Abstract
Treatment of Parkinson disease (PD) with l-3,4-dihydroxyphenylalanine (l-DOPA) dramatically relieves associated motor deficits, but l-DOPA–induced dyskinesias (LID) limit the therapeutic benefit over time. Previous investigations have noted changes in striatal medium spiny neurons, including abnormal activation of extracellular signal-regulated kinase1/2 (ERK). Using two PD models, the traditional 6-hydroxydopamine toxic lesion and a genetic model with nigrostriatal dopaminergic deficits, we found that acute dopamine challenge induces ERK activation in medium spiny neurons in denervated striatum. After repeated l-DOPA treatment, however, ERK activation diminishes in medium spiny neurons and increases in striatal cholinergic interneurons. ERK activation leads to enhanced basal firing rate and stronger excitatory responses to dopamine in striatal cholinergic neurons. Pharmacological blockers of ERK activation inhibit l-DOPA–induced changes in ERK phosphorylation, neuronal excitability, and the behavioral manifestation of LID. In addition, a muscarinic receptor antagonist reduces LID. These data indicate that increased dopamine sensitivity of striatal cholinergic neurons contributes to the expression of LID, which suggests novel therapeutic targets for LID.
Keywords: aphakia mouse, choline acetyltransferase, SL-327, dicyclomine, A2A antagonist
Dopaminergic drugs are effective treatments for the motor symptoms of Parkinson disease (PD), but long-term therapy is limited by disabling abnormal involuntary movements, referred to as l-DOPA–induced dyskinesias (LID) (1). Thus, understanding the molecular and cellular mechanisms underlying LID will help identify more effective treatments for PD, and may also help elucidate the role of dopamine (DA) signaling in motor control.
Several biochemical markers of LID have been studied in striatum using animal models. FosB/δFosB expression show a long-term temporal correlation with LID development in DA denervation PD models (2). The increased FosB expression persists over days or weeks and may contribute to the development of LID, but does not correlate with the l-DOPA–induced episodes of dyskinesia that follow each dose. To mediate expression of LID directly, cell signals should grow stronger with repeated l-DOPA treatment and show temporal correlation with acute dopaminergic stimulation. Acute administration of l-DOPA or dopamine agonists activates ERK1/2 by phosphorylation in striatal neurons of DA-denervated animals (3–7). In animals with unilateral 6-hydroxydopamine (6-OHDA) lesions, coadministration of inhibitors of ERK1/2 phosphorylation during repeated l-DOPA treatments reduce LID development (4, 8). Studies on these molecular changes have focused on the predominant cell type in the striatum, medium spiny neurons (MSN). In addition, although the unilateral 6-OHDA lesion has been the standard model for the PD phenotype, and particularly for LID, the abrupt nature of the lesion and extreme depletion of dopaminergic afferents has posed limitations in behavioral and biochemical studies.
In this study, we used both a unilateral 6-OHDA lesion model and a genetic model, the Pitx3-deficient aphakia mouse (Pitx3ak/ak) (9–11) for PD. The lack of transcription factor Pitx3 results in selective loss of nigrostriatal DA projections in a remarkably similar pattern to the neuroanatomical features of PD along with parkinsonian motor deficits (10, 12–15). There are several advantages and complementary features of the Pitx3ak/ak mouse over the more traditional PD models involving toxin-induced unilateral lesion. First, Pitx3ak/ak mice have more selective depletion of nigrostriatal DA projections than lesion models in that the terminals are lost in the dorsal striatum with relative sparing of ventral striatum. Second, unlike lesion models, the extent of the DA deficit is very similar between individuals, limiting an important source of intersubject variability. Third, the denervation of striatal DA is bilateral in Pitx3ak/ak mice, whereas it is difficult with lesion models to achieve bilateral DA depletion without excessive mortality. Fourth, Pitx3ak/ak mice lack nigrostriatal DA projections throughout development, which may favor the conditions for LID induction, as human PD patients with early-age onset and children with an impaired ability to produce DA show more pronounced LID than those who develop the condition later in life (16, 17). Consistent with this view, the molecular and cellular measures of LID seen in lesion models have also been demonstrated in Pitx3ak/ak mice (12, 13, 18–20).
In this article, we investigated the effects of acute and repeated l-DOPA treatment on striatal ERK phosphorylation, and tested its role in akinesia improvement and LID expression in Pitx3ak/ak mice and in a unilateral parkinsonian mouse model. Our behavioral, anatomical, and electrophysiological investigations support a critical role of striatal cholinergic neurons in the expression of LID.
Results
Repeated l-DOPA Exposure Induces ERK Phosphorylation in the Choline Acetyltransferase Interneurons of Dopamine Depleted Dorsal Striatum of Pitx3ak/ak Mice.
