Abstract
The neuromodulatory function of dopamine (DA) is an inherent feature of nervous systems of all animals. To learn more about the function of neural DA in Drosophila, we generated mutant flies that lack tyrosine hydroxylase, and thus DA biosynthesis, selectively in the nervous system. We found that DA is absent or below detection limits in the adult brain of these flies. Despite this, they have a lifespan similar to WT flies. These mutants show reduced activity, extended sleep time, locomotor deficits that increase with age, and they are hypophagic. Whereas odor and electrical shock avoidance are not affected, aversive olfactory learning is abolished. Instead, DA-deficient flies have an apparently “masochistic” tendency to prefer the shock-associated odor 2 h after conditioning. Similarly, sugar preference is absent, whereas sugar stimulation of foreleg taste neurons induces normal proboscis extension. Feeding the DA precursor l-DOPA to adults substantially rescues the learning deficit as well as other impaired behaviors that were tested. DA-deficient flies are also defective in positive phototaxis, without alteration in visual perception and optomotor response. Surprisingly, visual tracking is largely maintained, and these mutants still possess an efficient spatial orientation memory. Our findings show that flies can perform complex brain functions in the absence of neural DA, whereas specific behaviors involving, in particular, arousal and choice require normal levels of this neuromodulator.
Keywords: neurotransmitters, locomotor activity, memory formation, choice behavior, feeding behavior
An important challenge in neuroscience is to understand the roles of specific neurotransmitter systems on brain homeostasis and functioning. Dopamine (DA), a biogenic amine biosynthesized from tyrosine, is an essential neuromodulator in the mammalian central nervous system that is involved in attention, movement control, motivation, and cognition. Studies in Drosophila melanogaster indicate that DA also plays central regulatory roles in insects, specifically in the neural networks controlling locomotor activity and stereotypical behaviors (1–3), sleep and arousal (4–7), registration of salient stimuli (4, 8, 9), and associative olfactory learning (10–15). Some of these studies were based on genetic inactivation or overactivation of dopaminergic neurons. Dopaminergic neurons can corelease other neuroactive agents, such as neuropeptides, however. Therefore, one must ensure that the behavioral phenotypes observed specifically result from the lack of DA release to draw firm conclusions on brain DA function.
Nearly all neuropil regions of the insect CNS receive dense dopaminergic innervation. In particular, the Drosophila adult brain contains six paired clusters of dopaminergic neurons, some of which specifically project to higher brain centers, such as the central complex and the mushroom bodies (1, 10, 12, 13, 16–18). Tyrosine hydroxylase (TH) catalyzes the first and rate-limiting step in DA biosynthesis (Fig. S1A). Because DA is also required in Drosophila as a precursor substrate for cuticle sclerotization and melanization, inactivating mutations of the genomic TH, alias pale (ple) locus, results in unpigmented cuticle and late embryonic lethality (19).
Alternative splicing of Drosophila TH (DTH) produces two enzyme isoforms, DTH1 and DTH2 (20, 21) (Fig. S1B). DTH1 is selectively expressed in DA neurons within the CNS, whereas DTH2 is expressed in peripheral nonnervous tissues, which include the hypodermal cells that secrete the cuticle matrix (20, 22). Here, we take advantage of this tissue-specific alternative splicing to construct a mutated transgene, DTHgFS±, that only expresses an active TH enzyme in nonneural cells. Homozygous ple mutants are rescued by this transgene to adult stage, generating Drosophila essentially devoid of DA in the adult brain. We then studied the consequences of this neural-specific DA depletion on adult survival and behavior.
Results
Generation of Viable DA-Deficient Drosophila.
We first established the conditions for full rescue of DTH deficiency with the GAL4-upstream activation sequence (UAS) binary expression system by expressing genomic DTH (DTHg) driven by TH-GAL4 and Ddc-GAL4, each of which contains regulatory sequences from genes involved in DA biosynthesis (Fig. S1A). The combined action of the Ddc-GAL4 and TH-GAL4 drivers is required for full rescue, with neither GAL4 driver alone being sufficient for full rescue to adult stage (Table S1).
We then used in vitro mutagenesis to introduce frameshift mutations in DTHg (Fig. S1 C and D and SI Materials and Methods). DTHgFS+ contains one additional base in a hypoderm-specific exon, and DTHgFS± contains the same mutation plus another compensating mutation that removes one base in an adjacent common exon. When transcribed, DTHgFS+ only expresses active TH in neural tissues and, conversely, DTHgFS± only expresses active TH in nonneural tissues. No ple rescue could be observed with DTHgFS+ (Table S2), confirming that an active DTH2 isoform producing cuticular DA is required for Drosophila development (22). In contrast, expression of DTHgFS± yielded full rescue of ple to adulthood (Table S2), indicating that biosynthesis of neural DA is not essential for fly development and survival.
