 | 8 | 0.4813 |
| Heterozygosity of offspring | |||||
| G. fortis | 0.665 ± 0.105 | 0.645 ± 0.121 | 1.42 | 66 | 0.1609 |
| G. scandens | 0.636 ± 0.142 | 0.622 ± 0.099 | 0.47 | 6 | 0.6540 |
Mean heterozygosities are proportions of 14 autosomal microsatellite loci that are heterozygous.
Age Effects of Extrapair Mates.
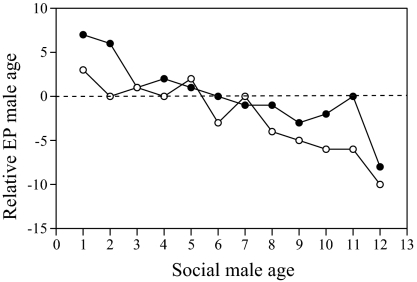
Females of both species are least likely to have extrapair mates when young, that is, 1 or 2 y old (SI Results and Fig. S1). Most extrapair mates of G. fortis females (n = 97) were the same age (44.3%) as their social mates or older (40.2%). Observed ages of the extrapair males were greater on average than the randomly expected ages across all age groups of social males, with minor exceptions (paired t11 = 3.68; P = 0.004; Fig. 2); see Methods for expectations. In contrast, most G. scandens extrapair mates (66.7%; n = 15) were younger than the social mates (Fig. S2). The difference from random expectation is not significant (paired t4 = 0.36; P = 0.74), but the sample of comparisons is small. The probability of a G. fortis male gaining paternity from EPM is close to 0 until age 5–6 y, and gains exceed losses on average only when males reach age 7 or 8 y (Fig. S2). This is an advantage of a long lifespan.
Fig. 2.
Observed median ages of G. fortis extrapair (EP) males (solid circles) compared with the median ages expected from random sampling of all males (open circles). Median values are expressed as deviations from social male ages. This graph shows that even though extrapair males are younger than old social males, they are not as young as expected. The sample size of extrapair males is n = 97.
Discussion
Globally, most land bird species are tropical. They differ from temperate zone species in being long-lived (44) and reproducing slowly, typically with a clutch of two eggs in lowland rainforests and more in mesic habitats (44, 45). In effect, their long life is lived at a slow pace (45–47). Darwin's finches deviate from the standard tropical pattern of a slow pace of life by combining tropical (long lifespan) and temperate (large clutch size) characteristics. Our study of fitness shows why this is so in terms of selective pressures (favoring high fledgling production and longevity) and ecological constraints and opportunities (pulsed food supply and relatively low predation). They live in a fluctuating environment, in strong contrast to birds of the humid tropics, and mortality in the nonbreeding season is occasionally very high. In these circumstances, the fitness of a breeder is a function of the number of offspring that it produces over its lifetime, and this function varies among cohorts according to an unpredictable temporal pattern in the environmental fluctuations: at what ages they experience times of plenty and times of scarcity. For example, on average a female G. fortis hatched in 1983 had to live for 6 y and produce 10 fledglings to replace herself with two recruits, whereas a female of the 1978 cohort achieved replacement in 2.5 y with five fledglings.
The strong and repeated effect of reproductive output on fitness (13) can be partitioned into clutch and fledgling components. Of the two, the fledgling component is the more important, because it is more variable. The modal clutch size is three or four eggs in most years, varies from as low as two in dry years, and up to five in El Niño years of prolific breeding (13, 48), but there is little variation among individuals within a breeding season. In contrast, fledgling numbers vary substantially among individuals, because some pairs are much more successful than others in converting eggs into fledglings (48). Their success stems from an ability to cope with interference from intruding finches and not from predation, which is rare and largely restricted to recently fledged offspring.
Thus, there are two components of biological success, in addition to chance, that have a bearing on the combination of life history traits. The first component is an ability to find food (seeds) in dry years when food is scarce and there is no breeding. The second is an ability to find food (insects and spiders) and avoid interference at the nest from intruders during breeding. Identifying the components, which are two different suites of behavioral and physiological traits, shows where further research is needed to gain a more detailed understanding of how fitness is maximized. Such research may yield insight into the question of how lifespan/reproduction trade-offs evolve differently in different tropical habitats that vary in seasonality, elevation, structure, and climate and also between tropical and temperate zones due to differences in ecology and seasonality, as well as other correlates of latitude (47, 49, 50).