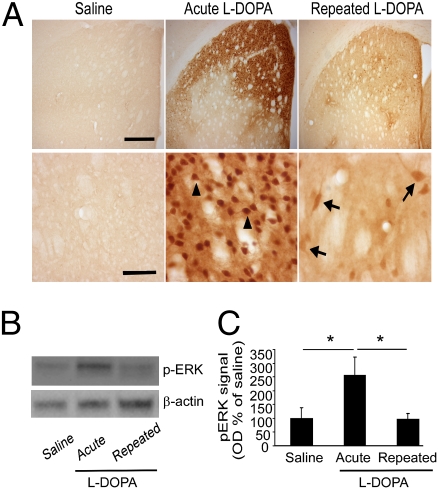
Based on previous studies associating ERK activation and l-DOPA treatment (3, 6), we hypothesized that striatal ERK phosphorylation should increase with repeated l-DOPA treatment, in parallel with the increasing phenotypic expression of LID. In contrast to our expectations, we found a profound reduction in striatal ERK activation following repeated l-DOPA treatment of homozygous Pitx3ak/ak mice for 7 wk (25 mg/kg, twice a day, i.p.) compared with that noted after the first exposure to l-DOPA (Fig. 1A). Western blot analysis showed that acute l-DOPA treatment produced more than a twofold increase of phosphorylated ERK1/2 (pERK) compared with untreated controls (Fig. 1B). Most recently, a similar reduction in striatal pERK associated with dyskinesia has been noted following prolonged l-DOPA in the MPTP primate model of PD (7). Close examination of the pERK-expressing cells in the striatum after acute l-DOPA showed that most of these had the gross morphology of MSN (Fig. 1A). In contrast, repeated l-DOPA treatment for >7 wk resulted in significantly lower pERK levels in response to an l-DOPA challenge approaching that seen in the saline group (Fig. 1 B and C). Cellular analyses revealed only a few scattered neurons with ERK phosphorylation after 7 wk of repeated l-DOPA treatment, and many of them had large (≥ 20-μm long diameter) cell bodies that are characteristic of cholinergic interneurons, together with some smaller MSN with much weaker pERK expression (Fig. 1A). Double immunostaining for GABAergic interneurons expressing parvalbumin or calretinin did not show colocalization of pERK in these cells following acute or repeated l-DOPA exposure (Fig. S1 B and C). These described changes were observed only in the dorsal striatum, where Pitx3ak/ak mice have selective depletion of DA (Fig. S2B), and not in those treated with saline (Fig. 1A and Fig. S3B). These cholinergic interneurons make up about 2% of the neurons in striatum (21), which is why pERK elevation in these cells did not contribute significantly to the total protein levels (Fig. 1 B and C).
Fig. 1.
ERK phosphorylation in the dorsal striatum of Pitx3ak/ak mice treated with l-DOPA. Mice received either repeated saline or l-DOPA (25 mg/kg, twice a day, i.p.) treatment for 5 to 7 wk and were killed 15 min after the last injection of saline or l-DOPA. (A) Immunohistochemical staining for pERK in repeated saline/saline control, repeated saline/acute l-DOPA, and repeated l-DOPA/l-DOPA treatment groups. (Scale bars, Upper, 400 μm; Lower, 50 μm.) Arrowheads demonstrate examples of presumed MSNs and arrows point to presumed larger cholinergic interneurons. (B) Western blot analysis of pERK in dorsal striatal tissues from the above treatment groups. (C) Quantitation of the pERK Western blots (n = 3–4, mean ± SEM; *P < 0.05, one-way ANOVA with Tukey posthoc test).
Littermate heterozygous Pitx3ak/+ mice were used as controls, as they do not exhibit a loss of DA in dorsal striatum or reduction in midbrain dopaminergic neurons relative to wild-type mice (Fig. S2 A and B), nor do they express dyskinesia or pERK following treatment with l-DOPA (Fig. S3 A and B).
ERK Phosphorylation in Cholinergic Neurons Correlates with Behavioral Manifestation of Dyskinesia.
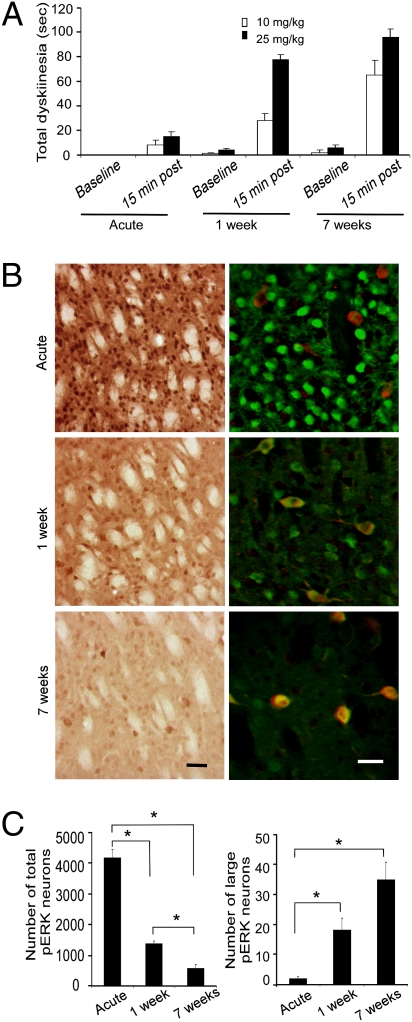
To assess the role of l-DOPA–induced ERK activation in striatal cholinergic neurons in the development of LID behaviors, we examined the effects of both dose and duration of l-DOPA treatment in Pitx3ak/ak mice. As we previously described (20), the paw dyskinesia developed over time with repeated l-DOPA administration in a time- and dose-dependent manner (Fig. 2A). Analysis of striatal ERK activation showed the number of pERK-labeled large-diameter neurons [corresponding to choline acetyltransferase (ChAT)-positive cells] increased with length of l-DOPA treatment, but the total number of pERK-expressing cells (presumably MSN) diminished over time (Fig. 2 B and C).
Fig. 2.
Development of dyskinesia and expression of pERK-labeled striatal cells with repeated l-DOPA administration in Pitx3ak/ak mice. Mice were treated either acutely, or repeatedly for 1 wk or for 7 wk with either 10 or 25 mg/kg of l-DOPA (twice a day, intraperitoneally). (A) The total dyskinesia was assessed by measuring the time animals spent on both two paw and three paw dyskinesias during a 2-min sampling period. Dyskinesia increased after l-DOPA treatment (P = 0.05), greater with 25 mg/kg compared with 10 mg (P < 0.05), and with 7-wk treatment compared with 1-wk treatment (P < 0.05 by three-way ANOVA; n = 5–9 per group). (B) Immunoperoxidase staining of neuronal ERK activation in the dorsal striatum at different time points following treatment with 25 mg/kg of l-DOPA (Left). Double immunofluorescent staining for pERK (green) and ChAT (red) demonstrates that the large-sized cells with increased ERK activation following repeated l-DOPA treatment correspond to cholinergic neurons (Right). [Scale bar, 50 μm in (Left) and 30 μm (Right).] (C) Quantitation of immunoperoxidase labeled pERK cells showed that the number of large-diameter, putative cholinergic pERK-positive cells in dorsal striatum increased with repeated drug treatment (Right), but the total number of pERK-labeled cells diminished (Left) (*P < 0.05 by one-way ANOVA) (n = 5–9/group). The data for A and C represent the mean ± SEM.