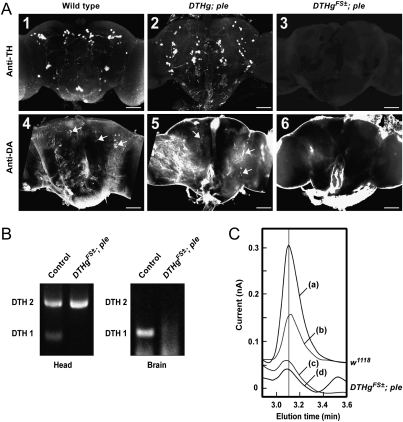
Immunohistochemistry confirmed that TH and DA are not detectable in adult brain of the DTHgFS±-rescued ple mutants (Fig. 1A 3 and 6), whereas they are present in WT flies (Fig. 1A 1 and 4) or ple mutants rescued by the native DTHg construct (Fig. 1A 2 and 5). Accordingly, RT-PCR shows that the neural-specific DTH1 mRNA is absent in head and brain extracts of DTHgFS±; ple (Fig. 1B). Similarly, no TH is present in the larval CNS of DTHgFS±; ple, whereas it is expressed at this stage in DTHg; ple(Fig. S2A). In contrast, DA is detected in larval neurons of both strains, although fewer positive cells could be seen in larval brain hemispheres of DTHgFS±; ple versus DTHg; ple (Fig. S2 A3 and A4). This suggests that systemic DA produced by peripheral TH can partially supply the mutant larval CNS with the missing biogenic amine.
Fig. 1.
Lack of TH expression and DA in the brain of DTHgFS±-rescued ple Drosophila. (A) Anti-TH immunostaining on whole-mount adult brains of WT Drosophila (1) and DTHg (2) or DTHgFS±-rescued (3) ple mutants. No TH immunoreactivity was detected in the brain of DTHgFS±; ple. Anti-DA immunostaining. DA-positive cells can be observed in the brains of WT (4) and DTHg; ple (5) flies (arrows) but not in the brains of DTHgFS±; ple flies (6). (B) Detection of the DTH1- and DTH2-specific spliced mRNAs by RT-PCR in heads (Left) and dissected brains (Right) of control or DTHgFS±; ple flies. No DTH transcript is present in the brain of DTHgFS±-rescued flies. (C) Representative graphs of DA assay by HPLC in brain extracts of control w1118 (a and b) and DTHgFS±; ple (c and d) detected with an optimal potential of +500 mV (a and c) or reduced to +420 mV (b and d). (d) Reduced potential did not decrease the amplitude of the residual “DA” peak of neural TH-deficient flies, indicating that this peak is not DA. The small additional peak on the right corresponds to a contaminant from eye pigments.
DA was then assayed by HPLC to determine the absolute magnitude of reduction of DA level in adult brain (Fig. 1C and Tables S3 and S4). Brain extracts from control flies and DTHg; ple showed equivalent levels of DA per brain (52.0 and 48.9 pg per brain, respectively), indicating that expression of the DTHg transgene quantitatively restored brain DA. In contrast, DTHgFS±; ple flies apparently had 7.5 pg of DA per brain, suggesting a strong (∼85%) but incomplete reduction of DA level in these mutants. HPLC was then repeated using a decreased detector potential of +420 mV, near the half-maximal oxidation potential of DA. Whereas the DA peak in an extract from a control brain behaves as expected (relative DA peak area at +420 vs. 500 mV: 0.43), the residual peak in the DTHgFS±; ple strain was unaffected by the reduced detector potential (relative “DA” peak area: 1.05) (Fig. 1C and Tables S3 and S4). We conclude that the residual peak in this strain is largely, if not totally, a non-DA contaminant and that brains of DTHgFS±; ple flies lack detectable DA, with an estimated sensitivity limit of 2% WT levels.
Nevertheless, adult brain of DA-deficient flies contains differentiated dopa decarboxylase (Ddc)-positive neurons that do not express serotonin and so are most likely “dopaminergic” neurons devoid of TH and DA. These cells indeed distribute in clusters identical to the WT pattern of dopaminergic neurons (Fig. S2B1–3). Moreover, characteristic dopaminergic innervation of the mushroom bodies appears normal in the mutant (Fig. S2B4–6). Therefore, maintenance of the adult brain dopaminergic system does not seem to depend on the activity of TH or the presence of DA in Drosophila.
