Abstract
Juvenile hormone (JH) plays crucial roles in many aspects of insect life. The Methoprene-tolerant (Met) gene product, a member of the bHLH-PAS family of transcriptional regulators, has been demonstrated to be a key component of the JH signaling pathway. However, the molecular function of Met in JH-induced signal transduction and gene regulation remains to be fully elucidated. Here we show that a transcriptional coactivator of the ecdysteroid receptor complex, FISC, acts as a functional partner of Met in mediating JH-induced gene expression. Met and FISC appear to use their PAS domains to form a dimer only in the presence of JH or JH analogs. In newly emerged adult female mosquitoes, expression of some JH responsive genes is considerably dampened when Met or FISC is depleted by RNAi. Met and FISC are found to be associated with the promoter of the early trypsin gene (AaET) when transcription of this gene is activated by JH. A juvenile hormone response element (JHRE) has been identified in the AaET upstream regulatory region and is bound in vitro by the Met-FISC complex present in the nuclear protein extracts of previtellogenic adult female mosquitoes. In addition, the Drosophila homologs of Met and FISC can also use this mosquito JHRE to activate gene transcription in response to JH in a cell transfection assay. Together, the evidence indicates that Met and FISC form a functional complex on the JHRE in the presence of JH and directly activate transcription of JH target genes.
Keywords: development, endocrinology, chromatin immunoprecipitation
Juvenile hormones (JHs) are sesquiterpenoid molecules synthesized and secreted by the corpora allata in insects. JHs are essential for development, reproduction, diapause, caste differentiation, migratory behavior, and longevity in many insect species (1–4). The prominent role of JH is maintaining the status quo in juvenile insects and preventing an insect from precociously turning into an adult. During larval development, ecdysone (the molting hormone) causes larval–larval molts in the presence of JH in the hemolymph. After the corpora allata stop secreting JH in the final larval instar, insect tissues change their commitment, and ecdysone triggers the larval–pupal and pupal–adult molts (5).
JH appears to harness a variety of signal transduction pathways to exert its function. Some effects of JH are mediated via membrane receptors and the protein kinase C signaling pathway (4, 6), whereas more evidence suggests that JH acts through intracellular receptors to modulate gene expression (7–10). In some cases, JH seems to exert its functions by modulating the ecdysteroid signaling pathway (11–17).
A leading candidate for the JH receptor (or a component of the receptor) is the product of the Methoprene-tolerant (Met) gene, which was originally isolated in Drosophila melanogaster (18). Met belongs to the basic helix–loop–helix (bHLH)-Per-Arnt-Sim (PAS) family of transcription factors that also includes the hypoxia inducible factor 1α (HIF-1α), aryl hydrocarbon receptor (AhR), aryl hydrocarbon nuclear translocator (Arnt), and CLOCK proteins. In vitro-synthesized Drosophila Met protein binds to JH-III with high affinity (19). Flies carrying the Met mutations show resistance to both the toxic and morphogenetic effects of JH and several JH analogs, including methoprene (18). Recent studies suggest that Met and its paralogous gene in Drosophila, germ cell expressed (gce), have overlapping but not identical functions in JH signaling (20, 21). In Tribolium castaneum, it has been clearly demonstrated that the Met ortholog of this beetle (TcMet) plays an essential role in mediating the classical antimetamorphic effect of JH during molting. RNAi suppression of TcMet expression causes larvae to pupate prematurely, before reaching their final instar (22). It remains unclear how Met protein mediates JH signaling at the molecular level.
JH plays important roles in the control of various aspects of adult reproduction in mosquitoes (23). Secretion of JH-III begins soon after emergence of the adult. JH-III levels increase during the first 2 d and remain high until a blood meal is taken. Upon blood feeding, the hemolymph JH-III titers drop precipitously, whereas 20E titers begin to rise and reach their maximum level at 18–24 h after a blood meal (23). Our previous study has shown that the mosquito ortholog of Met is required for the JH-induced expression of the Krüppel homolog 1 (AaKr-h1) gene and the early trypsin (AaET) gene in newly emerged adult female mosquitoes (24). Although the function of AaKr-h1 is unknown in mosquitoes, AaET is a female-specific protease involved in initial digestion of blood proteins in the midgut (25). Transcription of AaET is activated by JH after adult eclosion, but the AaET protein is produced only after blood ingestion (26). Here we report that Met binds to another bHLH-PAS domain protein only in the presence of JH. Both proteins are required for the proper expression of JH target genes after adult emergence. We also provide evidence indicating that the heterodimer directly binds to the regulatory regions of the target genes and activates their transcription in response to JH.