To further confirm that pERK is expressed primarily in striatal cholinergic interneurons after repeated l-DOPA treatment, double-fluorescence immunostaining for pERK and ChAT was performed. In animals treated with l-DOPA for the first time, very few pERK-expressing cells were cholinergic (Fig. 2B), whereas a considerable number of pERK-positive cells were cholinergic following repeated 7-wk l-DOPA treatment (Fig. 2B). Thus, repeated l-DOPA treatment in Pitx3ak/ak mice, which produces LID, correlates with increased ERK phosphorylation in striatal cholinergic interneurons and a decrease in MSN.
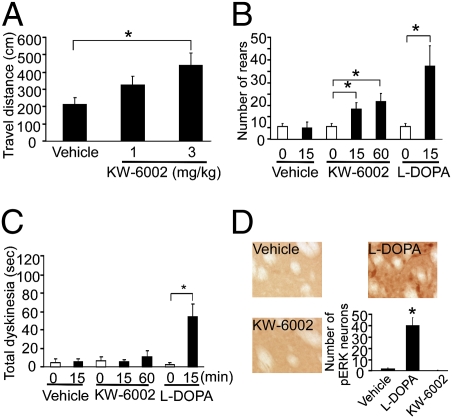
To further confirm the correlation of ERK phosphorylation in striatal cholinergic neurons with l-DOPA–induced behavioral expression of dyskinesia, we used a selective A2A receptor antagonist, which ameliorates akinesia in human PD patients without producing dyskinesia after repeated treatment (22). The selective A2A antagonist, KW-6002, significantly improved akinesia in Pitx3ak/ak mice, as evidenced by both open-field test and rearing activity (Fig. 3 A and B), without the expression of dyskinesia or striatal ERK phosphorylation after 7 wk of repeated l-DOPA treatment (Fig. 3 C and D). Therefore, the A2A antagonist not only selectively relieves parkinsonian symptoms without producing dyskinesia, but also does not produce ERK activation, suggesting that ERK phosphorylation in cholinergic neurons occurs only with the expression of l-DOPA–induced dyskinesias.
Fig. 3.
Behavioral and biochemical effects of a selective A2A antagonist KW-6002 in Pitx3ak/ak mice. Mice were treated repeatedly with l-DOPA (25 mg/kg, twice a day, i.p.) for 7 wk and then evaluated for behavioral response to the A2A antagonist, KW-6002. (A) Total distance traveled over 3 h in the open-field test (*P < 0.05, one-way ANOVA; n = 12 per group). (B) Total number of rearing events assessed for 2 min as a measure of akinesia. (C) Abnormal paw movement was assessed for 2 min in a cylinder as a measure of dyskinesia, as described previously (20). Data in B and C were collected 15 or 60 min after the last injection of vehicle (8% Tween-80 in saline), KW6002 (3 mg/kg, i.p.), or l-DOPA (25 mg/kg, i.p.) (*P < 0.05, one-way ANOVA with post hoc Bonferroni t test for KW-6002, or a t test for l-DOPA; n = 3–6 per group). (D) The pERK-positive cells were only observed in l-DOPA–, but not in vehicle- or KW-6002–treated animals. The graph represents the relative number of pERK-labeled neurons in the dorsal striatum from each treatment group. As there was no positive pERK signal at either 15 or 60 min after KW-6002 administration, the data were pooled (*P < 0.05 compared with vehicle or KW-6002 group, one-way ANOVA with post hoc Bonferroni t test; n = 3–5 per group). The data for A to D represent the mean ± SEM.
Inhibition of ERK Phosphorylation by MEK Inhibitor SL-327 Attenuates LID, Without Reducing Akinesia Improvement in Pitx3ak/ak Mice.
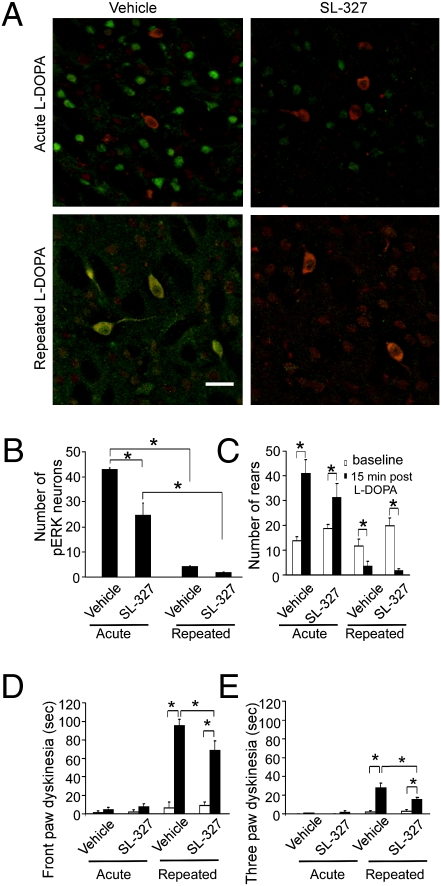
To establish a functional relationship between ERK phosphorylation in cholinergic interneurons and dyskinesia, we tested whether inhibition of ERK phosphorylation could reduce LID expression in Pitx3ak/ak mice. ERK1/2 is so far the only known substrate of mitogen-activated protein kinase kinase1/2 (MEK1/2) (23) and the administration of the specific MEK1/2 inhibitor, SL-327, or Ras inhibitor, lovastatin, concurrently with repeated l-DOPA treatment significantly attenuates l-DOPA–induced ERK phosphorylation and LID in unilateral DA-lesioned animals (4, 8), suggesting that ERK phosphorylation is necessary for the induction of dyskinesia. The inhibitory effect of SL-327 treatment on ERK phosphorylation was confirmed by a decrease in the intensity of pERK immunostaining in cholinergic neurons by SL-327 (Fig. 4A) and in the number of striatal pERK-expressing cells in the dorsal striatal area of Pitx3ak/ak mice acutely treated with l-DOPA (Fig. 4B). Behaviorally, SL-327 appeared to attenuate the acute effects of l-DOPA on akinesia (Fig. 4C), but this did not reach statistical significance. A similar statistically insignificant trend has been observed with this agent on general locomotion (4). SL-327 did, however, significantly decrease LID in Pitx3ak/ak mice with repeated l-DOPA treatment (Fig. 4 D and E). The effect of SL-327 on LID was likely not because of inhibition of other MAP kinases, as neither phospho-p38 or phospho-JNK were activated by l-DOPA in the dorsal striatum of Pitx3ak/ak mice (Fig. S4).