DA Deficiency Reduces Activity and Arousal.
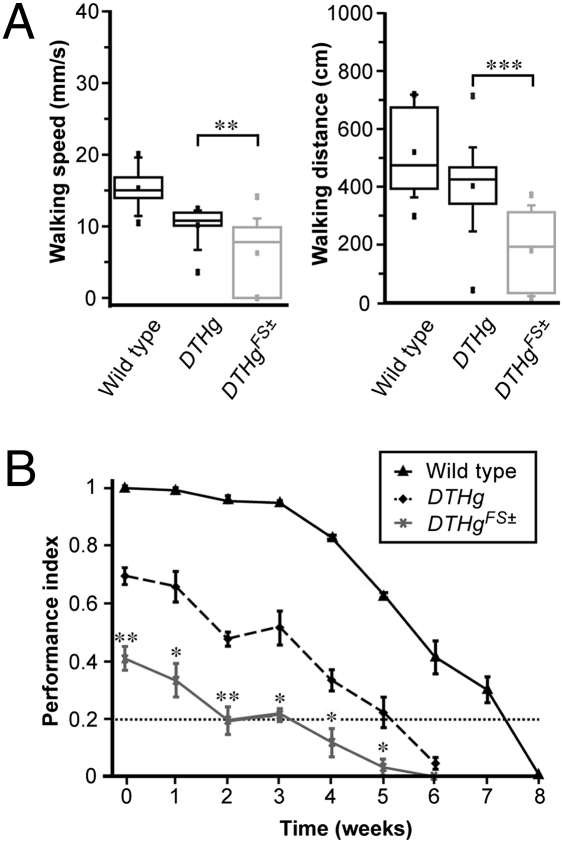
Unexpectedly, we observed that both DTHg; ple and DTHgFS±; ple lines show similar and normal adult life expectancy (Fig. S3A), demonstrating that the lack of neural DA has no detrimental effect on Drosophila lifespan. This enabled us to compare the behaviors of these lines. First, we studied the effect of neural DA deficiency on walking behavior of young adult flies. DTHg FS±; ple show reduced walking speed (median = 7.8 mm/s) compared with DTHg; ple (median = 10.8 mm/s) and WT (median = 15 mm/s) (Fig. 2A, Left). Similarly, the distance covered for 15 min is much shorter in DTHg FS±; ple (median = 193 cm) than in DTHg; ple (median = 425 cm) and WT (median = 474 cm) (Fig. 2A, Right). Therefore, flies deficient in neural DA show markedly decreased locomotor behavior.
Fig. 2.
Neural DA-deficient flies show locomotor deficits. (A) Box-and-whisker plots showing the distribution of walking speed (Left) and covered distance (Right) during spontaneous locomotor behavior of 5-d-old adult flies monitored in an open arena. The DA-deficient DTHgFS±; ple flies show a statistically significant reduction compared with rescued DTHg; ple in both walking speed (P = 0.015) and covered distance (P = 0.001). DTHg; ple show a difference compared with WT flies for walking speed (P = 0.0003) but not walking distance (P = 0.130). Plots represent the median (horizontal line), mean (square), 25% and 75% quartiles (box), 10% and 90% quantiles (whiskers), and extreme values (crosses) (**P < 0.02 and ***P < 0.005). (B) Climbing abilities of WT flies, DTHg; ple, and DTHgFS±; ple monitored by startle-induced negative geotaxis as a function of adult age. The robust negative geotactic behavior of young WT Drosophila declines steadily over time after 3 wk. DTHgFS±; ple show from the eclosion a strong impairment in this behavior; after 2 wk, their mean PI was only 0.2 (horizontal dotted line). DTHgFS±; ple values were compared for significance with DTHg; ple values (*P < 0.05 and **P < 0.02, Student's t test).
Startle-induced negative geotaxis monitors climbing ability and excitability in Drosophila, and DA modulates this behavior (1, 23). WT flies placed in a vial or a column respond to a gentle mechanical shock by fast climbing. This behavior is stable over the first 3 wk of adult life and then progressively declines with age, such that by day ∼55, WT flies show no response (Fig. 2B). The DTHgFS±; ple flies do not perform well in this test, although they are definitely able to climb. Just after eclosion, their performance index (PI) was ∼0.4, and at 15–21 d of age, their PI was only ∼0.2, comparable to the PI of 50-d-old WT flies (Fig. 2B, dotted line). DTHg; ple show PIs between those of WT and DA-deficient flies (Fig. 2B).