Results
Identification of a Met-Interacting Protein.
bHLH-PAS proteins tend to use the bHLH-PAS domains to form homodimers or heterodimers with other bHLH-PAS proteins (27). DmMet has been shown to form Met–Met and Met–GCE dimers in vitro, although formation of the two protein complexes are greatly reduced in the presence of JH or JH analogs (28). We performed a yeast two-hybrid screening to isolate mosquito proteins that are specifically associated with Met only in the presence of JH. A chimeric protein consisting of the bHLH-PAS domain of the Aedes aegypti Met (AaMet1-505) and the Gal4 DNA-binding domain was used as bait. The yeast transformants were selected on solid medium contained 10−6 M methoprene. The screening yielded a single clone encoding the bHLH-PAS domain (amino acid residues 1–539) of FISC, a mosquito protein which has been previously characterized as a coactivator of the ecdysteroid receptor (29).
The yeast two-hybrid assay indicated that the interaction between the bHLH-PAS domains of AaMet and AaFISC was methoprene-dependent as the cotransformants were unable to grow on the selection medium without the presence of methoprene (Fig. S1). To examine the potential AaMet–AaMet interaction, we cloned AaMet1-505 into the pGAD10 Gal4 activation domain fusion vector and used it in the two-hybrid assays for comparison. It appeared that AaMet formed a homodimer only in the absence of methoprene, consistent with a previous report by Godlewski et al. (28).
JH-Dependent Protein Interaction.
To validate the Met-FISC interaction in insect cells and to characterize its dependence on juvenile hormone, we used a modified two-hybrid system developed by Peter and Lucy Cherbas' laboratory (30). pCMA-GAD and pCMA-GBD are expression vectors for recombinant proteins fused to the GAL4 activation domain and binding domain, respectively. A cDNA fragment encoding the amino terminal bHLH-PAS domain of AaMet was cloned into pCMA-GBD, whereas the bHLH-PAS domain of AaFISC was cloned into pCMA-GAD.
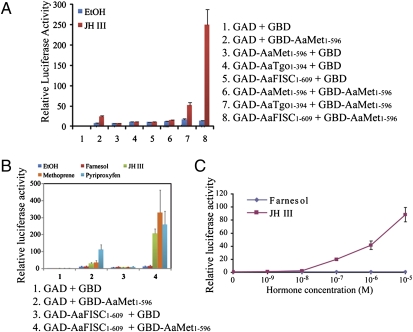
The GAD-AaFISC fusion was not able to activate the UAS×4–188-cc-Luc reporter gene, regardless of the presence of JH-III (Fig. 1A). GBD-AaMet was activated by JH-III and stimulated expression of the luciferase reporter gene, reminiscent of the transcriptional activity of DmMet in a similar experiment (19). The JH-dependent activation of the reporter gene by GBD-AaMet was further boosted when the GBD-AaMet and GAD-AaFISC fusion proteins were expressed together, suggesting a physical interaction between the bHLH-PAS regions of AaMet and AaFISC. In contrast, similar experiments implied that AaMet did not form either a homodimer or a heterodimer with other bHLH-PAS proteins, such as AaTgo (the mosquito ortholog of Drosophila Tango) in the L57 cells (Fig. 1A).
Fig. 1.
Interaction between AaMet and AaFISC in Drosophila L57 cells. (A) Modified two-hybrid assays. cDNA fragments encoding the bHLH-PAS domains of AaMet, AaFISC, and AaTgo were cloned into the pCMA-GBD and pCMA-GAD vectors. L57 cells were transfected by the reporter construct UAS×4–188-cc-Luc together with the indicated GAD and GBD fusion constructs. Transfected cells were cultured in the presence of 5 × 10−6 M JH-III or ethanol (solvent; EtOH) for 16 h. Activity of the reporter gene was measured by dual luciferase reporter assay. The two-hybrid assays were also used to examine the hormone specificity (B) and JH dose-dependence (C) of the AaMet-AaFISC interaction. L57 cells were cotransfected by pCMA-GBD-AaMet, pCMA-GAD-AaFISC and UAS×4–188-cc-Luc. Transfected cells were cultured in the presence of 5 × 10−6 M JH-III, methoprene, pyriproxyfen, farnesol, or ethanol (B) or in medium with indicated concentrations of JH-III or farnesol (C). The mean average of three independent experiments is shown, with error bars representing SD.