Fig. 4.
MEK inhibitor, SL-327, reduces pERK staining and the dyskinetic, but not antiparkinsonian effect of l-DOPA in Pitx3ak/ak mice. Mice were treated repeatedly for 7 wk with l-DOPA (25 mg/kg, twice a day, i.p.) or with saline and then evaluated for improvement in akinesia or expression of dyskinesia following a final challenge dose of l-DOPA with or without SL-327 pretreatment. The animals were perfused and striatal tissue sections were double-immunostained for pERK and for ChAT. (A) ChAT (red) colocalizes with pERK (green) in dorsal striatal area of Pitx3ak/ak mice exposed repeatedly to l-DOPA (Lower), but not with the first, acute exposure of l-DOPA (Upper). Either vehicle (8% Tween-80 in saline) or specific MEK inhibitor, SL-327 (75 mg/kg, i.p.) were administered 45 min before l-DOPA challenge. SL-327 reduced both the intensity of pERK staining and the number of pERK-expressing cells in both acute (Upper, Right) and repeated L-DOPA groups (Lower, Right). (Scale bar, 30 μm.) (B) SL-327 pretreatment decreased pERK-immunofluorescent neurons in acute l-DOPA treatment group in the dorsal striatal area (n = 5–8 per group; *P < 0.05 by two-way ANOVA followed by Tukey post hoc test). (C) The total number of rearing events increase with acute l-DOPA, reflecting the reversal of akinesia (n = 8 per group; *P < 0.05, two-way ANOVA followed by Tukey post hoc test). The duration of front-paw (D) and three-paw (E) dyskinesias in Pitx3ak/ak mice treated repeatedly with l-DOPA reflect moderate and severe forms of dyskinesia, respectively (20). (D and E: n = 8 per group; *P < 0.05, two-way ANOVA followed by Tukey post hoc test). The data for B to E represent the mean ± SEM.
ERK phosphorylation has numerous possible downstream targets. Our laboratory and others have noted FosB expression in the denervated striatum of dyskinetic animals (2, 3, 20, 24). DA- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) is also involved in DA-stimulated signal transduction and DARPP-32 knockout mice have significantly attenuated LID (4). However, DARPP-32 and FosB expression in Pitx3ak/ak mice treated repeatedly with l-DOPA did not overlap with striatal cholinergic interneurons (Fig. S1A), suggesting that these effects of l-DOPA are confined to MSN. These data suggest that striatal cholinergic interneurons have different downstream signaling pathways that mediate the expression of dyskinesia. Further delineation of this pathway will be addressed in future studies.
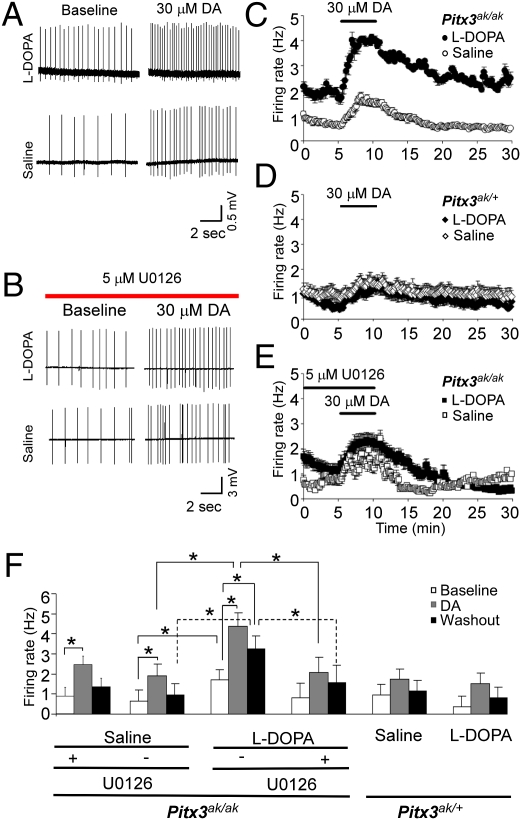
Repeated l-DOPA Exposure Is Associated with ERK-Dependent Increased Baseline Firing Rate and Stronger DA-Induced Excitation in Pitx3ak/ak Mice.