Monitoring activity over the light/dark cycle indicates that DTHgFS±; ple are overall less active and possibly sleep more frequently than DTHg; ple during the day (Fig. S3B). Because both strains showed frequent periods of relative inactivity, we also monitored sleep by direct observation. The results demonstrate that DTHgFS±; ple do sleep more than DTHg; ple during both day and night (Fig. S3C). Furthermore, we found that sleeping DTHgFS±; ple flies respond less frequently to mild and moderate mechanical stimuli (Fig. S3D), whereas response to strong stimuli was comparable to that of DTHg; ple flies. Overall, these results argue for a decreased arousal state in the absence of brain DA.
Treatment with 3-iodotyrosine (3IY), a TH inhibitor, decreases activity and increases resting periods in WT flies (4), whereas caffeine has the opposite effects (24). We observed that the neural TH-deficient DTHgFS±; ple, as expected, are not responsive to 3IY, whereas DTHg; ple flies reacted like WT (Fig. S5). In contrast, caffeine was found to decrease resting periods of both DTHg; ple and DTHgFS±; ple (Fig. S4), suggesting that caffeine does not act by modulating DA release in Drosophila.
Impaired Aversive Olfactory Learning and Feeding Behavior.
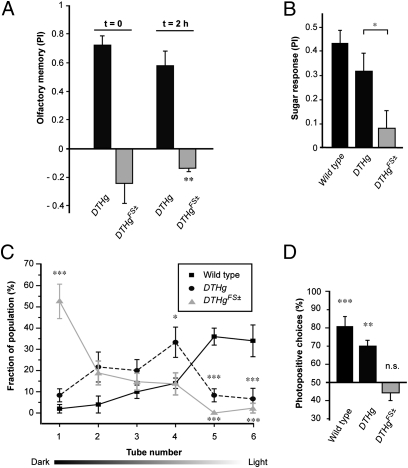
We used classical olfactory conditioning to evaluate the ability of DTHgFS±; ple flies to associate an aversive stimulus (electrical shock) with an odor. Either immediately (t = 0) or 2 h after training, these flies did not show any avoidance of the shock-associated odor (Fig. 3A). This is in contrast to the DTHg-rescued controls that demonstrate associative memory (PI = ∼0.6) (Fig. 3A), similar to WT (25). Interestingly, the DA-deficient flies showed an inverse tendency to choose the shock-associated odor during testing, an effect that was significant 2 h after training (PI = −0.13 ± 0.05, n = 9). We checked that shock avoidance (Fig. S5A) and odor perception (Fig. S5B) are preserved in DA-deficient as well as DTHg-rescued flies.
Fig. 3.
Behaviors disrupted in neural DA-deficient Drosophila. (A) Aversive olfactory learning. The PI of flies tested either immediately (t = 0) or 2 h after electrical shock training is shown. Whereas DTHg; ple show normal conditioned avoidance of the aversive odor, the neural DA-deficient DTHgFS±; ple do not learn. They rather show an abnormal preference for the shock-associated odor that is statistically significant 2 h after training (P = 0.312 at t = 0 and **P = 0.012 at t = 2 h, Wilcoxon signed-rank test against 0 as random behavior). (B) Sugar preference. After a 21-h starvation period, flies were tested in a T-maze for their response to sucrose. WT and DTHg-rescued Drosophila show positive responses to sugar, whereas DTHgFS±; ple flies appear not to be attracted (*P < 0.05, Student's t test). The sugar response of the DTHgFS±; ple flies was not different from 0 as random behavior (Wilcoxon signed-rank test). (C) Phototaxis assayed by countercurrent distribution. WT Drosophila display strong phototactic behavior and mostly distributes toward the light source in tubes 5 and 6. In contrast, most DTHgFS±; ple flies remain in tube 1. This behavioral impairment is partially rescued in DTHg; ple flies [***P < 0.0001, one-way ANOVA (Bonferroni-corrected) compared with WT values]. (D) Single-fly phototaxis assay. Flies were allowed to distribute freely between an illuminated tube and a dark tube. WT and DTHg-rescued Drosophila showed a strong preference for the illuminated tube. In contrast, the neural DA-deficient DTHgFS±; ple distributed equally [***P = 6.1 × 10−5, **P = 0.012, and P = 0.499 (not significant [n.s.]) for WT, DTHg; ple, and DTHgFS±; ple, respectively, Wilcoxon signed-rank test; mean tested against reference constant (50% for equal distribution)].