Next, we used the same cell transfection system to study the hormone-specificity and JH dose–response of the protein interaction between AaMet and AaFISC. Formation of the AaMet-AaFISC dimer was induced by JH-III and two JH agonists (methoprene and pyriproxyfen), but not by farnesol (a biosynthetic intermediate for JH-III) (Fig. 1B). In subsequent hormonal treatment experiments, farnesol was used as negative control. Marked activation of the reporter gene by the AaMet-AaFISC interaction was observed when the transfected cells were exposed to JH-III at a concentration of 10−7 M, and the reporter activity continued to increase in a JH-dose dependent manner (Fig. 1C). Together, these results demonstrated that the AaMet-AaFISC interaction is a JH-specific response.
PAS Domains Essential for the Met-FISC Interaction.
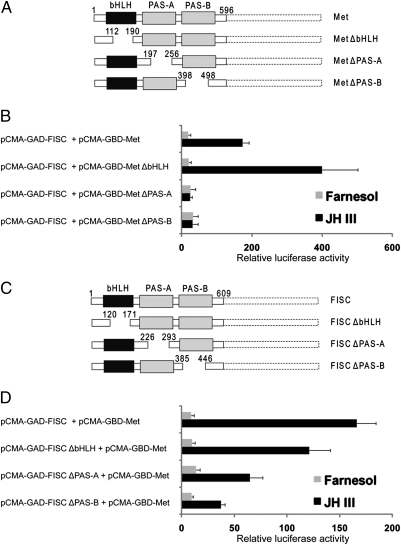
Having demonstrated that the bHLH-PAS regions of AaMet and AaFISC were sufficient for their JH-dependent dimerization, we started to delineate the functional domains in the bHLH-PAS regions. Derivatives of the pCMA-GBD-AaMet1-596 vectors were generated to produce GBD-Met fusion proteins with truncations of bHLH, PAS-A or PAS-B domains (Fig. 2A). Similar deletion mutations in GAD-AaFISC1-609 fusion protein were created. Two-hybrid assays were performed in the L57 cells as described above with these unique expression vectors. In the absence of bHLH domain, GBD-MetΔbHLH showed even stronger binding to GAD-AaFISC1-609 (Fig. 2B), indicating this domain in AaMet is not required for the JH-dependent Met-FISC interaction. Truncations of the two PAS domains in AaMet all significantly diminished formation of the Met-FISC complex, implicating the PAS domains in binding of JH and/or in protein–protein interaction. On the other hand, the bHLH, PAS-A and PAS-B domains of AaFISC all seemed to contribute to the Met-FISC interaction in response to JH, although the PAS-A and PAS-B domains appeared to play a bigger role in the binding of AaFISC to AaMet (Fig. 2D).
Fig. 2.
Roles of bHLH and PAS domains of AaMet and AaFISC in their JH-dependent dimerization. (A) Schematic diagram of truncations introduced into the bHLH-PAS region of AaMet. Similar truncations were introduced into the corresponding regions of AaFISC (C). L57 cells were transfected by the reporter construct UAS×4–188-cc-Luc, together with the indicated expression vectors that produced the truncated GBD-Met (B) or GAD-FISC (D). Transfected cells were cultured in medium with 5 × 10−6 M of JH-III or farnesol.
Roles of AaMet and AaFISC in Expression of the JH Target Genes.
Our previous studies have detected expression of AaMet and AaFISC genes in the fat body, midgut, and ovaries of adult female mosquitoes during posteclosion development (24, 29). AaMet and AaFISC, two bHLH-PAS family transcription factors, form a heterodimer in response to JH, suggesting that the AaMet-AaFISC complex may function in modulating transcriptional response to JH. After injecting double-stranded RNA corresponding to AaMet or AaFISC into adult female mosquitoes within 30 min after eclosion, we examined expression of four JH target genes that are normally up-regulated in the midgut after eclosion (24). Knockdown of either AaMet or AaFISC caused a considerable decrease in mRNA transcripts of AaET and AaKr-h1 in the midgut (Fig. 3A and Fig. S2). Expression of AAEL002576 and AAEL002619 were not markedly reduced in the AaMet RNAi mosquitoes, whereas impaired function of AaFISC affected the mRNA levels of AAEL002619, but not AAEL002576. Consistent with a diminished JH response, RNA interference of AaMet and AaFISC also significantly reduced the number of eggs oviposited by each female mosquito after a blood feeding (Fig. S3). These results indicated that both AaMet and AaFISC play important roles in modulating JH-regulated gene expression in adult female mosquitoes.
Fig. 3.
