Abstract
Plant cytokinesis deploys a transport system that centers cell plate-forming vesicles and fuses them to form a cell plate. Here we show that the adaptin-like protein TPLATE and clathrin light chain 2 (CLC2) are targeted to the expanding cell plate and to the equatorial subregion of the plasma membrane referred to as the cortical division zone (CDZ). Bimolecular fluorescence complementation and immunodetection indicates that TPLATE interacts with clathrin. Pharmacological tools as well as analysis of protein targeting in a mutant background affecting cell plate formation allowed to discriminate two recruitment pathways for TPLATE and CLC2. The cell plate recruitment pathway is dependent on phragmoplast microtubule organization and the formation and transport of secretory vesicles. The CDZ recruitment pathway, on the other hand, is activated at the end of cytokinesis and independent of trans-Golgi–derived vesicle trafficking. TPLATE and CLC2 do not accumulate at a narrow zone central of the CDZ. We have dubbed this subdomain the cortical division site and show that it corresponds precisely with the position where the cell plate merges with the parental wall. These data provide evidence that the plasma membrane is subject to localized endocytosis or membrane remodeling processes that are required for the fusion of the cell plate with a predefined region of the plasma membrane.
Keywords: adaptin, clathrin-mediated endocytosis, plant cell divison, caffeine, tyrphostin
Cytokinesis in plants, in contrast to that process in animals and yeasts, starts with targeted secretion and fusion of vesicles between the separated chromosomes at the center of the cell (1, 2). During the initial phases of plant cytokinesis, syntaxin-mediated vesicle fusion, together with callose biosynthesis within the fused vesicles, creates a transient membrane compartment at the central plane of the phragmoplast microtubules (2). After this initial stationary phase of cytokinesis, de novo microtubule formation at the outer border, together with depolymerization of microtubules at the center, allows the centrifugal expansion and guidance of the cell plate toward the parental cell wall (3). Animal cells, but also yeasts, which, like plant cells, are enwound in a polysaccharide wall, use a contractile actin ring that pulls in the plasma membrane (PM) at the division plane by means of an acto-myosin–based mechanism that involves the formation of a cleavage furrow (4). This centripetal narrowing of the division plane reaches a final point when the remaining gap is closed by means of syntaxin-mediated membrane fusion, a process that was designated as abscission (5).
Very little is known about membrane trafficking at the end of eukaryotic cytokinesis (6, 7). In plants, this involves the connection of the cell plate with the parental plasma membrane and the maturation of the cell plate into a cell wall. Electron microscopic and mutant analysis revealed multiple finger-like fusion tubes mediating cell plate anchoring and the importance of a timely deposition of callose (2, 8).
TPLATE, a plant-specific protein with similarity to the Adaptin/Coatomer proteins, was originally identified in a survey for phragmoplast-targeted proteins (9) and was proposed to be relevant for the final steps of plant cytokinesis because down-regulation of tobacco TPLATE (NtTPLATE) in tobacco BY-2 cells caused cell plate-anchoring defects (10).
Here we show that TPLATE colocalizes at the cell plate and at the cortical division zone (CDZ) with CLC2 during cell plate anchoring, whereas both proteins are specifically excluded from the actual cell plate insertion site. TPLATE interacts with both clathrin light chain 2 (CLC2) and clathrin heavy chain 1 (CHC1) in bimolecular fluorescence complementation (BiFC) and with Arabidopsis CHC by coimmunoprecipitation, pointing to a role for TPLATE in clathrin-mediated endocytosis. Chemical and genetic interference with cell plate recruitment of TPLATE and CLC2 in BY-2 cells and Arabidopsis roots suggests that a specific recruitment pathway acting at the CDZ is activated during anchoring of the cell plate with the parental plasma membrane. This pathway involves TPLATE and CLC2 and is differently regulated compared with recruitment events occurring during the initial phases of cell plate formation.
Results and Discussion
TPLATE Is Targeted to the CDZ.
Previously, we showed that TPLATE-GFP accumulated in the plasma membrane surrounding the insertion site during anchoring of the cell plate in Arabidopsis root cells (10). Time-lapse analysis in dividing BY-2 suspension cells revealed the timely accumulation of TPLATE at the CDZ during anchoring but before depolymerization of the phragmoplast microtubules (Movie S1). The CDZ is marked by the kinesin interacting with the A-type cyclin-dependent kinase CDKA;1 (KCA1), which accumulates in the plasma membrane in dividing BY-2 cells and is specifically depleted at the CDZ following preprophase band (PPB) breakdown (Fig. S1A). This zone, termed the kinesin-depleted zone (KDZ), also corresponds to the actin-depleted zone (ADZ) and the former position of the PPB, a ring of microtubules encircling the premitotic nucleus. Together, the ADZ, KDZ, and PPB specify the CDZ from prophase throughout cytokinesis (11). Coexpression of TPLATE-RFP and GFP-KCA1 showed that TPLATE accumulates at the CDZ (Fig. S1B), suggesting a complementary recruitment mechanism allowing the accumulation of TPLATE and the exclusion or elimination of KCA1.
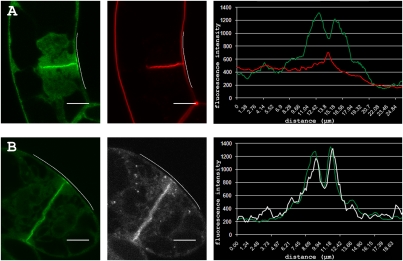
Detailed microscopic observations showed that TPLATE-GFP marks the flanks of the CDZ but not the center that corresponds to the exact site where the cell plate connects with the plasma membrane (Fig. 1A). The uneven distribution of TPLATE-GFP along the CDZ during the final steps in cytokinesis is illustrated by fluorescence intensity plots of TPLATE-GFP and the lipophylic dye FM4-64 (n > 30; Fig. 1A). FM4-64, callose, auxin-induced in root cultures 9 (AIR9), and formin 5 (FH5) mark the exact site where the cell plate connects with the PM (Fig. S1).