One interesting consequence of a long lifespan is that the social bonds of breeding pairs may persist for many years. Alternatively, a female might have several different social mates in her lifetime despite being socially monogamous at any one time, thereby enhancing the genetic diversity of her offspring from a combination of several social and extrapair mates. Darwin's finches, like birds of the humid tropics, have potentially long lifespans and may have several social mates (13, 51) as a result of death or desertion of mates. Repeated repairing with social mates in addition to EPM increases the genetic diversity of an individual's offspring. An extreme example in our study is a female G. scandens that lived 10 y, paired with nine social mates in her lifetime, and fledged 23 within-pair offspring and two additional offspring sired by two extrapair mates. In producing 25 offspring, she combined her chromosomes with 11 sets of others. High numbers and diversity of offspring should give high fitness to the female that produces them, especially in fluctuating environments such as the Galápagos.
Perhaps it does, but we found little evidence of an advantage to multiple mating. Diversity of offspring, as reflected in lifetime number of mates, did not predict numbers of recruits after controlling for the effects of fledgling production. One likely reason for this is that diversity or quality effects were small and were overwhelmed by stochastic events between the fledging of offspring and these offsprings’ achievement of reproductive maturity. An analysis of EPM led to the same conclusion. Although we paid careful attention to the possibility of female choice of extrapair mates, because this is less restricted than the choice of a social mate (SI Results), we found no evidence of genetic benefit or an overall fitness gain from EPM, in contrast to the many reports for other birds in the literature (15, 20, 23). Instead, we found a high incidence of EPM in females with old males and, to a disproportionate extent, in G. fortis (see also SI Results). This could have resulted from the females’ choice of old males (52, 53), but just as likely from an avoidance of young males, whose territories tend to be small and hence unrewarding in food. EPM could be a factor favoring long life in males of this species, given that a net fitness benefit (gains > losses) is realized only when males reach age 7 or 8 y (Fig. S2).
Females apparently choose mates nonselectively within a broad range of morphological and song cues of species identity in this system (ref. 16; see also SI Results and Table S2). The incidence of EPY in G. fortis nests is high among near neighbors and is highest where the nests are at high density and near large open areas where females feed (SI Results). EPM appears to be opportunistic and stochastic to some degree, as suggested elsewhere (54). When preparing to form a clutch of eggs, a female needs energy and sperm, both of which may be obtained in part by feeding occasionally in another territory in the absence of her social mate. We suggest that a female's choice of an extrapair male is not so much a choice of him as an incidental effect of choice of where to feed. In other species that are highly colored, ornamented, and subject to strong sexual selection, there are fitness benefits to be gained by selecting particular extrapair mates (24, 55, 56). A predictive approach to the question of lifetime fitness in these species, as adopted here, would be helpful in quantifying the relative contribution of selective mating to overall fitness.
Some have suggested that fitness differences arising from mate choice occur only under stressful environmental conditions (57, 58). We evaluated this possibility by comparing fitness of young raised under the contrasting conditions of low and high density and short and long breeding seasons, and failed to find a difference. The number of mates predicted the number of fledglings produced by males and females of the 1983 cohorts of both species, but not in other cohorts. That year was unusual in terms of prolific breeding, breeding of cohort members that had hatched at the beginning of the year, and breeding in the following year. In contrast, very few birds both hatched and bred in 1987, the other El Niño year, and there was no further breeding until 1990. The connection between genetic diversity of offspring and fitness (recruitment) may be expressed only at unusual times of El Niño events (12, 13).
Conclusion.
In a fluctuating environment such as the Galápagos Islands, there should be strong selection to survive droughts and live long enough to breed many times. In support of this expectation, we found that lifetime fitness (recruitment) was strongly predicted in four cohorts of two species of Darwin's finches by the lifetime production of number of fledglings. Behavioral factors that affect reproductive success on the one hand and the finding of food in dry seasons of scarcity on the other hand must contribute importantly to these two fundamentally different components of biological success. Survival of fledglings to the time of breeding introduces extra, uncorrelated variation, as shown by the stronger coefficients of determination (R2) in models predicting fledgling production than in models predicting recruitment. Variation at this life history stage is partly stochastic. It weakens any association between natal experience and recruitment, but is not so large as to overwhelm the influence of fledgling production on recruitment. Thus, the predictive analysis of lifetime fitness of Darwin's finches in terms of fledgling production and longevity provides insight into the selective pressures that have caused them to deviate from the standard pattern for tropical species of a slow pace of life. An unanswered question is how the coupling of long life and high reproduction is brought about physiologically.