AaMet and AaFISC are required for expression of JH target genes in the midgut of adult female mosquitoes. (A) Double-stranded RNA (dsRNA) induced gene knockdown. A 0.5-μg quantity of dsRNA for either AaMet or AaFISC was injected into newly emerged female mosquitoes within 30 min after eclosion. DsRNA for bacterial malE gene was used as control. Then, 4 d after injection, midguts were collected from the mosquitoes. Total RNA was extracted and subjected to quantitative RT-PCR analysis. Results are expressed as percentage of mRNA levels in the uninjected (UGAL) mosquitoes. (B) Schematic structure of the AaET gene. Three pairs of primers were designed to amplify the distal upstream region (ET2), the proximal promoter region (ETv), and the coding region of AaET (ETc6). Association of AaMet (C) and AaFISC (D) with the AaET promoter was measured by chromatin immunoprecipitation assays. Amount of immunoprecipitated DNA in each sample was represented as signal relative to the total amount of input chromatin. PBM, post blood meal; PE, posteclosion.
Detection of AaMet and AaFISC on a JH-Activated Promoter.
To examine whether AaMet and AaFISC directly regulate the promoter of AaET, we performed ChIP assays. The presence of AaMet and AaFISC in the proximal regulatory regions of AaET was at a background level at 2 h posteclosion (Fig. 3 C and D), when endogenous JH concentration had not yet increased in the newly emerged mosquitoes. At 30 h posteclosion, when the JH titers were near their peak, occupancy of the AaET promoter by either AaMet or AaFISC increased significantly. The association of AaMet and AaFISC with the AaET proximal promoter was concomitant with the active transcription of AaET at this stage (26). Binding of either AaMet or AaFISC to the AaET promoter went down to the background level again at 4 h after a blood meal (Fig. 3 C and D), when the JH concentrations declined precipitously and transcription of AaET was shut down. These results showed that AaMet and AaFISC act directly on the AaET promoter to activate its transcription.
Identification of a JH Response Element.
We cloned a 2.0-kb promoter region of AaET into the pGL3 basic luciferase reporter vector, and used transient transfection assays to test whether AaMet and AaFISC activated the AaET promoter in response to JH-III. Expression of either AaMet or AaFISC alone in L57 cells had no substantial effect on the activity of the pAaET-Luc reporter gene (Fig. S4A). When the two proteins were expressed together, the reporter gene was activated significantly if JH-III was present in the cultural medium. Serial deletion analysis of the promoter region revealed that the proximal region (nt −540 to −165) was crucial for the JH-induced activation of the reporter gene (Fig. S4B). Bioinformatic analysis of this region revealed a sequence (CCACACGCGAAG) similar to the binding site of the mammal AhR/Arnt bHLH-PAS heterodimer (Fig. S5). To test the function of this DNA element, we inserted four copies of this sequence and the minimal core promoter of AaET into the pGL3 basic luciferase reporter vector. Although the minimal core promoter alone was not responsive to JH treatment (Fig. S6), expression of the unique reporter gene (4×JHRE-luc) was considerably activated in L57 cells by the AaMet-AaFISC complex in the presence of JH-III (Fig. 4A), suggesting that this 12-nucleotide sequence acted as a juvenile hormone response element (JHRE). Furthermore, gel shift assays suggested the existence of a protein complex containing both AaMet and AaFISC in the nuclear extracts of adult female mosquitoes (Fig. 4B). Binding of the protein complex to JHRE was abolished by antibodies against either AaMet or AaFISC, presumably by blocking dimerization or DNA binding of these two proteins. The protein complex was detected in mosquitoes at 30 h after eclosion, but not in the newly emerged mosquitoes or the blood-fed mosquitoes. The appearance of the AaMet-AaFISC complex seems to correlate well with endogenous JH concentrations and the expression profile of AaET in the adult female mosquitoes.
Fig. 4.
AaMet and AaFISC bind to JHRE identified in AaET upstream regulatory region. (A) L57 cells were transfected with the 4×JHRE-luc reporter plasmid, together with the indicated expression vectors. After transfection, cells were cultured in medium with 5 × 10−6 M JH-III or farnesol. (B) EMSA experiments. Nuclear proteins were extracted from abdomens of adult female mosquitoes at the indicated time points and incubated in vitro with JHRE labeled by [γ-32P] ATP. For competition reactions, nuclear proteins were incubated with an approximate 100× molar excess of unlabeled probe or a nonspecific double-stranded oligonucleotide for 20 min before incubation with labeled probe. Identity of complex was verified by directly adding polyclonal antibodies against AaMet and AaFISC to the binding reactions.
Conserved Mechanism for JH Signaling and Transcriptional Regulation.