Fig. 1.
TPLATE and clathrin light chain accumulate at the CDZ during cell plate anchoring. (A) TPLATE-GFP expressing BY-2 cell stained with FM4-64 during cell plate anchoring and corresponding fluorescence intensity profile (TPLATE-GFP, green; FM4-64, red) along the plasma membrane spanning the division zone (white line). TPLATE-GFP accumulates in the plasma membrane of the division zone with reduced fluorescence intensity at the insertion site (peak of FM4-64 signal). (B) Colocalization of TPLATE-GFP (green) and CLC2-mCHERRY (white) with corresponding fluorescence intensity profiles (TPLATE-GFP, green; CLC2-mCHERRY, white) spanning the division zone (white line). TPLATE and CLC2 colocalize at the cell plate and the plasma membrane of the division zone with reduced fluorescence intensity at the insertion site. (Scale bars: 10 μm).
Coexpression of GFP-AIR9 and TPLATE-RFP shows that GFP-AIR9 accumulates at the exact insertion site whereas TPLATE accumulates at a region surrounding AIR9 (Fig. S1E). To make a clear distinction between the two cortical plasma membrane domains, a nomenclature is proposed here. The cortical division site (CDS) refers to the site where the cell plate connects with the parental wall and where TPLATE is absent, whereas the CDZ refers to the site where TPLATE accumulates and also corresponds to the position of the preprophase band, the ADZ, and the KDZ (Fig. 1 and Fig. S1 A, B, D, and E). By this definition, the CDZ corresponds to the initial broad Arabidopsis TANGLED ring, which narrows down to a punctate signal corresponding to the CDS during cytokinesis (12).
Clathrin Colocalizes and Interacts with TPLATE.
Because endocytosis is thought to occur at the CDZ following cell plate anchoring (13), we speculated that clathrin would be specifically targeted to the CDZ. CLC2 has been observed in dynamic foci at the cell cortex together with dynamin proteins (14–16), and it binds mammalian clathrin hubs, suggesting that it is a genuine factor of clathrin-mediated endocytosis (17). We therefore determined the subcellular distribution of CLC2 in dividing BY-2 cells.
In agreement with previous studies in Arabidopsis roots, CLC2-GFP localized at the cell plate (Movie S2) (18–20). Coexpression of TPLATE-GFP and CLC2-mCHERRY showed prominent colocalization at the cell plate and at the CDZ shortly before fusion of the cell plate with the parental wall (Fig. 1B; Fig. S2). The cell plate localization of TPLATE and CLC2 was most pronounced a few micrometers behind the leading edge of the expanding cell plate (Fig. S2, red brackets; Movie S3). Subsequent to fusion of the cell plate with the parental wall, TPLATE and CLC2 disseminated from the CDZ and the cell plate (Movies S1–S3). TPLATE and CLC2 are therefore not essential for initial vesicular trafficking to the cell plate but rather are involved in processes related to the maturation of the cell plate membrane.
The apparent colocalization suggests interaction between TPLATE and the clathrin lattice. To determine the putative binding of TPLATE and clathrin, we performed BiFC experiments. TPLATE, CLC2, and CHC1 were fused to N- and/or C-terminal halves of EGFP, and pair-wise interaction was analyzed using transient Agrobacterium-mediated transfection of Nicotiana benthamiana epidermal cells. N- and C-terminal fusions of TPLATE, CLC2, and CHC1 with the N terminus of EGFP (headGFP) were combined with C-terminal fusion proteins with the C terminus of EGFP (tailGFP). Full-length fusion constructs and free GFP were used as controls. An overview of the results is shown in Table 1 and the corresponding representative images in Fig. S3. In addition to the interaction between CLC2 and CHC1, TPLATE interacted with CLC2 and CHC1 (Table 1 and Fig. S3). Also, homo-dimerization of TPLATE, CLC2, and CHC1 could be observed using this system. To confirm the observed interaction between TPLATE and clathrin, coimmunoprecipitation (co-IP) experiments were performed using a functional TPLATE-GFP fusion protein in the homozygous tpl mutant background (10) and an antibody against soybean CHC (21). Endogenous Arabidopsis CHC was repeatedly detected by Western blotting in the pull-down fraction of TPLATE-GFP (Fig. S4). Pull-down experiments using 35S::GFP-MBD (microtubule binding domain of MAP4) served as negative control. The low amount of clathrin present in the co-IP experiments compared with the amount of TPLATE in the pellet fraction suggests that the interaction between TPLATE and clathrin is weak or that processing of the material causes dissipation of the interaction. BiFC, on the other hand, may stabilize the TPLATE–clathrin interaction, which facilitates the detection of the complexed proteins.
Table 1.
Overview of BiFC interactions between TPLATE, CLC2, and CHC1
| BiFC combination | TPLATE-tailGFP | CLC2-tailGFP | CHC1-tailGFP |
| TPLATE-headGFP | + | + | + |
| HeadGFP-TPLATE | + | + | + |
| CLC2-headGFP | + | + | + |
| HeadGFP-CLC2 | + | + | + |
| CHC1-headGFP | – | + | – |
| HeadGFP-CHC1 | – | – | + |
Pairwise interaction analysis between TPLATE, CLC2, and CHC1 was tested by BiFC via transient Agrobacterium-mediated transfection of Nicotiana benthamiana leaf epidermal cells. Positive interactions are marked with a plus sign; combinations for which no interaction was detected are indicated with a minus sign.