We sought evidence for an additional contribution to the prediction of recruitment by nonrandom mate choice. Results of regression analyses and comparisons of offspring sired by extrapair and social mates provided no support for a hypothesis of selective mating or an effect of mate choice on the prediction of recruitment. This does not mean that such effects do not exist. They may be relatively small and difficult to discern or rare. They would repay further study, given that advantages to females of mating with several males have been reported in a variety of organisms (31, 33, 59, 60).
Methods
Daphne Major is 0.34 km2 in area, 0.75 km long, and 120 m high. Finches breed when ~20 mm of rain falls, typically between January and April, but ~50 mm of rain is required for successful breeding, and this amount does not fall in every year (12). An attempt was made to find every nest and identify the parents in every year of breeding from 1976 onward. Chicks were banded in the nest at age 8 d with a combination of one numbered metal band and three colored leg bands. When fully grown at age 60 d or older, the chicks were captured in mist nets placed in all habitats and weighed, and the wings, tarsi, and beaks were measured (61). From 1988 onward, a small drop of blood was obtained from the brachial vein of 8-d-old chicks in the nest and adults captured in mist nets. Dried blood samples were stored on EDTA-soaked filter paper in a jar of Drierite for later analysis of variation at 14 autosomal microsatellite loci performed at Princeton University (36, 62) or by Ecogenics (16).
Survival and details of breeding were recorded in 1978–1998 for all members of the cohorts produced in 1978, 1981, 1983, and 1987. We used a multiple regression step-down procedure for each cohort to predict the lifetime production of fledglings and lifetime fitness, measured as the number of recruits to the breeding population. The procedure was halted when only significant predictors remained. Predictors for the analysis of fledglings were lifetime number of clutches that produced at least one fledgling, lifetime number of mates, and longevity. For the analysis of lifetime fitness, we used the same variables except for number of clutches, which was replaced by lifetime number of fledglings. Maximum lifetime values were 16 successful clutches, 46 fledglings, 13 recruits, 13 social mates, and 17 y for longevity, although not all of these values apply to the same individual. Of note, several finches that hatched in 1983 bred in that same year. Those that died in the same year were given a value of 0.5 for longevity. All predictor variables were log-transformed before analysis (13).
Details of the genotyping techniques as well as primer sequences have been given previously (63). Microsatellite profiles of offspring matched the profiles of all adult females (mothers) that fed them, but not all putative fathers, that is, the social mates of the mothers. A 2-bp difference between offspring and the putative parent was treated as a scoring error, whereas greater differences were considered to exclude the adult as a parent (16, 36). Estimated exclusion probabilities were >0.996 in previous analyses with 8 of the 14 loci (36, 62). The percentages of extrapair fathers of G. fortis that could be identified unambiguously by microsatellite matching in the present study were 56.5 in 1987 (n = 46), 73.1 in 1991 (n = 108), 67.7 in 1992 (n = 15), 74.2 in 1993 and 1995 (n = 31), and 34.8 in 1998 (n = 23). Others could not be identified because not all males were genotyped.
We used genetic data in two ways. First, we corrected the offspring of the 1987 cohort for EPY and reran the multiple regression analyses. Second, we used all genetic data from 1988–1998 to test three hypotheses: (i) Females differ more from their extrapair mates than from their social mates, (ii) extrapair mates are more heterozygous than social mates, and (iii) offspring sired by extrapair mates are more heterozygous than those sired by social mates. We used Nei's D as a metric of genetic difference for test (i) and used the fraction of the 14 loci that were heterozygous for each individual for tests (ii) and (iii). For a given female with a single social mate, the effects of two extrapair mates on fitness were averaged and counted once.
We estimated the random expectation in a given year of a female mating outside the pair bond with a male older, younger, or the same age as the social mate from the frequencies of nesting males of each age (1–12 y) in the population at that time. There are no floating males without a territory in the study species. We then compared and averaged the age-specific probabilities in the 6 years. For each age of the social mate, and for both G. fortis (n = 97) and G. scandens (n = 15), we compared the median expected age of extrapair males with the median observed age of the extrapair males. There are gaps in the G. scandens data; for example, there are no data for ages 2, 3, 6–9, and 11 y, owing to small sample sizes and drought years of no breeding.
We performed all analyses using JMP (SAS Institute). We used all of the data available for each test; that is, we were not selective except where explained. Differences in sample sizes in different analyses arise from incomplete data. See SI Results for a discussion of causes and consequences of EPM, as well as potential biases in the estimation of frequencies. All tests were two-tailed.
