Abstract
Kawasaki Disease is a vasculitis of young childhood that particularly affects the coronary arteries. Molecular analysis of the oligoclonal IgA response in acute KD led to production of synthetic KD antibodies. These antibodies identify intracytoplasmic inclusion bodies in acute KD tissues. Light and electron microscopic studies indicate that the inclusion bodies are consistent with aggregates of viral proteins and RNA. Advances in molecular genetic analysis and completion of the human genome project have sparked a worldwide effort to identify genes associated with KD. A polymorphism of one such gene, ITPKC, a negative regulator of T cell activation, confers susceptibility to KD in Japanese populations and increases the risk of developing coronary artery abnormalities in both Japanese and U.S children. Identification of the etiologic agent and of genes conferring KD susceptibility are the best means to improving diagnosis and therapy, and allow for prevention of this important disorder of childhood.
Keywords: IgA, Intracytoplasmic inclusion bodies, ITPKC, Coronary artery aneurysms, Vasculitis, Pediatrics
Introduction
Kawasaki Disease (KD) is a multisystem inflammatory illness of young childhood that can result in acute vasculitis, most strikingly of the coronary arteries. Rarely, KD can lead to myocardial infarction and sudden death. Without treatment, approximately 25% of children with KD develop coronary artery abnormalities. Therapy with intravenous gammaglobulin and aspirin within the first ten days of fever onset reduces the prevalence of coronary artery abnormalities from about 25% in untreated patients to about 5% (1). KD is unusual among serious vasculitis disorders in that steroids are not indicated for primary therapy (2). The leading theory of KD etiology is that a ubiquitous infectious etiologic agent that usually results in asymptomatic infection causes KD in a small subset of genetically predisposed children. New etiologic and genetic studies of KD hold great promise for improved diagnosis, therapy, and prevention of this important vascular disorder of childhood.
The Etiology of KD
Many proposed etiologies of KD have been suggested since Dr. Kawasaki’s initial description of the illness in Japan in the 1960s (3). The most widely proposed theories have been in the categories of environmental toxin exposure, autoimmune pathogenesis, and infectious diseases.
Environmental Toxin Theory
Although rug shampooing was reported to be a risk factor for KD in some studies in the United States (4), it is not a risk factor in Japan (5), the nation with the highest reported incidence of KD (6). It seems highly unlikely that an illness that presents with the same distinctive clinical features in all nations would have different etiologies in individual nations. Mercury poisoning shares some clinical features with KD, but is not etiologically related (7–8). The lack of recurrence of disease upon re-entering the home after hospitalization for KD argues against a household toxin as the etiology.
Autoimmune pathogenesis theory
Although the immune response to KD likely plays a role in the pathology, as it does in most infectious diseases, a primary autoimmune etiology seems unlikely given the self-limited febrile phase of illness, the generally non-recurring nature of KD and the lack of indication for corticosteroids in primary therapy of KD (2). Although immune complexes can be detected in serum of KD patients 2–3 weeks after fever onset, they do not appear to play a role in the development of coronary artery disease (9); immune complex deposits are not observed in inflamed KD arterial tissue (10).
Data supporting an infectious etiology of KD
The clinical findings of fever, rash, conjunctival injection, cervical adenitis, and erythematous pharynx in KD and the fact that these symptoms resolve spontaneously, even without treatment, support an infectious etiology. The young age group affected, with peak incidence in males age 9–11 months (6, 11), and the very high prevalence of the illness in Japan, where 1% of children develop KD by age 5 (6), also are more consistent with an infectious cause than with other potential etiologies. Well-documented epidemics of illness with geographic wave-like spread are particularly indicative of an infectious etiologic agent spreading through the population (12). A winter-spring predominance of cases in non-temperate climates also supports an infection (11), particularly a respiratory infection, and a preceding history of respiratory illness in KD patients in some outbreaks of the illness also supports this theory (7–8). Although many infectious agents have been proposed as the etiology of KD (13), none has been consistently associated with the illness. Current investigations focus on a superantigen/bacterial toxin etiology or a viral etiology characterized by intracytoplasmic inclusion bodies in KD tissues. To explain these theories, it is helpful to briefly review important aspects of the immune response in acute KD.
The innate and adaptive immune responses in KD
Production of many cytokines in the acute febrile phase of KD attests to activation of the innate immune response (14). In addition, oligoclonal CD8 T lymphocyte (15) and IgA and IgM B lymphocyte (16–17) responses indicate an antigen-driven adaptive immune response. Oligoclonal IgA plasma cells infiltrate vascular tissue in acute KD (10, 16, 18), strongly suggesting that they are targeting specific antigen.