To test whether the observed differences in ERK phosphorylation in striatal cholinergic interneurons correlate with differences in excitability before and after repeated l-DOPA administration, we recorded the firing rate of striatal cholinergic neurons from both homozygous (Pitx3ak/ak) and heterozygous (Pitx3ak/+) mice previously treated with either l-DOPA (25 mg/kg, twice a day, i.p.) or saline for at least 7 wk. Cholinergic interneurons were identified on the basis of soma diameter and firing properties (SI Materials and Methods). The baseline firing rate of striatal cholinergic neurons was significantly higher in l-DOPA–treated homozygous Pitx3ak/ak mice compared with saline-treated controls of the same genotype (Fig. 5). DA increased firing rate of cholinergic neurons in both l-DOPA– and saline-treated homozygous Pitx3ak/ak mice, but to a much higher rate in mice treated repeatedly with l-DOPA than in mice treated with saline (Fig. 5 A, C, and F). Although the percent-increase in firing was similar because of a higher baseline in the l-DOPA–treated Pitx3ak/ak mice, the absolute magnitude of the firing rate change from the baseline to the peak was much greater in this group (2.05 ± 0.20 Hz) compared with the saline control group (0.92 ± 0.27 Hz, P = 0.011). As transmitter release is highly sensitive to neuronal firing rate (25), the l-DOPA–induced changes in activity and DA sensitivity are expected to profoundly affect striatal acetylcholine release. In addition, the DA-induced elevation of firing rate in l-DOPA–treated Pitx3ak/ak mice lasted longer than other treatment groups, maintaining increased excitability 4 to 5 min after DA washout, when the firing rate in saline-treated Pitx3ak/ak mice returned to baseline levels (Fig. 5C). To assess the functional connection between ERK phosphorylation and increased excitability, a selective MEK1/2 inhibitor, U0126, was applied before acute DA administration in the slice preparation. U0126 is commonly used for in vitro physiology experiments and efficiently inhibits MEK (26). Because U0126 does not penetrate the blood-brain barrier, systemically active SL-327 was used in in vivo experiments described above (Fig. 4). U0126 significantly attenuated the acute DA-induced excitation of striatal cholinergic neurons from repeated l-DOPA–treated Pitx3ak/ak mice, compared with those without U0126 treatment (Fig. 5B, E, and F). U0126 blocked the increased firing after DA, and following DA washout in striatal cholinergic neurons in Pitx3ak/ak mice with repeated l-DOPA treatment (Fig. 5 E and F). The excitation seen after inhibition of ERK activation was similar to that seen in tissue from naive Pitx3ak/ak mice untreated with l-DOPA. U0126 had no inhibitory effect on acute DA-induced firing of striatal cholinergic interneurons from Pitx3ak/ak mice treated repeatedly with saline (Fig. 5F). Together, these data demonstrate that ERK phosphorylation is necessary for enhanced excitability of striatal cholinergic neurons after repeated l-DOPA treatment.
Fig. 5.
Repeated administration of l-DOPA to Pitx3ak/ak mice increases firing rate of striatal cholinergic neurons. Mice were treated repeatedly with l-DOPA (25 mg/kg, twice a day, i.p.) or saline for 8 wk, but no treatment was given on the day of experiment. Extracellular recordings were made from cholinergic interneurons in the dorsal striatal area of parasagittal slices from Pitx3ak/ak homozygous mice or from the corresponding striatal area of Pitx3ak/+ heterozygous mice. Dopamine (30 μM) was applied from minutes 5 to 10. To inhibit ERK phosphorylation, 5 μM U0126 was applied 10 min before exposure to DA. Then, U0126 and DA were washed out after 5 min of incubation. Representative firing rate data before and after 30 μM DA from saline and l-DOPA–treated Pitx3ak/ak mice in the absence (A) or presence of U0126 (B). DA induces stronger excitation of striatal ChAT interneurons in repeated l-DOPA treated Pitx3ak/ak mice than in controls (A). Time course of average firing rate before and after DA from Pitx3ak/ak (C) and Pitx3ak/+ (D) mice treated repeatedly with l-DOPA or saline. (E) Time course of average firing rate before and after DA in the presence of U0126 from Pitx3ak/ak mice treated repeatedly with l-DOPA or saline. (F) Comparison of baseline firing rate, DA-induced firing, the firing rate after DA washout among groups of Pitx3ak/+ and Pitx3ak/ak mice pre-exposed repeatedly with either saline or l-DOPA, as well as the effect of U0126 on the firing rate. Data used for statistical analysis were from a 1-min interval: baseline was collected during the minute immediately before the onset of the drug effect; the DA response was centered on the peak firing rate between minutes 6.5 and 13.5; and the recovery was collected between 14 and 15 min after the onset of recording (n = 4–9 per group, mean ± SEM; *P < 0.05, homozygous comparisons, two-way ANOVA followed by Tukey post hoc test).
Muscarinic Receptor Antagonism Attenuates LID, and Enhances l-DOPA–Induced Akinesia Improvement in Pitx3ak/ak Mice.
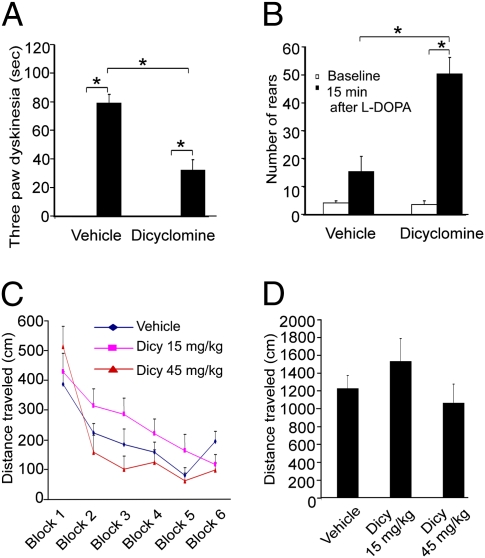
Striatal cholinergic neurons express M4 muscarinic receptors (27, 28) and striatal projection neurons express both M1 and M4 muscarinic receptors (28). We hypothesized that the enhanced striatal cholinergic transmission could affect striatal projection neuron output through muscarinic receptors to mediate the expression of LID. The muscarinic receptor antagonist, dicyclomine (15 mg/kg) (29), was administered to Pitx3ak/ak mice that had previously undergone 7 wk of l-DOPA treatment and significantly reduced LID in these animals (Fig. 6A). On the other hand, dicyclomine enhanced the akinesia improvement produced by l-DOPA administration, as shown by enhanced rearing (Fig. 6B). Dicyclomine alone did not have significant impact on general locomotor activity (Fig. 6 C and D). Our data support the hypothesis that LID is mediated by elevated cholinergic tone, which can be partially inhibited by muscarinic antagonism.