Tyrphostin and Wortmannin Affect PM Recruitment of TPLATE.
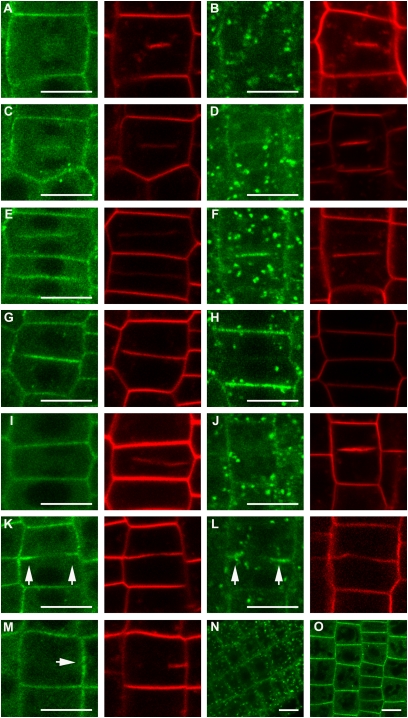
As clathrin is a main component of endocytosis-related processes, we anticipated that TPLATE would be involved in membrane trafficking or membrane remodeling. In Arabidopsis roots, TPLATE-GFP labels the PM in a punctate manner (10) (Fig. S5). We analyzed the localization of TPLATE under conditions that block internalization of FM4-64 as readout of inhibited endocytosis. Tyrphostin A23 is an inhibitor of the loading of cargo into clathrin-coated vesicles (22, 23). It was shown to affect the dynamics and foci number of several Arabidopsis dynamin proteins at the PM (14, 16) and to inhibit internalization of FM4-64 in BY-2 (24). FM4-64 internalization can also be reduced by Wortmannin, a specific inhibitor of the phosphatidylinositol 3-kinase (25).
In Arabidopsis root cells expressing a genomic fusion of TPLATE-GFP, Tyrphostin A23 specifically inhibited FM4-64 uptake at concentrations of 75 and 100 μM (Fig. S5 B-D), which resulted in a strong decrease of TPLATE recruitment to the PM (Fig. S5 A and E). Wash-out experiments restored PM recruitment of TPLATE and FM4-64 internalization, indicating that the drug treatment did not permanently damage membrane trafficking in seedlings (Fig. S5G). Wortmannin (30 μM) also resulted in a block of FM4-64 internalization and caused reduction of TPLATE PM recruitment (Fig. S5I). The strong reduction of PM labeling of TPLATE under conditions that block FM internalization by drugs that are known to affect endocytosis argues for a role for TPLATE in this process.
TPLATE and CLC2 Are Similarly Recruited to the CDZ and Cell Plate.
To determine the mechanism by which TPLATE and CLC2 are targeted, we applied drugs that could potentially interfere with their recruitment to the cell plate and CDZ. Propyzamide (6 μM) and Latrunculin B (20 μM) were used to assess the involvement of the cytoskeleton in TPLATE recruitment, and caffeine (5 mM) was used as a cytokinesis drug (26). Latrunculin B (Lat B) added to BY-2 cells during metaphase did not interfere with the progression of cytokinesis and did not affect the recruitment of TPLATE to the cell plate or the CDZ (n = 5). Lat B sensitive actin filaments are therefore not essential for TPLATE recruitment. Propyzamide added to BY-2 cells immediately after cell plate initiation blocked cell plate expansion completely and instantly (n = 2; Fig. S6). The fraction of TPLATE recruited to the early plate remained bound despite the absence of phragmoplast microtubules. TPLATE did not accumulate further into the cell plate nor was it recruited to the CDZ, indicating that intact microtubules are not required for the membrane association of TPLATE; however, intact microtubules and/or phragmoplast expansion are required for TPLATE recruitment to the CDZ.
Caffeine is well known to disrupt cell plate consolidation, resulting in cytokinesis defects and aberrant cell plate formation in multicellular plants, but the underlying mechanisms affected are not uncovered (26–29). Time-lapse observation of cytokinesis using interference contrast and fluorescence microscopy of FH5-GFP expressing BY-2 cells showed that application of caffeine (5 mM) slowed down centrifugal plate expansion, but it did not prevent cytokinesis from taking place (Fig. S7A) in agreement with previous data (30). Also, caffeine-treated BY-2 cells incorporated FM4-64 in the cell plate (Fig. S7D), and CLC2-GFP accumulation at endosomal compartments was similar to nontreated cells (Fig. 3 and Fig. S8).
Fig. 3.
Central cell plate recruitment of TPLATE-GFP and CLC2-GFP in Arabidopsis root cells is sensitive to caffeine in contrast to recruitment during anchoring. (A–H) Representative images of TPLATE-GFP (A, C, E, and G) and CLC2-GFP (B, D, F, and H) localization during the different phases of cytokinesis in nontreated roots stained with FM4-64. TPLATE and CLC2 are absent from very young cell plates (A and B) and gradually accumulate into the expanding plates (C–H). (I–L) Representative images of TPLATE-GFP and CLC2-GFP localization after 2 h treatment with caffeine (5 mM). Both TPLATE and CLC2 are excluded from unattached cell plates (I and J) and accumulate at the outer rim of the cell plate after anchoring (K and L, arrows) when the cell plate membrane is still continuous as visualized by FM. (M) Specific accumulation of TPLATE-GFP at the CDZ (arrow) with reduced intensity at the cell plate insertion site when prolonged treatment with caffeine leads to destruction of the cell plate. (N and O) Epidermal section of CLC2-GFP (N) and TPLATE-GFP (O) showing that interphase cell localizations of both proteins remain unaffected by caffeine treatment. (Scale bars: 10 μm).