Supplementary Material
Acknowledgments
We thank the many field assistants for their help; Lukas Keller, Nat Wheelright, and Jane Reid for discussion; and Michaela Hau, Dick Holmes, and Martin Wikelski for comments on the manuscript. We also thank the Charles Darwin Foundation and the Galápagos National Parks Service for their support of our research. This work was funded by grants from the US National Science Foundation and Class of 1877 funds from Princeton University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018080108/-/DCSupplemental.
References
- 1.Falconer DS, Mackay T. An Introduction to Quantitative Genetics. London: Longman; 1996. [Google Scholar]
- 2.Wagner GP. The measurement theory of fitness. Evolution. 2010;64:1358–1376. doi: 10.1111/j.1558-5646.2009.00909.x. [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth B. Evolution in Age-Structured Populations. Cambridge, UK: Cambridge Univ Press; 1980. [Google Scholar]
- 4.Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin's finches. Science. 2002;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- 5.Packer C, et al. Ecological change, group territoriality, and population dynamics in Serengeti lions. Science. 2005;307:390–393. doi: 10.1126/science.1105122. [DOI] [PubMed] [Google Scholar]
- 6.Ellegren H, Sheldon BC. Genetic basis of fitness differences in natural populations. Nature. 2008;452:169–175. doi: 10.1038/nature06737. [DOI] [PubMed] [Google Scholar]
- 7.Reznick D, Brygga H, Endler JA. Experimentally induced life-history evolution in a natural population. Nature. 1990;346:357–359. [Google Scholar]
- 8.Sinervo B. The evolution of maternal investment in lizards: An experimental and comparative analysis of egg size and its effect on offspring performance. Evolution. 1990;44:279–294. doi: 10.1111/j.1558-5646.1990.tb05198.x. [DOI] [PubMed] [Google Scholar]
- 9.Palkovacs EP, et al. Experimental evaluation of evolution and coevolution as agents of ecosystem change in Trinidadian streams. Philos Trans R Soc Lond B. 2009;364:1617–1628. doi: 10.1098/rstb.2009.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant PR, Grant BR. Evolution of character displacement in Darwin's finches. Science. 2006;313:224–226. doi: 10.1126/science.1128374. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs HL, Grant PR. Oscillating selection on a population of Darwin's finches. Nature. 1987;327:511–513. [Google Scholar]
- 12.Grant PR, Grant BR. How and Why Species Multiply: The Radiation of Darwin's Finches. Princeton, NJ: Princeton Univ. Press; 2008. [Google Scholar]
- 13.Grant PR, Grant BR. Non-random fitness variation in two populations of Darwin's finches. Proc R Soc B. 2000;267:131–138. doi: 10.1098/rspb.2000.0977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akcay E, Roughgarden J. Extra-pair paternity in birds: Review of the genetic benefits. Evol Ecol Res. 2007;9:855–868. [Google Scholar]
- 15.Kempenaers B. Mate choice and genetic quality: A review of the heterozygosity theory. Adv Stud Behav. 2007;37:189–278. [Google Scholar]
- 16.Grant PR, Grant BR. Pedigrees, assortative mating and speciation in Darwin's finches. Proc R Soc B. 2008;275:661–668. doi: 10.1098/rspb.2007.0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber SK, De León LF, Hendry AP, Bermingham E, Podos J. Reproductive isolation of sympatric morphs in a population of Darwin's finches. Proc R Soc B. 2007;274:1709–1714. doi: 10.1098/rspb.2007.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs HL, Grant PR. Inbreeding in Darwin's medium ground finches (Geospiza fortis) Evolution. 1989;43:1273–1284. doi: 10.1111/j.1558-5646.1989.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 19.Freeman-Gallant CR, Wheelright NT, Meikeljohn KE, Sollecito SV. Genetic similarity, extrapair paternity, and offspring quality in Savannah sparrows (Passerculus sandwichensis) Behav Ecol. 2006;17:952–958. [Google Scholar]
- 20.Suter SM, Keiser M, Feignoux R, Meyer DR. Reed bunting females increase fitness through extra-pair mating with genetically dissimilar males. Proc R Soc B. 2007;274:2865–2871. doi: 10.1098/rspb.2007.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffiths SC, Immler S. Female infidelity and genetic compatibility in birds: The role of the genetically loaded raffle in understanding the function of extrapair paternity. J Avian Biol. 2009;40:97–101. [Google Scholar]