Fig. 6.
Muscarinic antagonist, dicyclomine, enhances l-DOPA's antiparkinsonian effect and attenuates dyskinesia in Pitx3ak/ak mice. Dicyclomine (15 mg/kg, i.p.) was given 30 min before the final injection of l-DOPA (25 mg/kg) in Pitx3ak/ak mice treated repeatedly with l-DOPA (25 mg/kg, twice a day, i.p.) for 5 wk. (A) Three-paw dyskinesia was markedly decreased by dicyclomine. (B) Dicyclomine significantly enhanced rearing activity compared with vehicle/l-DOPA. (n = 12–16 per group; *P < 0.05, two-way ANOVA followed by Tukey post hoc test). (C and D) The muscarinic receptor antagonist dicyclomine (Dicy) had no impact on general locomotor activity of Pitx3ak/+ heterozygous mice in the open-field test for either individual time blocks (5 min, C) or the total time (30 min, D) tested compared with vehicle (n = 12 per group). The data for A to D represent the mean ± SEM.
Cholinergic Neuronal Hyperactivity Correlates with LID in Unilateral Parkinsonian Mice.
To confirm our findings in Pitx3ak/ak mice of increased cholinergic tone associated with LID, we used the unilateral 6-OHDA lesion of median forebrain bundle, which has been widely used to model PD and LID (30). The extent of neurotoxin-induced DA denervation in unilateral lesioned mice was about 94% by densitometry of tyrosine hydroxylase-immunoreactive fibers (Fig. S5 A and B) (n = 26). Repeated l-DOPA treatment for 5 wk enhanced limb dyskinesia, compared with the first exposure to l-DOPA, although even acute l-DOPA exposure induced significant limb and axial dyskinesia (Fig. S5C), consistent with the literature using this model (2, 30–34). Acute treatment with l-DOPA–induced pERK in predominantly medium-sized neurons and repeated l-DOPA administration reduced the number of medium-sized pERK-labeled cells by 72% and increased the number of large-diameter pERK-labeled neurons by 3.4-fold in lesioned striatum (Fig. S5D). Double immunofluorescent staining confirmed expression of pERK predominantly in ChAT-labeled neurons following repeated l-DOPA treatment (Fig. S5E). Neither acute nor repeated l-DOPA administration induced significant ERK activation in the intact striatum of 6-OHDA–lesioned mice (Fig. S5D). Administration of dicyclomine significantly attenuated LID without interfering with the antiakinetic, therapeutic action of l-DOPA on forepaw adjusting steps (Fig. S5F). Thus, in both the unilateral lesion model and Pitx3ak/ak mice, repeated l-DOPA treatment increases ERK activation in striatal ChAT neurons and diminishes ERK phosphorylation in MSN, and muscarinic antagonism attenuates LID without affecting l-DOPA's beneficial antiparkinsonian action.
Discussion
To date, investigations of the molecular pathways underlying LID have focused primarily upon MSNs, which are the striatal projection neurons. Direct D1R stimulation in the denervated striatum of PD animals activates ERK by phosphorylation (5, 35, 36). Our data show that l-DOPA–induced ERK activation shifts from being predominantly in MSN to cholinergic interneurons concomitant with behavioral LID expression in both unilateral 6-OHDA lesion and the Pitx3ak/ak model. Therefore, this shift of ERK activation from MSN to cholinergic interneurons indicates a potentially maladaptive neural mechanism, where l-DOPA responses become sensitized and lead to LID. Previous studies suggest that ERK activation by l-DOPA in MSNs is important in the induction of LID, as inhibiting ERK activation with each dose of l-DOPA attenuates LID development (4, 8). On the other hand, our findings show that ERK activation in cholinergic neurons is important in the actual expression of LID, as inhibition of ERK activation after LID development reduces LID expression. Although changes in cholinergic neurons may be compensatory, resulting from primary alterations in MSNs or corticostriatal excitatory drive during induction of LID, our data suggest that cholinergic neurons may play a central role upstream of output MSNs once LID is established.
Although Pitx3ak/ak mice and unilateral-lesioned PD mice both exhibited a similar biochemical response to repeated l-DOPA treatment—namely, a shift in ERK phosphorylation from MSN to striatal cholinergic neurons—we noted that the acute behavioral response to l-DOPA differed between the two mouse models. Pitx3ak/ak mice showed little dyskinetic behavior upon the first exposure to l-DOPA, whereas dyskinesia was expressed by unilateral lesioned mice. The degree of behavioral response to acute l-DOPA may depend upon the extent of DA denervation between the two PD models. Unilateral 6-OHDA lesions used in this study produced massive striatal DA depletions of 94%, covering the entire striatum, whereas striatal DA depletion is limited to the most dorsal aspect of dorsal striatum in Pitx3ak/ak mice. LID has been reported with the first exposure to l-DOPA in unilateral 6-OHDA–lesioned rats (2, 30–34) and the level of dyskinetic behavior is influenced by the amount of DA loss (34). Therefore, we believe the Pitx3ak/ak mice show the time course more consistent with mild to moderate PD, whereas 6-OHDA–lesioned mice model a more severe stage of PD.