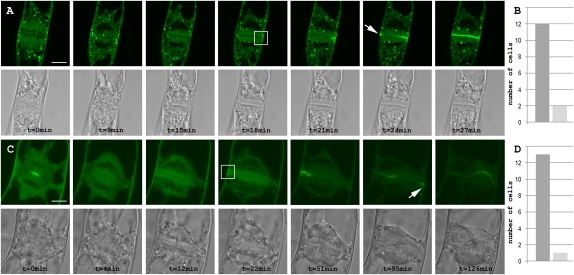
These observations indicate that caffeine does not have a gross impact on membrane trafficking and does not interfere with the transport of cell plate-forming vesicles to the cell plate. To address the question of whether caffeine treatment affected recruitment of TPLATE and CLC2, we treated TPLATE-GFP (n = 14)- and GFP-CLC2 (n = 13)-expressing BY-2 cells with caffeine and observed cytokinesis until cell plate anchoring (Fig. 2). Remarkably, TPLATE and CLC2 dissociated from the cell plate within minutes following caffeine treatment (Fig. 2 and Fig. S8). Cell plate expansion occurred without recruitment of TPLATE and CLC2 to the cell plate in almost all cells monitored (Fig. 2 B and D), indicating that the central cell plate localization of neither of these proteins is essential for cell plate expansion under these conditions. Following the fusion of the cell plate with the parental wall, TPLATE and CLC2 were recruited specifically to the CDZ (Fig. 2 and Fig. S7E). Subsequently, the TPLATE and CLC2 GFP fluorescence accumulated first at the cell plate border and then along the plate as a centripetal wave toward the center of the cell plate (Fig. 2). The gradual increase of TPLATE-GFP and GFP-CLC2 at the midplane can be the result of either migration from the plasma membrane into the cell plate or direct cell plate recruitment from the cytoplasm following a signal coming from the CDZ. Recruitment at the CDZ and migration into the cell plate or simple diffusion from the PM to the anchored cell plate would, however, require a constitutive presence of TPLATE and CLC2 at the CDZ, which is not the case (Fig. 2). Therefore, cell plate recruitment is likely caused by a centripetal signal activated upon anchoring.
Fig. 2.
In the presence of caffeine, TPLATE and CLC2 are recruited to the CDZ upon anchoring of the cell plate. (A) Time-lapse photos of a GFP-CLC2–expressing BY-2 cell, treated with 5 mM caffeine at anaphase (t = 0 min) and followed throughout cytokinesis. The cell plate localization of GFP-CLC2 is highly sensitive to caffeine whereas the punctate endomembrane localization of GFP-CLC2 remains unaffected. Accumulation of GFP-CLC2 upon contact of the cell plate with the plasma membrane (white box) is followed by the centripetal reappearance of fluorescence at the cell plate. GFP-CLC2 also accumulates at the opposite side of the plate upon anchoring (arrow). (B) Quantification of the caffeine sensitivity of CLC2. Of a total of 14 cells followed through cytokinesis, 12 cells lost CLC2 signal at the cell plate followed by reappearance during anchoring whereas 2 cells were insensitive to the treatment. (C) Time-lapse photos of a TPLATE-GFP–expressing BY-2 cell, treated with 5 mM caffeine during the initial stage of cell plate formation. Cell plate accumulation of TPLATE-GFP is rapidly lost and returns at the division zone upon anchoring (white box), followed by centripetal reappearance of fluorescence at the cell plate. Fluorescence returns at the opposite side during anchoring (arrow). (D) Quantification of the caffeine sensitivity of TPLATE. Of a total of 14 cells followed through cytokinesis, 13 cells lost TPLATE signal at the cell plate followed by reappearance during anchoring and 1 cell was insensitive to the treatment. (Scale bars: 10 μm).
To determine the specificity of the caffeine effect, we analyzed the behavior of three peripheral cell plate markers: GFP-KCA1, FH5-GFP, and a biosensor for phosphatidylinositol 4-phosphate (11, 31, 32). The microtubule motor protein KCA1, the formin FH5, and the pleckstrin homology domain of the human phosphatidylinositol-4-phosphate adaptor protein-1 (FAPP1; mVENUS–PHFAPP1) did not delocalize upon caffeine treatment (Fig. S7). Also phragmoplastin, a soybean dynamin-related protein that has been implicated in membrane recycling at the cell plate, has been reported to remain bound to the cell plate in the presence of caffeine (30).
Our observations together with the phragmoplastin data show that caffeine affects only a subpopulation of the cell plate peripheral proteins that include TPLATE and CLC2. Although TPLATE and CLC2 recruitment to the central cell plate is selectively altered by caffeine, recruitment to the CDZ is not. This indicates the existence of two distinct recruitment pathways active during plant cytokinesis: one implicated in central cell plate formation, which is sensitive to caffeine, and another in cell plate anchoring, which is caffeine-insensitive.