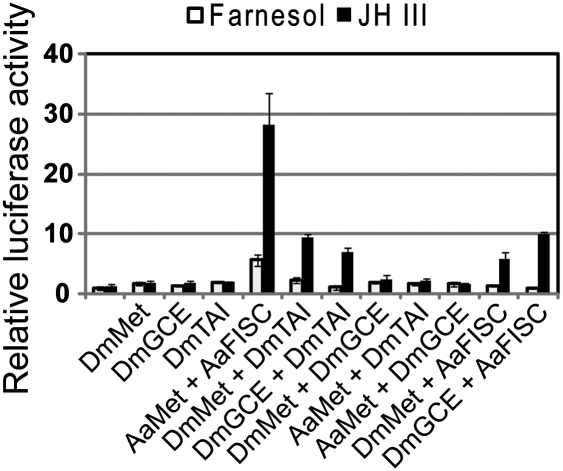
The JHRE shares a high degree of sequence similarity with a common motif that has been previously identified in a group of Drosophila JH-responsive promoters (9). Using transient transfection assays, we tested the functions of Drosophila Met, GCE, and Taiman (TAI; the Drosophila ortholog of AaFISC) in mediating JH signaling in the L57 cells. None of the three bHLH-PAS proteins alone had any significant effect on the expression of the 4×JHRE-luc reporter gene (Fig. 5). Coexpression of DmTAI with either DmMet or DmGCE led to significant induction of the reporter gene by JH-III. In contrast, the combination of DmMet and DmGCE was not able to activate the reporter gene in response to JH-III. This evidence suggests that binding of the Met-FISC complex to the JHRE is a conserved mechanism in activating expression of JH target genes.
Fig. 5.
JH-induced transcriptional activation by the Drosophila homologs of Met and FISC. L57 cells were transfected by 4×JHRE-luc and expression vectors for the indicated proteins. Transfected cells were cultured in medium with 5 × 10−6 M JH-III or farnesol. Average results of three independent experiments are shown, with error bars representing SD.
Discussion
Genetic studies have shown that Met is required for proper expression of JH target genes in fruit flies, red flour beetles, and mosquitoes (10, 24, 31). Although the protein structure of Met suggests that it may act as a JH-activated transcriptional regulator, the binding of Met to JH-responsive promoters has not been definitively demonstrated so far. In this study, a chromatin immunoprecipitation experiment indicated that Met was indeed associated with the early trypsin promoter when this gene was activated by endogenous juvenile hormone in the newly emerged adult female mosquitoes. This is a unique demonstration of Met directly regulating a JH target gene.
To elucidate the molecular roles of Met in JH signaling, a number of proteins have been tested in vitro or in the cultured insect cells for their abilities to bind Met (9, 28, 32). The protein interactions with Met were largely independent of the presence of JH, or even repressed by JH. Using a library screening approach, we have identified a mosquito bHLH-PAS protein (FISC) that binds to Met in a JH-dependent manner. EMSA and ChIP experiments have demonstrated that the Met-FISC complex forms in vivo and binds to a JH-regulated promoter in previtellogenic mosquitoes only in the presence of high titers of juvenile hormone. This observation is consistent with the RNAi results showing that both Met and FISC are required in adult mosquitoes for activation of JH target genes, such as AaET and AaKr-h1. In Fig. 1, the GBD-Met fusion (without the GAD-FISC fusion) activated the UAS×4–188-cc-Luc reporter gene after the JH treatment. This activation also relied on the endogenous Taiman protein in the L57 cells as the JH induction was severely dampened when Taiman was depleted by RNAi (Fig. S7). Formation of the Met-FISC complex thus constitutes a key step in signal transduction of juvenile hormone. It is also worth noting that not all of the JH target genes are affected by RNAi knockdown of Met or FISC (Fig. 3A), implying that JH might act through several distinct pathways even in a single tissue at a particular developmental stage.
Transient transfection and gel shift assays indicated that Met-FISC activated the AaET promoter by binding to the JHRE. It is currently under investigation whether the two proteins are directly binding to the JHRE or are recruited to the JHRE via protein interaction with other transcription factors. Because of the relative large sizes of the two proteins, it is difficult to obtain full-length and functional recombinant Met and FISC proteins. EMSA experiments using in vitro-synthesized proteins turned out to be problematic, because both rabbit reticulocyte lysate and wheat germ extract displayed high background binding to the labeled JHRE. In a separate experiment, our preliminary study showed that the JH-induced transcriptional activation by Met-FISC was completely abolished in cell transfection assays if the DNA binding domain (bHLH region) of either Met or FISC was truncated. However, we cannot rule out the possibility that the bHLH regions are also required for interactions with other proteins.