- 22.Brown JL. A theory of mate choice based on heterozygosity. Behav Ecol. 1997;8:60–65. [Google Scholar]
- 23.Foerster K, Delhey K, Johnsen A, Lifjeld JT, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. [DOI] [PubMed] [Google Scholar]
- 24.Fossøy F, Johnsen A, Lifjeld JT. Multiple genetic benefits of female promiscuity in a socially monogamous passerine. Evolution. 2008;62:145–156. doi: 10.1111/j.1558-5646.2007.00284.x. [DOI] [PubMed] [Google Scholar]
- 25.García-Navas V, Ortego J, Sanz JJ. Heterozygosity-based assortative mating in blue tits (Cyanistes caeruleus): Implications for the evolution of mate choice. Proc R Soc B. 2009;276:2931–2940. doi: 10.1098/rspb.2009.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson DS, Komdeur J, Burke T, von Schantz T. MHC-based patterns of social and extra-pair mate choice in the Seychelles warbler. Proc R Soc B. 2005;272:759–767. doi: 10.1098/rspb.2004.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neff BD, Pitcher TE. Cell-mediated immunity and multi-locus heterozygosity in bluethroat nestlings. J Evol Biol. 2009;22:424–429. doi: 10.1111/j.1420-9101.2009.01790.x. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson JL. Extrapair copulations in western bluebirds (Sialia mexicana): Female receptivity favors older males. Behav Ecol Sociobiol. 2001;50:423–429. [Google Scholar]
- 29.Schmoll T, Mund V, Dietrich-Bischoff V, Winkle W, Lubjuhn T. Male age predicts extrapair and total fertilization success in the socially monogamous coal tit. Behav Ecol. 2007;18:1073–1081. [Google Scholar]
- 30.Davies NB. Dunnock Behaviour and Social Evolution. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 31.Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc. 2000;75:21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- 32.Yasui Y. Female multiple mating as a genetic bet-hedging strategy when mate choice criteria are unreliable. Ecol Res. 2001;16:605–616. [Google Scholar]
- 33.Hosken DJ, Stockley P. Benefits of polyandry: A life history perspective. Evol Biol. 2003;33:173–194. [Google Scholar]
- 34.Griffith SC, Owens IPF, Thuman KA. Extra pair paternity in birds: A review of interspecific variation and adaptive function. Mol Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- 35.Westneat DF, Stewart IRK. Extra-pair paternity in birds: Causes, correlates, and conflicts. Annu Rev Ecol Syst. 2003;34:365–396. [Google Scholar]
- 36.Keller LF, Grant PR, Grant BR, Petren K. Heritability of morphological traits in Darwin's finches: Misidentified paternity and maternal effects. Heredity. 2001;87:325–336. doi: 10.1046/j.1365-2540.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 37.Macedo RH, Karubian J, Webster MS. Extrapair paternity and sexual selection in socially monogamous birds: Are tropical birds different? Auk. 2008;125:769–777. [Google Scholar]
- 38.Augustin J, Blomqvist D, Szép T, Szabó D, Wagner RH. No evidence of genetic benefits from extra-pair fertilizations in female sand martins (Riparia riparia) J Ornithol. 2007;148:189–198. [Google Scholar]
- 39.Schmoll T, Schurr FM, Winkel W, Epplen JT, Lubjuhn T. Lifespan, lifetime reproductive performance and paternity loss of within-pair and extra-pair offspring of the coal tit. Periparus ater. Proc R Soc B. 2009;276:337–346. doi: 10.1098/rspb.2008.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson DS, Jury FL, Blaakmeer K, Komdeur J, Burke T. Parentage assignment and extra-group paternity in a cooperative breeder: The Seychelles warbler (Acrocephalus sechellensis) Mol Ecol. 2001;10:2263–2273. doi: 10.1046/j.0962-1083.2001.01355.x. [DOI] [PubMed] [Google Scholar]
- 41.Postma E, van Noordwijk AJ. Gene flow maintains a large genetic difference in clutch size at a small spatial scale. Nature. 2005;433:65–68. doi: 10.1038/nature03083. [DOI] [PubMed] [Google Scholar]
- 42.Reid JM, Arcese P, Keller LF. Individual phenotype, kinship, and the occurrence of inbreeding in song sparrows. Evolution. 2008;62:887–899. doi: 10.1111/j.1558-5646.2008.00335.x. [DOI] [PubMed] [Google Scholar]