Caffeine has been reported to cause cytokinesis defects in planta, which are dependent on the timing of drug addition and on the concentration used (26–30). The cytokinesis defects observed in BY-2 are likely caused by a defect in plate consolidation as caffeine does not disrupt initial vesicle fusion events but affects the fuzzy-coat formation and budding of clathrin-coated vesicles (26). This is in agreement with the very fast cell plate dissociation of CLC2-GFP upon caffeine application (Fig. S8). We evaluated the effect of caffeine on Arabidopsis roots expressing the genomic fusion of TPLATE-GFP (10) and 35S::CLC2-GFP (16). Cell plate recruitment of TPLATE and CLC2 during cytokinesis gradually increased during plate expansion as compared with the endocytic tracer FM4-64 (Fig. 3 A–H). In Arabidopsis, TPLATE is primarily recruited to the PM and the cell plate, in contrast to CLC2, which also accumulates at endosomal compartments (Fig. 3). Similar to the caffeine effect on BY-2 cells, TPLATE and CLC2 recruitment in Arabidopsis roots is abolished during early cell plate formation (Fig. 3 I and J). PM targeting and recruitment of CLC2 to endosomal compartments was not affected (Fig. 3 N–O). Both proteins were also specifically recruited to the periphery of FM-stained fused cell plates (Fig. 3 K and L). Caffeine treatments exceeding 2 h caused the disintegration of the central part of the cell plate, resulting in cell wall stubs. Also in these cells, TPLATE was observed to accumulate specifically at the CDZ before cell plate recruitment (Fig. 3M, arrow). Thus, concerning the dynamics of TPLATE targeting, caffeine had similar effects in Arabidopsis roots as in tobacco BY-2 cells, corroborating two independent TPLATE/CLC2 recruitment pathways—one at the cell plate and one at the CDZ.
CDZ Recruitment of TPLATE Occurs in the Absence of Trans Golgi Network (TGN) Trafficking.
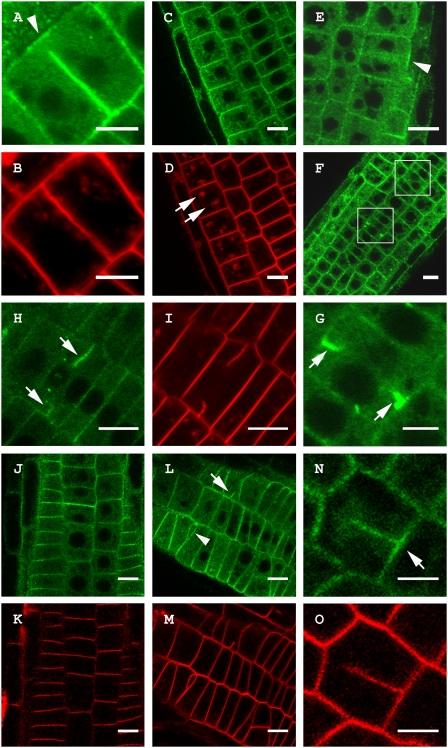
As TPLATE was specifically recruited to the CDZ during cell plate anchoring in BY-2 and Arabidopsis (Fig. 4A and Fig. S2), we subsequently evaluated the interdependence of the central plate and CDZ recruitment pathways by a genetic approach that eliminates central cell plate formation through conditional inactivation of endoplasmic reticulum (ER)-to-Golgi trafficking. Blocking ER-to-Golgi trafficking in the Arabidopsis gnom-like1 (gnl1-1) guanine-nucleotide exchange factor for ADP-ribosylation factor GTPases (ARF-GEF) mutant background with the fungal toxin Brefeldin A (BFA) causes the inability to form cell plates and leads to the formation of cell wall stubs (33–35). Cytokinesis was observed in root segments of gnl1-1 Arabidopsis seedlings expressing the genomic TPLATE-GFP fusion. TPLATE recruitment to the PM is not sensitive to BFA (Fig. 4 C and D). After BFA treatment of gnl1-1, TPLATE-GFP no longer accumulated at the midplane of dividing cells, but still associated with the CDZ (Fig. 4E). Extended incubation of gnl1-1 seedlings with BFA led to strong TPLATE-GFP labeling of cell wall stubs in dividing root cells (Fig. 4 F and G). Formation of cell wall stubs has previously been reported for gnl1-1 treated with BFA and is likely independent from TGN secretion (33). In agreement with this, Concanamycin A, an inhibitor of the V-ATPase blocking TGN trafficking, also causes cell wall stubs (33) to which TPLATE was specifically recruited (Fig. 4 H and I). These experiments affirm the presence of two independent recruitment pathways for TPLATE in Arabidopsis. The excessive accumulation of TPLATE-GFP at the cell wall stubs may be a consequence of the prolonged activation of the centripetal recruitment pathway resulting in abnormally large and thick stubs. Reduced endocytosis in the cyclopropylsterol isomerase1-1 (cpi1-1) mutant results in the accumulation of the cytokinetic syntaxin KNOLLE at the CDZ (13), similar to what was observed for TPLATE. To address whether TPLATE membrane recruitment is sensitive to reduced levels of sterols, we crossed the genomic fusion of TPLATE-GFP into CPI11-1/cpi1-1 and identified homozygous cpi1-1 mutants expressing TPLATE-GFP in the next generation by segregation of the cpi1-1 phenotype and screening for the presence of fluorescence. Homozygous cpi1-1 mutants showed strongly misregulated patterning of cells and cytokinesis defects, yet TPLATE was recruited to the PM (Fig. 4L) and the CDZ (Fig. 4N), similarly to wild-type (Fig. 4A) and heterozygous cpi1-1 mutants (Fig. 4J). Therefore, TPLATE targeting does not depend on CPI-controlled composition of sterols in the plasma membrane.
Fig. 4.