A distal regulatory region of AaET was also shown to be indispensable for JH-dependent activation of the AaET promoter (Fig. S4). Intriguingly, when four copies of JHRE were placed upstream of the minimal promoter (TATA box) of AaET, the JHRE seemed to be sufficient for the Met-FISC mediated JH activation (Fig. S6). This discrepancy implies that regulation of JH target genes is more sophisticated than the binding of Met-FISC to JHRE. More studies are needed to elucidate the underlying molecular mechanisms.
In vitro experiments have shown that Met can bind to both EcR and USP, two components of the ecdysteroid receptor (32). Here we find that FISC, a coactivator of the EcR/USP, also binds to Met and plays an important role in juvenile hormone signaling. Whether these protein interactions are involved in the crosstalk of ecdysone and JH signaling is awaiting further experimental evidence. Because the binding of FISC to EcR/USP and Met relies on the presence of 20-hydroxyecdysone and juvenile hormone respectively, the shuffling of FISC between the two signaling pathways may account for the antagonistic actions of these two hormones.
A sequence similar to the AaET JHRE is also found in the promoter region of AaJHA15, another JH-regulated gene in adult female mosquitoes (33). The common motif 2 discovered in a group of JH-activated Drosophila promoters also shares high sequence similarity with the AaET JHRE, suggesting an evolutionarily conserved mechanism underneath the JH-induced transcriptional activation. Indeed, the Drosophila Met and Taiman activated the 4×JHRE-luc reporter gene in a JH-dependent manner. Although DmMet-AaFISC appeared comparable to DmMet-DmTAI in mediating JH-induced gene expression, AaMet-DmTAI was completely unable to activate expression of the reporter gene after JH treatment. This observation suggests that the intricate protein interactions between Met and FISC/TAI determine the affinity of the dimers to the JHRE and/or their ability to activate transcription of the JH target genes.
Unlike mosquitoes, two Met-like genes (Met and gce) exist in fruit flies. Combination of gce and Taiman also led to considerable activation of the reporter gene in response to JH. This observation is in line with a recent report showing that gce can partially substitute for Met in vivo (20). It would be interesting to test next whether Met-TAI and gce-TAI preferentially bind to distinct JH responsive promoters in vivo.
Materials and Methods
Yeast Two-Hybrid Screen.
A yeast two-hybrid cDNA library was constructed in the pGAD10 Gal4 activation domain vector, according to the manufacturer's instructions (Clontech), using a total of 10 mg poly(A)+ RNA from abdomens of adult female mosquitoes. AaMet1-505 (bHLH-PAS domain) was cloned into the Gal4 DNA-binding domain vector pGBKT7 (Clontech). Yeast strain AH109 was sequentially transformed with pGBKT7-AaMet1-505 and with the mosquito cDNA library. All of the selection medium contained 10−6 M methoprene dissolved in DMSO. Colonies that appeared on the SD/-Trp/-Leu/-His plates (medium stringency) were transferred to the SD/-Trp/-Leu/-His/-Ade/Xα-Gal plates (high stringency). The library plasmids from positive clones that expressed HIS3, Ade and LacZ reporters were recovered and retransformed into yeast cells, together with the original bait, for testing the specificity of protein–protein interactions.
Transient Transfection Assay.
Drosophila L57-3–11 cells were transfected according to the instructions of Hu et al. (30). pCMA was used as the expression vector for all of the proteins described in the transfection assays. Truncated proteins were expressed by deletional mutagenesis using a method described by Li et al. (34). The ORFs for AaTgo, DmMet, and Dmgce were cloned by RT-PCR based on the cDNA sequences in the GenBank. Juvenile hormone III, methoprene, pyriproxyfen and farnesol (Sigma Aldrich) were dissolved in ethanol.
Double-Stranded RNA-Induced Gene Silencing.
RNAi knockdown of AaMet and AaFISC was performed as described previously (24, 29). Briefly, 0.5 μg dsRNA was injected into the newly emerged female Ae. aegypti mosquitoes within 30 min after eclosion. The mosquitoes were then maintained in the insectary under normal conditions. Then, 3–4 d after injection, the mosquitoes were dissected, and the mRNA extracted from the midgut was examined by quantitative RT-PCR (24).
Chromatin Immunoprecipitation Assay.
Polyclonal antibodies for AaMet and AaFISC have been reported previously (24, 29). Ae. aegypti mosquito abdomens were homogenized in PBS on ice, followed by the addition of formaldehyde to a final concentration of 1% and incubation at 37 °C for 10 min. Chromatin immunoprecipitation assays were performed using a QuikChIP kit (IMGENEX) according to the instruction manual. Mock immunoprecipitations using preimmune sera for each antibody were included as negative controls to determine the baseline of the nonspecific background. The precipitated DNA and DNA input were analyzed by using quantitative RT-PCR. PCR primers were as follows: ET2-F, 5′-GCTTGGTAGAACAGTCAATGGGTCAG-3′; ET2-R, 5′-AGAGTCCATCGGATAGGCATCACG-3′; ETv-F, 5′-GTTTTGAAATTACCCATCCCACACG-3′; ETv-R, 5′-GTCCATTCCTATGATGCGGATTCTT-3′; ETc6-F, 5′-GTAAGGATTCTTGCCAGGGAGACTC-3′; and ETc6-R, 5′-ATCCATTGGCGAACAGTGGACAC-3′.