Striatal cholinergic neurons represent only about 2% of the total striatal neuronal population (21), yet the large striatal cholinergic neurons have richly arborizing axons with large terminal fields within the striatum, suggesting the importance of these neurons in modulating striatal activity (37). Cholinergic tone contributes to DA and glutamate release locally via presynaptic nicotinic receptors on DA and glutamatergic terminals (38, 39). Striatal cholinergic interneuron activity also contributes to plasticity of glutamatergic and GABAergic inputs to this area via both muscarinic and nicotinic receptor activation (37, 40, 41). DA denervation modulates the cholinergic system, as M4 mRNA is reduced (42), along with M4 autoreceptor malfunction, resulting in a loss of negative feedback inhibition and increased acetylcholine (ACh) release (27). Among the five subtypes of muscarinic receptors, MSNs predominantly express M1 and M4 receptors (43). Striatal synaptic plasticity is dependent upon endogenous ACh acting specifically on M1 receptors, with high levels of ACh facilitating long-term potentiation and lower levels facilitating long-term depression (44). Long-term potentiation has been associated with dyskinetic behavior in animal models of PD (45).
We showed that repeated l-DOPA administration to Pitx3ak/ak mice results in enhanced baseline and DA-induced firing rate in striatal cholinergic neurons, compared with repeated saline-treated Pitx3ak/ak mice. In addition, the DA-induced firing rate of striatal cholinergic neurons is significantly greater in repeated l-DOPA–treated Pitx3ak/ak mice than in Pitx3ak/+ mice. Inhibition of ERK activation restores the baseline and DA-induced increases in firing rate l-DOPA–treated Pitx3ak/ak mice to that of untreated Pitx3ak/ak. Together, the electrophysiological data along with biochemical and behavioral results support the hypothesis that expression of LID following repeated l-DOPA exposure results from enhanced striatal cholinergic neuronal excitability, and that these changes are mediated by ERK activation.
The inhibition of LID with a muscarinic antagonist provides further evidence for the contribution of enhanced cholinergic signaling to this condition. These findings are consistent with earlier reports of exacerbated parkinsonian symptoms following treatment with the anticholinesterase inhibitor, physostigmine, and improved symptoms after treatment with centrally acting antimuscarinics, such as benztropine (46). Anticholinergic treatments are still used clinically for parkinsonian tremor and rigidity (47), but have been limited because of side effects and have not been explored for LID. Dicyclomine is a muscarinic antagonist with limited receptor subtype specificity (48) and development of more selective muscarinic receptor ligands or other novel approaches to modify striatal cholinergic signaling specifically may provide more effective treatment strategies for LID. Together, the electrophysiological data along with biochemical and behavioral results support the hypothesis that expression of LID following repeated l-DOPA exposure results from enhanced striatal cholinergic neuronal excitability, and that these changes are mediated by ERK activation within cholinergic neurons (Fig. S6).
Materials and Methods
For details regarding the experimental procedures used in these studies, please see SI Materials and Methods.
Drug Treatment and Behavioral Tests.
Homozygous Pitx3ak/ak and heterozygous Pitx3ak/+ mice were repeatedly treated with either saline or l-DOPA for various time periods. The 6-OHDA was used to create a unilateral PD model in Pitx3ak/+ mice (4–5 mo old). Abnormal paw movements exhibited in the cylinder were scored as previously detailed (20).
Immunohistochemistry.
Mice were perfused immediately following behavioral testing for immunohistochemical staining of pERK and other neuronal phenotype markers, as described previously (20).
Electrophysiology.
On-cell current-clamp recordings were obtained from cholinergic interneurons located in the dorsal lateral striatum, identified by their large size and spontaneous firing rate (between 0.1 and 7 Hz).
Supplementary Material
Acknowledgments
We thank Dr. Jacques Petzer (North-West University, Potchefstroom, South Africa) for providing KW-6002, Dr. Vytautas P. Bindokas (University of Chicago BSD Microscopy Core Facilities, Chicago, IL) for assistance with confocal microscopy, and Dr. William Lin for critical comments on the manuscript. This work was supported by National Institutes of Health Grants R01 NS064865 (to U.J.K.), R01 NS32080 (to U.J.K.), and American Parkinson Disease Association Advanced Center for Research (to U.J.K.), Grants DA015918 (to D.S.M.), F31DA023340 (to J.P.B.), and DA07255 (to S.A.O.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006511108/-/DCSupplemental.
References
- 1.Nutt JG. Motor fluctuations and dyskinesia in Parkinson's disease. Parkinsonism Relat Disord. 2001;8:101–108. doi: 10.1016/s1353-8020(01)00024-4. [DOI] [PubMed] [Google Scholar]
- 2.Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA–induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6:461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- 3.Pavón N, Martín AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with l-DOPA–induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59:64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 4.Santini E, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in l-DOPA–induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of l-DOPA–induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62:800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santini E, et al. Distinct changes in cAMP and extracellular signal-regulated protein kinase signalling in l–DOPA-induced dyskinesia. PLoS ONE. 2010;5:e12322. doi: 10.1371/journal.pone.0012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuster S, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of l-DOPA–induced abnormal involuntary movements in experimental Parkinson's disease. J Neurosci. 2008;28:4311–4316. doi: 10.1523/JNEUROSCI.4720-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunes I, Tovmasian LT, Silva RM, Burke RE, Goff SP. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci USA. 2003;100:4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Munckhof P, et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130:2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- 11.Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 12.Hwang DY, et al. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: Behavioral characterization of a novel genetic model of Parkinson's disease. J Neurosci. 2005;25:2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smits SM, Mathon DS, Burbach JP, Ramakers GM, Smidt MP. Molecular and cellular alterations in the Pitx3-deficient midbrain dopaminergic system. Mol Cell Neurosci. 2005;30:352–363. doi: 10.1016/j.mcn.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Kas MJ, et al. Phenotypic segregation of aphakia and Pitx3-null mutants reveals that Pitx3 deficiency increases consolidation of specific movement components. Behav Brain Res. 2008;186:208–214. doi: 10.1016/j.bbr.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 15.Beeler JA, et al. Dopamine-dependent motor learning: Insight into levodopa's long-duration response. Ann Neurol. 2010;67:639–647. doi: 10.1002/ana.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickremaratchi MM, Ben-Shlomo Y, Morris HR. The effect of onset age on the clinical features of Parkinson's disease. Eur J Neurol. 2009;16:450–456. doi: 10.1111/j.1468-1331.2008.02514.x. [DOI] [PubMed] [Google Scholar]