Pharmacological rescue experiments were performed in adult flies to distinguish between physiological or developmental effects of brain DA deficiency. Remarkably, treatment of adult DTHgFS±; ple with the DA precursor l-DOPA significantly improved their negative geotaxis behavior (Fig. S6A). This indicates that progressive locomotor impairments directly result from DA deficiency and not from developmental abnormalities. Similarly, l-DOPA feeding of adult DA-deficient flies substantially increased their learning performance index towards a positive value (PI = 0.26 ± 0.08 with l-DOPA compared to -0.11 ± 0.07 without l-DOPA at t = 0, n = 6) (Fig. S6B), confirming that the lack of neural DA induces an absolute defect in aversive olfactory memory.
Preparatory to appetitive conditioning trials, we tested the flies for sugar preference after a 21-h period of starvation. Whereas DTHg-rescued controls respond positively and comparably to WT, DTHgFS±; ple flies showed no significant response to sucrose (Fig. 3B). The proboscis extension reflex appears normal in these mutants (Movie S1), however, indicating that sugar perception and reflex appetitive reactions are preserved. This suggests that neural DA is specifically required for behavioral attraction to sugar. Quantification of food intake revealed that DTHgFS±; ple eat one-third as much as DTHg; ple (Fig. S6C), demonstrating that they are markedly hypophagic. Again, l-DOPA significantly improves this phenotype, whereas the drug has no effect on food intake of DTHg; ple flies. Interestingly, l-DOPA also has a positive effect on food intake of heterozygous ple flies (Fig. S6C), suggesting a dominant effect of ple on feeding behavior.
Lack of Phototaxis but Preserved Visual Orientation and Spatial Memory.
Positive phototaxis is a characteristic behavior of Drosophila and many other insect species. In countercurrent assay, DTHgFS±; ple flies were mainly recovered in the first tube of the apparatus, indicating that they are not attracted by light, whereas DTHg; ple showed partial rescue of phototactic behavior (Fig. 3C). The mutants have normal electroretinograms (Fig. S5C) and optomotor response (Fig. S5D), indicating that visual functions are preserved. We also performed individual assays to rule out the possibility that the lack of positive response in the countercurrent test was attributable to reduced activity. A single fly was placed at the entrance of a T-maze and allowed to choose freely between a dark tube and an illuminated tube until it reached one of the vials. Similar to WT, neurally rescued DTHg; ple show a significant preference toward the lighted tube. In contrast, DTHgFS±; ple single flies show no preference, choosing either tube with equal probability (Fig. 3D).
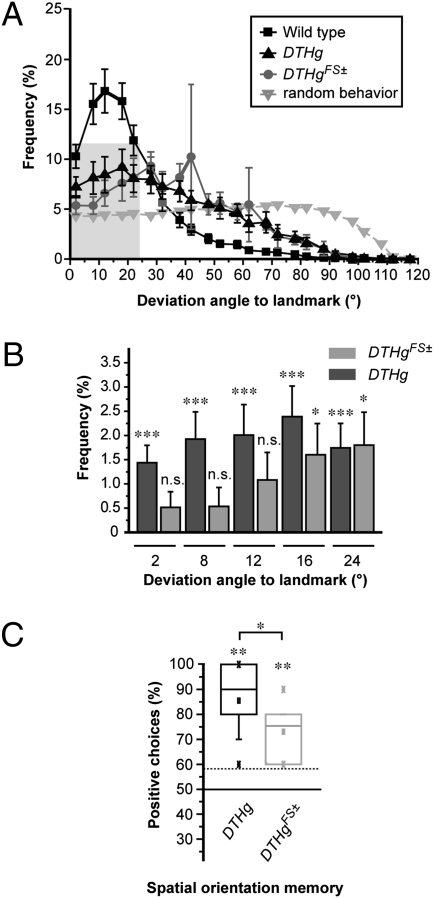
We further checked the flies for visual tracking and orientation in the Buridan's paradigm, a cylindrical virtual reality arena in which two dark vertical stripes are presented opposite to each other. Under standard conditions, WT flies patrol between two given visual targets for a considerable length of time. Whole orientation curves indicate that DTHg; ple and DTHg FS±; ple are comparably able to target the two stripes, with a fixation ability different from random behavior, although less efficiently than WT flies (Fig. 4A). A closer look at the curves shows that both mutants differ in their orientation ability at small deviation angles (Fig. 4B), suggesting that the lack of DA makes visual orientation slightly less accurate. Therefore, in Drosophila, the presence of neural DA improves visual tracking but, nevertheless, is dispensable for this behavior.
Fig. 4.