Electrophoretic Mobility Shift Assay.
Abdomens were collected from 200 adult female Ae. aegypti mosquitoes for each time point. Nuclear protein extraction was carried out as described by Miura et al. (35). Detailed method for EMSA experiments was described in SI Text.
Supplementary Material
Acknowledgments
We thank Dr. Lucy Cherbas (Indiana University, Bloomington, IN) for providing the pCMA, pCMA-GAD, pCMA-GBD, and UAS×4-188-cc-Luc plasmids, and Dr. Denise J. Montell (Johns Hopkins University School of Medicine, Baltimore) for supplying the cDNA clone and antibodies for Taiman. We also thank Dr. Honglin Jiang (Virginia Tech, Blacksburg, VA) for helping with the EMSA experiments. This work was partly supported by the startup fund from Virginia Tech (to J.Z.) and Grant J-929 from the Thomas F. and Kate Miller Jeffress Memorial Trust (to J.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013914108/-/DCSupplemental.
References
- 1.Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- 2.Nijhout HF. Insect Hormones. Princeton: Princeton University Press; 1994. [Google Scholar]
- 3.Riddiford LM. Juvenile hormone action: A 2007 perspective. J Insect Physiol. 2008;54:895–901. doi: 10.1016/j.jinsphys.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt GR, Davey KG. Cellular and molecular actions of juvenile hormone. II: Roles of juvenile hormone in adult insects. Adv Insect Physiol. 1996;26:1–155. [Google Scholar]
- 5.Riddiford LM, Hiruma K, Zhou X, Nelson CA. Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem Mol Biol. 2003;33:1327–1338. doi: 10.1016/j.ibmb.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto K, Chadarevian A, Pellegrini M. Juvenile hormone action mediated in male accessory glands of Drosophila by calcium and kinase C. Science. 1988;239:916–919. doi: 10.1126/science.3124270. [DOI] [PubMed] [Google Scholar]
- 7.Comas D, Piulachs MD, Bellés X. Fast induction of vitellogenin gene expression by juvenile hormone III in the cockroach Blattella germanica (L.) (Dictyoptera, Blattellidae) Insect Biochem Mol Biol. 1999;29:821–827. doi: 10.1016/s0965-1748(99)00058-2. [DOI] [PubMed] [Google Scholar]
- 8.Dubrovsky EB, Dubrovskaya VA, Bilderback AL, Berger EM. The isolation of two juvenile hormone-inducible genes in Drosophila melanogaster. Dev Biol. 2000;224:486–495. doi: 10.1006/dbio.2000.9800. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zhang Z, Robinson GE, Palli SR. Identification and characterization of a juvenile hormone response element and its binding proteins. J Biol Chem. 2007;282:37605–37617. doi: 10.1074/jbc.M704595200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minakuchi C, Zhou X, Riddiford LM. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev. 2008;125:91–105. doi: 10.1016/j.mod.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubrovsky EB, Dubrovskaya VA, Berger EM. Hormonal regulation and functional role of Drosophila E75A orphan nuclear receptor in the juvenile hormone signaling pathway. Dev Biol. 2004;268:258–270. doi: 10.1016/j.ydbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Henrich VC, Burns E, Yelverton DP, Christensen E, Weinberger C. Juvenile hormone potentiates ecdysone receptor-dependent transcription in a mammalian cell culture system. Insect Biochem Mol Biol. 2003;33:1239–1247. doi: 10.1016/j.ibmb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Parthasarathy R, Palli SR. Stage- and cell-specific expression of ecdysone receptors and ecdysone-induced transcription factors during midgut remodeling in the yellow fever mosquito, Aedes aegypti. J Insect Physiol. 2007;53:216–229. doi: 10.1016/j.jinsphys.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Richards G. Sequential gene activation by ecdysone in polytene chromosomes of Drosophila melanogaster. VI. Inhibition by juvenile hormones. Dev Biol. 1978;66:32–42. doi: 10.1016/0012-1606(78)90271-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhou B, et al. Regulation of the transcription factor E75 by 20-hydroxyecdysone and juvenile hormone in the epidermis of the tobacco hornworm, Manduca sexta, during larval molting and metamorphosis. Dev Biol. 1998;193:127–138. doi: 10.1006/dbio.1997.8798. [DOI] [PubMed] [Google Scholar]