- 43.Ricklefs RE. Parental investment and avian reproductive rate: Williams's principle reconsidered. Am Nat. 2010;175:350–361. doi: 10.1086/650371. [DOI] [PubMed] [Google Scholar]
- 44.Martin TE. Avian life-history evolution has an eminent past: Does it have a bright future? Auk. 2004;121:289–301. [Google Scholar]
- 45.Wikelski M, Spinney L, Schelsky W, Scheuerlein A, Gwinner E. Slow pace of life in tropical sedentary birds: A common-garden experiment on four stonechat populations from different latitudes. Proc R Soc B. 2003;270:2383–2388. doi: 10.1098/rspb.2003.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiersma P, Muñoz-Garcia A, Walker A, Williams JB. Tropical birds have a slow pace of life. Proc Natl Acad Sci USA. 2007;104:9340–9345. doi: 10.1073/pnas.0702212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson WD, et al. Diversification of life histories in New World birds. Auk. 2010;127:253–262. [Google Scholar]
- 48.Grant PR, Grant BR, Keller LF, Petren K. Effects of El Niño events on Darwin's finch productivity. Ecology. 2000;81:2442–2457. [Google Scholar]
- 49.Bennett PM, Owens IPF. Evolutionary Ecology of Birds: Life Histories, Mating Systems and Extinction. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 50.McNamara JM, Barta ZN, Wikelski M, Houston AI. A theoretical investigation of the effect of latitude on avian life histories. Am Nat. 2008;172:331–345. doi: 10.1086/589886. [DOI] [PubMed] [Google Scholar]
- 51.Grant PR, Grant BR. Demography and the genetically effective sizes of two populations of Darwin's finches. Ecology. 1992;73:766–784. [Google Scholar]
- 52.Bouman KM, van Dijk RE, Wijmenga JJ, Komdeur J. Older male reed buntings are more successful at gaining extrapair fertilizations. Anim Behav. 2007;73:15–27. [Google Scholar]
- 53.Lehtonen PK, Primmer CR, Laaksonen T. Different traits affect gain of extrapair paternity and loss of paternity in the pied flycatcher, Ficedula hypoleuca. Anim Behav. 2009;77:1103–1110. [Google Scholar]
- 54.Reyer H-U, Bollmann K, Schläpfer AR, Schymainda A, Klecack G. Ecological determinants of extra-pair fertilizations and egg dumping in alpine water pipits (Anthus spinoletta) Behav Ecol. 1997;8:534–543. [Google Scholar]
- 55.Veen T, et al. Hybridization and adaptive mate choice in flycatchers. Nature. 2001;411:45–50. doi: 10.1038/35075000. [DOI] [PubMed] [Google Scholar]
- 56.Albrecht T, et al. Extra-pair fertilizations contribute to selection on secondary male ornamentation in a socially monogamous passerine. J Evol Biol. 2009;22:2020–2030. doi: 10.1111/j.1420-9101.2009.01815.x. [DOI] [PubMed] [Google Scholar]
- 57.Schmoll T, et al. Paternal genetic effects on offspring fitness are context- dependent within the extrapair mating system of a socially monogamous passerine. Evolution. 2005;59:645–657. [PubMed] [Google Scholar]
- 58.Van Dongen WFD, Mulder RA. Multiple ornamentation, female breeding synchrony, and extra-pair mating success of golden whistlers (Pachycephala pectoralis) J Ornithol. 2009;150:607–620. [Google Scholar]
- 59.Simmons L. The evolution of polyandry: Sperm competition, sperm selection, and offspring viability. Annu Rev Ecol Evol Syst. 2005;36:125–146. [Google Scholar]
- 60.Gowaty PA, Kim Y-K, Rawlings J, Anderson WW. Polyandry increases offspring viability and mother productivity but does not decrease mother survival in Drosophila pseudoobscura. Proc Natl Acad Sci USA. 2010;107:13771–13776. doi: 10.1073/pnas.1006174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boag PT, Grant PR. Darwin's finches (Geospiza) on Isla Daphne Major, Galápagos: Breeding and feeding ecology in a climatically variable environment. Ecol Monogr. 1984;54:463–489. [Google Scholar]
- 62.Petren K, Grant PR, Grant BR. Low extrapair paternity in the cactus finch (Geospiza scandens) Auk. 1999;116:252–256. [Google Scholar]
- 63.Petren K. Microsatellite primers from Geospiza fortis and cross-species amplification in Darwin's finches. Mol Ecol. 1998;7:1782–1784. doi: 10.1046/j.1365-294x.1998.00518.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.