Superantigen/bacterial toxin theory
A superantigen/bacterial toxin etiology has been considered for KD primarily because of three observations: 1) KD patients experience peripheral desquamation, as is known to occur with scarlet fever and toxic shock syndrome, 2) in some studies, peripheral blood T lymphocytes in acute KD have shown expansion of specific T cell receptor Vβ chain families (19–21), and 3) cytokines can be detected in the peripheral blood in acute KD. However, desquamation is not specific for superantigen-mediated illnesses and occurs in measles and other disease processes, other investigators have failed to detect T cell receptor Vβ skewing in KD (22–24), and cytokines are detected in the peripheral blood in many infectious and inflammatory conditions. In addition, Vβ skewing can be observed in an antigen-driven response if individual patients experience expansion of a clone of T lymphocytes within a particular family that has a “good fit” for an antigen. Evidence of such clonal expansion can be determined by sequencing the CDR3 regions of the expanded Vβ family. Clonal expansion of CD8 T lymphocytes in the peripheral blood of acute KD patients has been demonstrated by this technique (15). A superantigen/bacterial toxin theory for KD continues to be investigated, but significant supportive data are currently lacking. In addition, a hallmark of superantigen-mediated illness, a paralysis of the adaptive immune response, is not observed in KD (see previous section). An initial study implicating toxic shock syndrome toxin-1 as being etiologically related to KD (25) was not confirmed in a subsequent multicenter study (26). Recent studies focusing on superantigen-producing bacteria in the gastrointestinal tract of KD patients as etiologic agents have suffered from a failure to control for antibiotic exposure in patients and controls, which impacts stool culture results, a failure to determine whether bacterial superantigens are actually produced more often within the gastrointestinal tracts of KD patients than controls, and a failure to determine the presence or absence of serum antibodies to the superantigens in KD patients and controls, to determine whether the children would be susceptible to their effects (27–28). Moreover, so far no bacterial toxin has been detected in peripheral blood of KD patients. Distribution of the toxin through the bloodstream would be required to explain the involvement of multiple organs and tissues in the acute KD inflammatory process.
Identifying the antigen targeted by KD oligoclonal IgA plasma cells
IgA plasma cells infiltrate inflamed tissues during acute KD, including coronary arteries and other arterial tissues, upper respiratory tract, kidney, and pancreas (10, 29). The presence of oligoclonal IgA B lymphocytes (16) and CD8 T lymphocytes (15, 30) in acute KD suggests an immune response to an intracellular pathogen with a respiratory portal of entry, such as a virus. Synthetic antibodies derived from prevalent IgA antibody sequences in acute KD arterial tissue detected antigen by immunohistochemistry in acute KD but not infant control tissues (31). Synthetic antibodies derived from the most prevalent IgA alpha heavy chain gene sequences appeared to bind more strongly than those derived from less prevalent sequences, consistent with an antigen-driven response (18). Antigen was detected in the apical cytoplasm of KD medium-sized ciliated bronchial epithelial cells but not in control infant bronchial epithelium, and in the cytoplasm of a subset of macrophages in inflamed KD tissues (31).