- 17.Pons R, et al. Aromatic l-amino acid decarboxylase deficiency: Clinical features, treatment, and prognosis. Neurology. 2004;62:1058–1065. doi: 10.1212/wnl.62.7.1058. [DOI] [PubMed] [Google Scholar]
- 18.van den Munckhof P, Gilbert F, Chamberland M, Lévesque D, Drouin J. Striatal neuroadaptation and rescue of locomotor deficit by l-dopa in aphakia mice, a model of Parkinson's disease. J Neurochem. 2006;96:160–170. doi: 10.1111/j.1471-4159.2005.03522.x. [DOI] [PubMed] [Google Scholar]
- 19.Singh B, et al. Motor deficits and altered striatal gene expression in aphakia (ak) mice. Brain Res. 2007;1185:283–292. doi: 10.1016/j.brainres.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Y, et al. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson's disease. Neurobiol Dis. 2007;27:11–23. doi: 10.1016/j.nbd.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- 22.Lundblad M, Vaudano E, Cenci MA. Cellular and behavioural effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA–induced dyskinesia. J Neurochem. 2003;84:1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 24.Andersson M, Westin JE, Cenci MA. Time course of striatal DeltaFosB-like immunoreactivity and prodynorphin mRNA levels after discontinuation of chronic dopaminomimetic treatment. Eur J Neurosci. 2003;17:661–666. doi: 10.1046/j.1460-9568.2003.02469.x. [DOI] [PubMed] [Google Scholar]
- 25.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 26.Chen-Roetling J, Li Z, Chen M, Awe OO, Regan RF. Heme oxygenase activity and hemoglobin neurotoxicity are attenuated by inhibitors of the MEK/ERK pathway. Neuropharmacology. 2009;56:922–928. doi: 10.1016/j.neuropharm.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding J, et al. RGS4-dependent attenuation of M4 autoreceptor function in striatal cholinergic interneurons following dopamine depletion. Nat Neurosci. 2006;9:832–842. doi: 10.1038/nn1700. [DOI] [PubMed] [Google Scholar]
- 28.Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caccamo A, et al. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Cenci MA, Lee CS, Björklund A. l-DOPA–induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- 31.Johansson PA, Andersson M, Andersson KE, Cenci MA. Alterations in cortical and basal ganglia levels of opioid receptor binding in a rat model of l-DOPA–induced dyskinesia. Neurobiol Dis. 2001;8:220–239. doi: 10.1006/nbdi.2000.0372. [DOI] [PubMed] [Google Scholar]
- 32.Delfino MA, et al. Behavioral sensitization to different dopamine agonists in a parkinsonian rodent model of drug-induced dyskinesias. Behav Brain Res. 2004;152:297–306. doi: 10.1016/j.bbr.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Nadjar A, Gerfen CR, Bezard E. Priming for l-dopa–induced dyskinesia in Parkinson's disease: A feature inherent to the treatment or the disease? Prog Neurobiol. 2009;87:1–9. doi: 10.1016/j.pneurobio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Putterman DB, Munhall AC, Kozell LB, Belknap JK, Johnson SW. Evaluation of levodopa dose and magnitude of dopamine depletion as risk factors for levodopa-induced dyskinesia in a rat model of Parkinson's disease. J Pharmacol Exp Ther. 2007;323:277–284. doi: 10.1124/jpet.107.126219. [DOI] [PubMed] [Google Scholar]
- 35.Darmopil S, Martín AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits l-DOPA–induced dyskinesia and histone activation. Biol Psychiatry. 2009;66:603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Santini E, et al. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 2009;108:621–633. doi: 10.1111/j.1471-4159.2008.05831.x. [DOI] [PubMed] [Google Scholar]
- 37.Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Kaiser S, Wonnacott S. Alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- 39.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 40.Pisani A, et al. Targeting striatal cholinergic interneurons in Parkinson's disease: Focus on metabotropic glutamate receptors. Neuropharmacology. 2003;45:45–56. doi: 10.1016/s0028-3908(03)00137-0. [DOI] [PubMed] [Google Scholar]
- 41.Bamford NS, et al. Repeated exposure to methamphetamine causes long-lasting presynaptic corticostriatal depression that is renormalized with drug readministration. Neuron. 2008;58:89–103. doi: 10.1016/j.neuron.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kayadjanian N, Schofield WN, Andren J, Sirinathsinghji DJ, Besson MJ. Cortical and nigral deafferentation and striatal cholinergic markers in the rat dorsal striatum: Different effects on the expression of mRNAs encoding choline acetyltransferase and muscarinic m1 and m4 receptors. Eur J Neurosci. 1999;11:3659–3668. doi: 10.1046/j.1460-9568.1999.00788.x. [DOI] [PubMed] [Google Scholar]
- 43.Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonsi P, et al. Loss of muscarinic autoreceptor function impairs long-term depression but not long-term potentiation in the striatum. J Neurosci. 2008;28:6258–6263. doi: 10.1523/JNEUROSCI.1678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Picconi B, et al. Loss of bidirectional striatal synaptic plasticity in l-DOPA–induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 46.Duvoisin RC. Cholinergic-anticholinergic antagonism in parkinsonism. Arch Neurol. 1967;17:124–136. doi: 10.1001/archneur.1967.00470260014002. [DOI] [PubMed] [Google Scholar]
- 47.Katzenschlager R, Sampaio C, Costa J, Lees A. Anticholinergics for symptomatic management of Parkinson's disease. Cochrane Database Syst Rev. 2003;(2):CD003735. doi: 10.1002/14651858.CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckley NJ, Bonner TI, Buckley CM, Brann MR. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989;35:469–476. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.