TPLATE-GFP accumulates at cell wall stubs in the Arabidopsis gnl1-1 mutant treated with BFA similar to Concanamycin A treatment but localization is not altered in the cpi1-1 background. (A) Untreated Arabidopsis root cell showing specific recruitment of TPLATE-GFP to the CDZ during cell plate anchoring independent of cell plate recruitment (arrowhead). (B) FM4-64 staining of the cell in A showing that the plate has contacted the PM and that the TPLATE-GFP label at the cell plate is absent from the outer rim of the plate. (C and D) Single section through a root expressing a genomic TPLATE-GFP treated with BFA (50 μM) and stained with FM4-64. TPLATE remains present at the PM and does not accumulate in BFA bodies (arrows in D). (E–G) Root epidermal cells of a gnl1-1 seedling expressing a genomic construct of TPLATE-GFP treated with 25 μM BFA. (E) Single plane confocal section of a dividing cell. TPLATE localizes to the plasma membrane and CDZ (arrowhead) but is absent between the reforming daughter nuclei. (F and G) Overview and close-up of gnl1-1 root epidermal cells showing TPLATE accumulation at cell wall stubs (white boxes, arrows). (H and I) TPLATE accumulation at cell wall stubs (arrows) in seedlings treated with 2 μM Concanamycin A. (J–O) Localization of a genomic TPLATE-GFP fusion in the cpi1-1 background stained with FM4-64. (J and K) TPLATE localization in the heterozygous cpi1-1 background. (L–O) TPLATE-GFP in the cpi1-1 mutant background. The cpi1-1 mutant shows altered division planes and cell wall stubs (arrowhead and arrow in L), yet TPLATE localization remains present at the PM (L) and the CDZ (arrow in N) in this background. (Scale bars: A, B, G, N, and O, 5 μm; C–F and H–M, 10 μm).
Concluding Remarks.
The presence of clathrin on membranes is generally associated with endocytotic activity (14–16, 18–20, 36–38). Enhanced endocytosis of FM4-64 was reported and clathrin-coated pits were recorded in close association with the PM underlying the preprophase band (39, 40), which corresponds to the CDZ throughout cytokinesis. Expression of a dominant-negative CHC in BY-2 cells suppressed endocytosis and resulted in forked cell plates anchoring outside the CDZ (20). Inhibition of TPLATE activity in BY-2 cells by means of RNAi also caused inadequate anchoring of the cell plate but did not affect initial cell plate formation (10). At this point, there is no direct evidence supporting a role for clathrin-dependent endocytosis in cell plate anchorage, and the observed specific recruitment of TPLATE and CLC2 to the CDZ could also serve to remodel the PM at the CDZ before anchoring. Recent experiments by Boutté and coworkers (13) show that genetic or pharmacologically altered plant sterol composition is required to prevent lateral diffusion of the cytokinesis-specific syntaxin KNOLLE (41) into the CDZ at the end of cytokinesis. The striking similarity between the accumulation of KNOLLE at the CDZ when endocytosis is impaired, the interaction of TPLATE with clathrin, the structural resemblance of TPLATE with large adaptin subunits, and the specific accumulation of TPLATE and CLC2 during cell plate anchoring favors the hypothesis that TPLATE plays a role in clathrin-mediated endocytosis. The pharmacological and genetic evidence presented here shows that there are two differently regulated pathways of endocytotic recruitment during plant cytokinesis: one at the central cell plate during the early phases of plant somatic cytokinesis and one that is activated at the CDZ during anchoring of the cell plate with the mother wall. TPLATE may play a role in trafficking events leading to cell plate maturation/consolidation, a process that involves both cell wall synthesis and PM identity establishment following cytokinesis. The existence of a centripetal recruitment pathway shows similarity to centripetal accumulation of several exocyst components at the cell plate following cytokinesis (42). Although speculative at this stage, TPLATE could be part of a mechanism counterbalancing exo84-dependent exocytosis events occurring during cell plate maturation. The future task will be to identify and characterize interactors of TPLATE to gain insight into the components involved in CDZ and cell plate membrane recruitment.
Materials and Methods
A detailed description is provided in SI Materials and Methods.
Cloning of Constructs.
TPLATE-GFP, FH5-GFP, GFP-MBD, RFP-TUA2, GFP-AIR9, and GFP-KCA1 are described elsewhere (9, 11, 31, 43–45). The Agrobacterium strain containing 35S::mVENUS-PHFAPP1 (32) was a kind gift from Joop Vermeer (University of Lausanne, Lausanne, Switzerland).
Plant Material.
Stable BY-2 transformation was carried out as described by Geelen and Inzé (46). The Arabidopsis line expressing 35S::CLC2-EGFP is described elsewhere (16). Arabidopsis plants expressing a genomic construct of TPLATE fused to GFP (10) were crossed with homozygous gnl1-1 plants (34) and heterozygous cpi1-1 plants (47).
Transient Expression of BiFC Constructs.
Wild-type Nicotiana benthamiana plants (3–4 wk old) were used to transiently express the various BiFC construct combinations by Agrobacterium tumefaciens (strain LBA4404)-mediated transient transformation of lower epidermal leaf cells as previously described (48).
Chemical Treatments.
Stock solutions were prepared of BFA (50 mM in DMSO, 1000–2000× stock; Molecular Probes), propyzamide (6 mM in DMSO, 1000× stock; Chem Service Inc.), caffeine (500 mM in water, 100× stock; Sigma Aldrich), Concanamycin A (2 mM in DMSO, 1000× stock; Sigma Aldrich), aniline blue (0.5% in water; Sigma Aldrich), Latrunculin B (20 mM in DMSO, 1000× stock; Sigma Aldrich), Tyrphostin A23 and A51 (50 mM in DMSO, 1000–500× stock; Sigma Aldrich), Wortmannin (20 mM in DMSO, 666× stock; Sigma Aldrich), and FM4-64 (2 mM in water, 500–250× stock; Molecular Probes).
Confocal Microscopy.
Image acquisition was obtained with either a Zeiss 100M, an Olympus FluoView1000, or a Zeiss 710 inverted confocal microscope.