Visual fixation and spatial memory are largely preserved in the absence of brain DA. (A) Whole orientation curves showing frequency of angle deviation between the fly trajectory and approached target. Mutant and rescue flies show a frequency distribution significantly different from the calculated random behavior (DTHg; ple: P = 0.007 and DTHgFS±: ple: P = 0.005, F test), whereas both strains do not differ from each other (P = 0.455; mean of 13 flies of each genotype). This confirms that DTHg; ple and DTHgFS±; ple flies are able to visualize and target the landmarks, although less consistently than WT flies (DTHg; ple: P = 0.028 and DTHgFS±; ple: P = 0.036, F test). (B) Differences between angles to the approached target and calculated random behavior for small deviations (derived from shaded area of curves in B are shown. Trajectories of DTHgFS±; ple target the landmarks but are not accurate, making angle frequency not significantly different from random behavior for small deviations [*P < 0.05, not significant (n.s.)]. This defect is rescued in DTHg; ple flies that contain neural DA (***P < 0.005). The Wilcoxon signed-rank test was used against 0 as random behavior. (C) Test of spatial orientation memory in the detour paradigm (details are provided in Materials and Methods). Ten flies per genotype and 10 tests per fly were recorded. The frequency of positive choices is represented by box-and-whisker plots showing the median (horizontal line), mean (square), 25% and 75% quartiles (box), 10% and 90% quantiles (whiskers), and extreme values (crosses). Random behavior would result in a reference value of 58% (horizontal dotted line). The DTHgFS±; ple flies still retain spatial orientation memory (**P = 0.0015, one-sample sign test against the random value), although their performance is reduced compared with the DTHg; ple flies (*P = 0.034, Mann–Whitney U test).
Orientation in a changing environment requires the ability to retain and recall the position of a target. Drosophila possesses such a spatial orientation memory that can be tested by the detour paradigm. In this test, a fly walks toward a landmark that suddenly disappears while, simultaneously, a vertical distracter stripe appears laterally to the fly. After the fly approaches this second landmark, the distracter stripe also disappears within 1 s. In this situation, WT flies will turn back to their previous, still invisible, landmark, demonstrating a spatial orientation memory for their former target with a median frequency of ∼80% (26). We observed that both DTHg; ple and DTHgFS±; ple do recall the position of the first landmark with a median frequency of 90% and 73%, respectively (Fig. 4C). This demonstrates that the lack of brain DA has only a minor impact on spatial orientation memory in Drosophila.
Discussion
Here, we describe specific genetic tools to study DA as a signaling molecule in the Drosophila brain. We show that the embryonic lethality of ple is fully rescued by a combination of dopaminergic drivers (Ddc-GAL4 and TH-GAL4) expressing a frameshift mutant of the TH gene (DTHgFS±). The DTHgFS±; ple mutants only express TH in nonneural tissues, principally the cuticle-producing cells of the hypoderm. This leads to the absence of DA in adult brain without apparent morphological disturbance of the dopaminergic system, as attested by l-DOPA rescue of defective behaviors. The resulting flies are fully viable with a normal lifespan and they show a number of distinctive phenotypes.
Mutants without neural DA show reduced locomotor activity and arousal, and they sleep significantly more than control flies during both day and night, consistent with previous reports on the role of DA in these behaviors (4–6). The lack of further effect of a TH inhibitor (3IY) demonstrates that spontaneous activity is not influenced by trace levels of neural DA or by cuticular DA in these flies. Other reports indicate that blockade of dopaminergic synaptic transmission increases activity, particularly startle-induced activity (1, 7, 27). This apparent discrepancy cannot be fully explained at present but might be related to the fact that only part of brain DA signaling is inhibited with the use of GAL4 drivers that do not express in the entire dopaminergic system.
The role of dopaminergic neurons in Drosophila aversive olfactory learning has been widely studied before (10–15). The present results show conclusively that DA is the critical signaling molecule released by these neurons that is required for this form of associative memory. Interestingly, although electrical shock avoidance is not altered, we observed that the DA-deficient flies have a striking tendency to choose the shock-associated odor 2 h after training, indicative of an inversion of the evaluation system. Such an apparently “masochistic” behavior could result from an inability of these flies to attribute a negative value to a reinforcing stimulus, leading to a bias toward an appetitive behavior. In a possibly related observation, Krashes et al. (12) recently reported that specific DA neurons can inhibit the expression of an appetitive memory performance.