- 16.Zhou B, Hiruma K, Shinoda T, Riddiford LM. Juvenile hormone prevents ecdysteroid-induced expression of broad complex RNAs in the epidermis of the tobacco hornworm, Manduca sexta. Dev Biol. 1998;203:233–244. doi: 10.1006/dbio.1998.9059. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Riddiford LM. Broad specifies pupal development and mediates the ‘status quo’ action of juvenile hormone on the pupal-adult transformation in Drosophila and Manduca. Development. 2002;129:2259–2269. doi: 10.1242/dev.129.9.2259. [DOI] [PubMed] [Google Scholar]
- 18.Ashok M, Turner C, Wilson TG. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA. 1998;95:2761–2766. doi: 10.1073/pnas.95.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura K, Oda M, Makita S, Chinzei Y. Characterization of the Drosophila Methoprene-tolerant gene product. Juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 2005;272:1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 20.Baumann A, Barry J, Wang S, Fujiwara Y, Wilson TG. Paralogous genes involved in juvenile hormone action in Drosophila melanogaster. Genetics. 2010;185:1327–1336. doi: 10.1534/genetics.110.116962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, et al. Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in Drosophila. Development. 2009;136:2015–2025. doi: 10.1242/dev.033712. [DOI] [PubMed] [Google Scholar]
- 22.Konopova B, Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc Natl Acad Sci USA. 2007;104:10488–10493. doi: 10.1073/pnas.0703719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagedorn HH. The endocrinology of the adult female mosquito. Adv Dis Vect Res. 1994;10:109–148. [Google Scholar]
- 24.Zhu J, Busche JM, Zhang X. Identification of juvenile hormone target genes in the adult female mosquitoes. Insect Biochem Mol Biol. 2010;40:23–29. doi: 10.1016/j.ibmb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Noriega FG, Wang XY, Pennington JE, Barillas-Mury CV, Wells MA. Early trypsin, a female-specific midgut protease in Aedes aegypti: Isolation, aminoterminal sequence determination, and cloning and sequencing of the gene. Insect Biochem Mol Biol. 1996;26:119–126. doi: 10.1016/0965-1748(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 26.Noriega FG, Pennington JE, Barillas-Mury C, Wang XY, Wells MA. Aedes aegypti midgut early trypsin is post-transcriptionally regulated by blood feeding. Insect Mol Biol. 1996;5:25–29. doi: 10.1111/j.1365-2583.1996.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 27.Partch CL, Gardner KH. Coactivator recruitment: A new role for PAS domains in transcriptional regulation by the bHLH-PAS family. J Cell Physiol. 2010;223:553–557. doi: 10.1002/jcp.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Godlewski J, Wang S, Wilson TG. Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem Biophys Res Commun. 2006;342:1305–1311. doi: 10.1016/j.bbrc.2006.02.097. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Chen L, Sun G, Raikhel AS. The competence factor beta Ftz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol Cell Biol. 2006;26:9402–9412. doi: 10.1128/MCB.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X, Cherbas L, Cherbas P. Transcription activation by the ecdysone receptor (EcR/USP): Identification of activation functions. Mol Endocrinol. 2003;17:716–731. doi: 10.1210/me.2002-0287. [DOI] [PubMed] [Google Scholar]
- 31.Parthasarathy R, Tan A, Palli SR. bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval-pupal metamorphosis. Mech Dev. 2008;125:601–616. doi: 10.1016/j.mod.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bitra K, Palli SR. Interaction of proteins involved in ecdysone and juvenile hormone signal transduction. Arch Insect Biochem Physiol. 2009;70:90–105. doi: 10.1002/arch.20281. [DOI] [PubMed] [Google Scholar]
- 33.Bian G, Raikhel AS, Zhu J. Characterization of a juvenile hormone-regulated chymotrypsin-like serine protease gene in Aedes aegypti mosquito. Insect Biochem Mol Biol. 2008;38:190–200. doi: 10.1016/j.ibmb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, et al. Site-directed mutagenesis by combination of homologous recombination and DpnI digestion of the plasmid template in Escherichia coli. Anal Biochem. 2008;373:389–391. doi: 10.1016/j.ab.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Miura K, Wang SF, Raikhel AS. Two distinct subpopulations of ecdysone receptor complex in the female mosquito during vitellogenesis. Mol Cell Endocrinol. 1999;156:111–120. doi: 10.1016/s0303-7207(99)00136-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.