GFP-Based Coimmunoprecipitation and Western Blotting.
Total protein extracts were obtained from liquid nitrogen-ground 6-d-old Arabidopsis seedlings, and co-IP was performed using GFP-Trap_A agarose beads (Chromotek). Protein detection after Western blotting was done with the Living Colors A.v. Monoclonal GFP antibody (1/5000) (JL-8; Clontech) or the soybean CHC antibody (1/500) (sc-57684; Santa Cruz Biotechnology).
Supplementary Material
Acknowledgments
We thank Masaru Fujimoto (University of Tokyo, Tokyo, Japan) and Satoshi Naramoto (VIB-Ghent University, Belgium) for kindly providing the CLC2 entry clones, Joop Vermeer (University of Lausanne, Lausanne, Switzerland) for the 35S::mVENUS-PHFAPP1, Corina Codreanu for cloning mCHERRY in pDONRP2P3R, Mansour Karimi for vector construction, Markus Grebe (Umeå University, Sweden) for the cpi1-1 mutant, Leentje De Puysseleyr for help with the BiFC experiments, Jiri Friml for critical reading of the manuscript and helpful comments, and Martine De Cock for help preparing the manuscript. A.G. is indebted to the Agency for Innovation by Science and Technology for a predoctoral fellowship. D.V.D. is a postdoctoral fellow of the Research Foundation-Flanders.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017890108/-/DCSupplemental.
References
- 1.Jürgens G. Plant cytokinesis: Fission by fusion. Trends Cell Biol. 2005;15:277–283. doi: 10.1016/j.tcb.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Samuels AL, Giddings TH, Jr., Staehelin LA. Cytokinesis in tobacco BY-2 and root tip cells: A new model of cell plate formation in higher plants. J Cell Biol. 1995;130:1345–1357. doi: 10.1083/jcb.130.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jürgens G. Cytokinesis in higher plants. Annu Rev Plant Biol. 2005;56:281–299. doi: 10.1146/annurev.arplant.55.031903.141636. [DOI] [PubMed] [Google Scholar]
- 4.Barr FA, Gruneberg U. Cytokinesis: Placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Low SH, et al. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 6.Montagnac G, Echard A, Chavrier P. Endocytic traffic in animal cell cytokinesis. Curr Opin Cell Biol. 2008;20:454–461. doi: 10.1016/j.ceb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Van Damme D, Inzé D, Russinova E. Vesicle trafficking during somatic cytokinesis. Plant Physiol. 2008;147:1544–1552. doi: 10.1104/pp.108.120303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thiele K, et al. The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J. 2008;58:13–26. doi: 10.1111/j.1365-313X.2008.03760.x. [DOI] [PubMed] [Google Scholar]
- 9.Van Damme D, Bouget F-Y, Van Poucke K, Inzé D, Geelen D. Molecular dissection of plant cytokinesis and phragmoplast structure: A survey of GFP-tagged proteins. Plant J. 2004;40:386–398. doi: 10.1111/j.1365-313X.2004.02222.x. [DOI] [PubMed] [Google Scholar]
- 10.Van Damme D, et al. Somatic cytokinesis and pollen maturation in Arabidopsis depend on TPLATE, which has domains similar to coat proteins. Plant Cell. 2006;18:3502–3518. doi: 10.1105/tpc.106.040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanstraelen M, et al. Cell cycle-dependent targeting of a kinesin at the plasma membrane demarcates the division site in plant cells. Curr Biol. 2006;16:308–314. doi: 10.1016/j.cub.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Walker KL, Müller S, Moss D, Ehrhardt DW, Smith LG. Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Curr Biol. 2007;17:1827–1836. doi: 10.1016/j.cub.2007.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutté Y, et al. Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J. 2010;29:546–558. doi: 10.1038/emboj.2009.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto M, et al. Arabidopsis dynamin-related proteins DRP2B and DRP1A participate together in clathrin-coated vesicle formation during endocytosis. Proc Natl Acad Sci USA. 2010;107:6094–6099. doi: 10.1073/pnas.0913562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto M, Arimura S-i, Nakazono M, Tsutsumi N. Imaging of plant dynamin-related proteins and clathrin around the plasma membrane by variable incidence angle fluorescence microscopy. Plant Biotechnol. 2007;24:449–455. [Google Scholar]
- 16.Konopka CA, Backues SK, Bednarek SY. Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell. 2008;20:1363–1380. doi: 10.1105/tpc.108.059428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheele U, Holstein SEH. Functional evidence for the identification of an Arabidopsis clathrin light chain polypeptide. FEBS Lett. 2002;514:355–360. doi: 10.1016/s0014-5793(02)02439-0. [DOI] [PubMed] [Google Scholar]
- 18.Seguí-Simarro JM, Austin JR, II, White EA, Staehelin LA. Electron tomographic analysis of somatic cell plate formation in meristematic cells of Arabidopsis preserved by high-pressure freezing. Plant Cell. 2004;16:836–856. doi: 10.1105/tpc.017749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seguí-Simarro JM, Staehelin LA. Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: A quantitative and spatial analysis. Planta. 2006;223:223–236. doi: 10.1007/s00425-005-0082-2. [DOI] [PubMed] [Google Scholar]
- 20.Tahara H, et al. Clathrin is involved in organization of mitotic spindle and phragmoplast as well as in endocytosis in tobacco cell cultures. Protoplasma. 2007;230:1–11. doi: 10.1007/s00709-006-0226-7. [DOI] [PubMed] [Google Scholar]