Our study reveals the requirement for neural DA in sugar preference and normal food intake in Drosophila. The fact that serotonin is also involved in feeding in flies (28) suggests that these two biogenic amines directly interact in the control of this behavior. The lack of sugar preference, despite the intact proboscis extension reflex, may suggest that DA is required for reward and motivation in flies, as is the case in mammals, but this would need further work to be established. Remarkably, several of the deficits we observe in DA-deficient Drosophila, (e.g., hypoactivity and hypophagy readily rescued by l-DOPA) are quite similar to phenotypes observed in DA-deficient mice (29). The same is true for the preservation of dopaminergic neuron circuits. This argues for partial conservation of brain DA functions between flies and mammals.
The lack of phototaxis does not result from blindness because flies without neural DA have normal electroretinograms and optomotor response. Circadian entrainment to dim light also requires neural DA (30). We suspect that this is not related to the phototaxis defect, which was tested under high levels of illumination. An unexpected result of this study is that visual tracking and spatial orientation memory are largely retained in Drosophila lacking neural DA. Although neural DA improves visual fixation and orientation toward a landmark, possibly by increasing arousal level and attention (8), this neuromodulator appears to be dispensable for these behaviors. Our results, in agreement with previous findings (26, 31), suggest that the neural circuits involved in the formation of spatial memory do not depend on neural DA.
In conclusion, we find that activity, feeding, and certain choice behaviors are markedly altered or abolished in neural DA-deficient flies, whereas other complex behaviors are surprisingly well maintained and might be similar to WT without the decrease in arousal. This suggests that DA is implicated in many but not all aspects of brain functioning in Drosophila. The specific behaviors that absolutely require DA could be related to the primordial and evolutionarily conserved functions of this essential neuromodulator in primitive nervous systems.
Materials and Methods
Further details and references of the procedures are provided in SI Materials and Methods.
Drosophila Strains.
DTHg- and DTHgFS±-rescued homozygous ple Drosophila were generated before each experiment by crossing a line containing the combined TH-GAL4 and Ddc-GAL4 drivers with the respective UAS transgene, each in a heterozygous ple mutant background. The Canton S strain was used as WT flies for comparison in behavior tests.
Immunohistochemistry.
Adult brains were dissected in ice-cold Drosophila Ringer's solution and processed for whole-mount immunostaining by standard procedure. The following primary antibodies were used: rabbit anti-DA (1:100; ImmunoStar), rabbit anti-serotonin (1:500; Sigma-Aldrich), mouse monoclonal anti-TH (1:50; ImmunoStar), rat anti-Ddc (1:200; prepared in the laboratory of J.H.), and rabbit anti-GFP (1:500; Invitrogen Molecular Probes).
DA Assay.
DA levels in Drosophila brain extracts were determined by HPLC coupled to electrochemical detection essentially using a mobile phase containing 4 mM decanesulfonic acid, 50 mM citrate/acetate (pH 4.5), and 20% (vol/vol) acetonitrile. Standard detection of DA was performed at a detector potential of +500 mV relative to an Ag/AgCl electrode. Electrochemical confirmation of the identity of the DA peaks was performed at a reduced potential of +420 mV.
Locomotor Behavior.
Two methods were used to test for spontaneous locomotor behavior of 2- to 5-d-old adult Drosophila. First, walking speed and covered distance were computed from video-based recordings of individual flight-disabled flies walking freely for 15 min in an open arena. Second, activity was monitored by recording infrared beam crossings in glass tubes (6.5-cm length, 3-mm inside diameter) using a Drosophila activity monitoring system (TriKinetics).
Olfactory Conditioning.
To test for aversive olfactory learning, groups of about 35 flies were conditioned in a barrel-type machine by sequential exposure to two odors, octanol and methylcyclohexanol, for 60 s with 45-s rest intervals between presentation of odors (25). Exposure to the first odor was paired with electrical shocks.
Phototaxis.
Phototactic behavior was tested by mass and single-fly assays. The mass assay was carried out in a dark room with the countercurrent procedure. The results were the mean of the scores from five trials. Data were statistically analyzed with a one-way ANOVA (Bonferroni-corrected). The single-fly phototaxis assay was performed using a T-maze system in which flies choose freely between an illuminated vial and a dark vial. The test was repeated five times with each fly (n = 16), and the percentage of photopositive choices was scored. Data were analyzed with the Wilcoxon signed-rank test.
Visual Fixation and Orientation.
Orientation toward a landmark was analyzed in the Buridan's paradigm on freely walking 5-d-old Drosophila with shortened wings. The angular deviation between fly trajectory and the approached target was measured every 0.2 s for 15 min. Spatial orientation memory was tested in the detour paradigm (26). Ten consecutive trials of at least 10 flies per genotype were recorded. Data were analyzed with the Kruskal–Wallis ANOVA test.
Supplementary Material
Acknowledgments
We thank Erich Buchner for help with the electroretinogram and optomotor assays, Isabelle Rivals for advice on statistical analyses, and Scott Waddell and Burkhard Poeck for helpful discussions. This work was supported by grants from the Centre National de la Recherche Scientifique and the Fondation de France (to S.B.), the National Institutes of Health (to J.H.), and Agence Nationale pour la Recherche (to T.P.), and by Deutsche Forschungsgemeinschaft Grant STR590-4 (to R.S.). Fellowships were provided by the Fondation pour la Recherche Médicale (to H.C. and T.R.) and Fondation Pierre-Gilles de Gennes (to L.S. and T.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010930108/-/DCSupplemental.
References
- 1.Friggi-Grelin F, et al. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003a;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 2.Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci USA. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 6.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebestky T, et al. Two different forms of arousal in Drosophila are oppositely regulated by the dopamine D1 receptor ortholog DopR via distinct neural circuits. Neuron. 2009;64:522–536. doi: 10.1016/j.neuron.2009.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Y, Xi W, Peng Y, Wang Y, Guo A. Long-term but not short-term blockade of dopamine release in Drosophila impairs orientation during flight in a visual attention paradigm. Eur J Neurosci. 2004;20:1001–1007. doi: 10.1111/j.1460-9568.2004.03575.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang K, Guo JZ, Peng Y, Xi W, Guo A. Dopamine-mushroom body circuit regulates saliency-based decision-making in Drosophila. Science. 2007;316:1901–1904. doi: 10.1126/science.1137357. [DOI] [PubMed] [Google Scholar]
- 10.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krashes MJ, et al. A neural circuit mechanism integrating motivational state with memory expression in Drosophila. Cell. 2009;139:416–427. doi: 10.1016/j.cell.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riemensperger T, Völler T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 14.Schwaerzel M, et al. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J Neurosci. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seugnet L, Suzuki Y, Vine L, Gottschalk L, Shaw PJ. D1 receptor activation in the mushroom bodies rescues sleep-loss-induced learning impairments in Drosophila. Curr Biol. 2008;18:1110–1117. doi: 10.1016/j.cub.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nässel DR, Elekes K. Aminergic neurons in the brain of blowflies and Drosophila: Dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 1992;267:147–167. doi: 10.1007/BF00318701. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 19.Neckameyer WS, White K. Drosophila tyrosine hydroxylase is encoded by the pale locus. J Neurogenet. 1993;8:189–199. doi: 10.3109/01677069309083448. [DOI] [PubMed] [Google Scholar]
- 20.Birman S, Morgan B, Anzivino M, Hirsh J. A novel and major isoform of tyrosine hydroxylase in Drosophila is generated by alternative RNA processing. J Biol Chem. 1994;269:26559–26567. [PubMed] [Google Scholar]
- 21.Vié A, Cigna M, Toci R, Birman S. Differential regulation of Drosophila tyrosine hydroxylase isoforms by dopamine binding and cAMP-dependent phosphorylation. J Biol Chem. 1999;274:16788–16795. doi: 10.1074/jbc.274.24.16788. [DOI] [PubMed] [Google Scholar]
- 22.Friggi-Grelin F, Iché M, Birman S. Tissue-specific developmental requirements of Drosophila tyrosine hydroxylase isoforms. Genesis. 2003b;35:175–184. doi: 10.1002/gene.10178. [DOI] [PubMed] [Google Scholar]
- 23.Bainton RJ, et al. Dopamine modulates acute responses to cocaine, nicotine and ethanol in Drosophila. Curr Biol. 2000;10:187–194. doi: 10.1016/s0960-9822(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 24.Andretic R, Kim YC, Jones FS, Han KA, Greenspan RJ. Drosophila D1 dopamine receptor mediates caffeine-induced arousal. Proc Natl Acad Sci USA. 2008;105:20392–20397. doi: 10.1073/pnas.0806776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pascual A, Préat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- 26.Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature. 2008;453:1244–1247. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- 27.Alekseyenko OV, Lee C, Kravitz EA. Targeted manipulation of serotonergic neurotransmission affects the escalation of aggression in adult male Drosophila melanogaster. PLoS ONE. 2010;5:e10806. doi: 10.1371/journal.pone.0010806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neckameyer WS. A trophic role for serotonin in the development of a simple feeding circuit. Dev Neurosci. 2010;32:217–237. doi: 10.1159/000304888. [DOI] [PubMed] [Google Scholar]
- 29.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 30.Hirsh J, et al. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr Biol. 2010;20:209–214. doi: 10.1016/j.cub.2009.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sitaraman D, et al. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci USA. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.