- 21.Blackbourn HD, Jackson AP. Plant clathrin heavy chain: Sequence analysis and restricted localisation in growing pollen tubes. J Cell Sci. 1996;109:777–786. doi: 10.1242/jcs.109.4.777. [DOI] [PubMed] [Google Scholar]
- 22.Banbury DN, Oakley JD, Sessions RB, Banting G. Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J Biol Chem. 2003;278:12022–12028. doi: 10.1074/jbc.M211966200. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz-Zapater E, Soriano-Ortega E, Marcote MJ, Ortiz-Masiá D, Aniento F. Trafficking of the human transferrin receptor in plant cells: Effects of tyrphostin A23 and brefeldin A. Plant J. 2006;48:757–770. doi: 10.1111/j.1365-313X.2006.02909.x. [DOI] [PubMed] [Google Scholar]
- 24.Lam SK, et al. BFA-induced compartments from the Golgi apparatus and trans-Golgi network/early endosome are distinct in plant cells. Plant J. 2009;60:865–881. doi: 10.1111/j.1365-313X.2009.04007.x. [DOI] [PubMed] [Google Scholar]
- 25.Emans N, Zimmermann S, Fischer R. Uptake of a fluorescent marker in plant cells is sensitive to brefeldin A and wortmannin. Plant Cell. 2002;14:71–86. doi: 10.1105/tpc.010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels AL, Staehelin LA. Caffeine inhibits cell plate formation by disrupting membrane reorganization just after the vesicle fusion step. Protoplasma. 1996;195:144–155. [Google Scholar]
- 27.Jones MG, Payne HL. Cytokinesis in Impatiens balsamina and the effect of caffeine. Cytobios. 1978;20:79–91. [PubMed] [Google Scholar]
- 28.López-Sáez JF, Mingo R, González-Fernández A. ATP level and caffeine efficiency on cytokinesis inhibition in plants. Eur J Cell Biol. 1982;27:185–190. [PubMed] [Google Scholar]
- 29.Yasuhara H. Caffeine inhibits callose deposition in the cell plate and the depolymerization of microtubules in the central region of the phragmoplast. Plant Cell Physiol. 2005;46:1083–1092. doi: 10.1093/pcp/pci121. [DOI] [PubMed] [Google Scholar]
- 30.Gu X, Verma DPS. Dynamics of phragmoplastin in living cells during cell plate formation and uncoupling of cell elongation from the plane of cell division. Plant Cell. 1997;9:157–169. doi: 10.1105/tpc.9.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingouff M, et al. Plant formin AtFH5 is an evolutionarily conserved actin nucleator involved in cytokinesis. Nat Cell Biol. 2005;7:374–380. doi: 10.1038/ncb1238. [DOI] [PubMed] [Google Scholar]
- 32.Vermeer JEM, et al. Imaging phosphatidylinositol 4-phosphate dynamics in living plant cells. Plant J. 2009;57:356–372. doi: 10.1111/j.1365-313X.2008.03679.x. [DOI] [PubMed] [Google Scholar]
- 33.Reichardt I, et al. Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr Biol. 2007;17:2047–2053. doi: 10.1016/j.cub.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 34.Richter S, et al. Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature. 2007;448:488–492. doi: 10.1038/nature05967. [DOI] [PubMed] [Google Scholar]
- 35.Teh O-K, Moore I. An ARF-GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature. 2007;448:493–496. doi: 10.1038/nature06023. [DOI] [PubMed] [Google Scholar]
- 36.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 37.Dhonukshe P, et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 38.Dhonukshe P, et al. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell. 2006;10:137–150. doi: 10.1016/j.devcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Dhonukshe P, Mathur J, Hülskamp M, Gadella TWJ., Jr. Microtubule plus-ends reveal essential links between intracellular polarization and localized modulation of endocytosis during division-plane establishment in plant cells. BMC Biol. 2005;3:11.1–11.15. doi: 10.1186/1741-7007-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karahara I, et al. The preprophase band is a localized center of clathrin-mediated endocytosis in late prophase cells of the onion cotyledon epidermis. Plant J. 2009;57:819–831. doi: 10.1111/j.1365-313X.2008.03725.x. [DOI] [PubMed] [Google Scholar]
- 41.Lauber MH, et al. The Arabidopsis KNOLLE protein is a cytokinesis-specific syntaxin. J Cell Biol. 1997;139:1485–1493. doi: 10.1083/jcb.139.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fendrych M, et al. The Arabidopsis exocyst complex is involved in cytokinesis and cell plate maturation. Plant Cell. 2010;22:3053–3065. doi: 10.1105/tpc.110.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marc J, et al. A GFP-MAP4 reporter gene for visualizing cortical microtubule rearrangements in living epidermal cells. Plant Cell. 1998;10:1927–1940. doi: 10.1105/tpc.10.11.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buschmann H, et al. Microtubule-associated AIR9 recognizes the cortical division site at preprophase and cell-plate insertion. Curr Biol. 2006;16:1938–1943. doi: 10.1016/j.cub.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 45.Van Damme D, et al. In vivo dynamics and differential microtubule-binding activities of MAP65 proteins. Plant Physiol. 2004;136:3956–3967. doi: 10.1104/pp.104.051623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geelen DNV, Inzé DG. A bright future for the Bright Yellow-2 cell culture. Plant Physiol. 2001;127:1375–1379. [PMC free article] [PubMed] [Google Scholar]
- 47.Men S, et al. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat Cell Biol. 2008;10:237–244. doi: 10.1038/ncb1686. [DOI] [PubMed] [Google Scholar]
- 48.Boruc J, et al. Functional modules in the Arabidopsis core cell cycle binary protein-protein interaction network. Plant Cell. 2010;22:1264–1280. doi: 10.1105/tpc.109.073635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.