Intracytoplasmic inclusion bodies are present in KD tissues
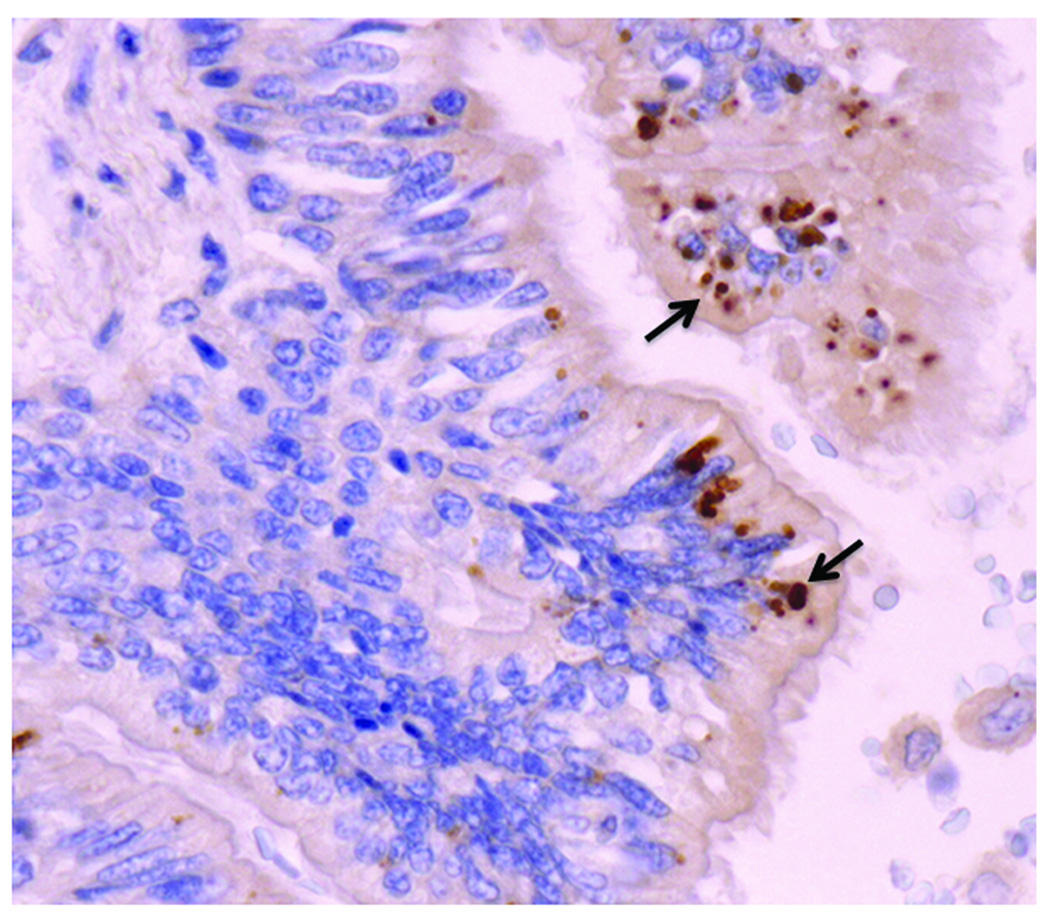
Light and electron microscopic studies localized the antigen identified by synthetic KD antibodies to intracytoplasmic inclusion bodies (ICI) that were consistent with aggregates of viral proteins and nucleic acids (32) (Figure 1). These homogeneous ICI do not have the substructure characteristic of bacterial inclusion bodies (33). Hematoxylin and eosin stains reveal amphophilic ICI, indicating the presence of both nucleic acid and protein in the ICI (32). Methyl green pyronin and Feulgen stains revealed RNA, but not DNA, within the ICI (34). Hopefully, high-throughput sequencing and bioinformatics analysis of KD tissues containing ICI will soon reveal sequence(s) of the putative etiologic agent, hypothesized to be a previously unidentified RNA virus with limited or no homology to known viruses.
Figure 1.
Intracytoplasmic inclusion bodies (ICI, arrows, in brown) in ciliated bronchial epithelium of an infant with acute fatal Kawasaki Disease, detected by immunohistochemistry using KD synthetic antibody. Nuclei stain blue with the hematoxylin counterstain. The ICI are consistent with aggregates of viral protein and RNA, and are likely the result of infection with a “new” RNA virus.
Genetics and KD
Historical aspects
The high prevalence of KD in Asian, particularly Japanese, children, strongly supports a genetic predisposition to developing KD; Japanese children who live a Western lifestyle continue to experience the same increased risk of KD (8). For decades, KD researchers attempted to identify candidate genes conferring susceptibility to the illness, particularly human leukocyte antigens (35). Many of these studies suffered from the small numbers of patients included, and by failure to confirm the findings in independent case-control studies. This led to many publications suggesting a KD gene association that often could not be reproduced in a different cohort of the same or different ethnic groups (35).
Epidemiologic aspects supporting a genetic influence on KD
Over time, data has continued to show a likely genetic influence on KD susceptibility and outcome. The incidence of KD is at least ten times higher in Japan than in Western populations (36–37). Siblings of children with KD have a tenfold higher risk of developing the illness than that of the general population, and children of parents who had KD have a twofold increased incidence (38–39).
The new era of molecular genetics and KD
With the completion of the human genome project and with advances in molecular genetics, a reassessment of genetic susceptibility to KD was made possible. It is highly likely that KD is polygenic, and that different genes may affect susceptibility in different ethnic groups. A genome-wide linkage study of siblings with KD and their healthy parents has identified ten chromosomal loci that appear to be associated with KD in Japanese populations (40). Linkage disequilibrium mapping of single nucleotide polymorphisms (SNPs) has been used to further identify potential genes of interest within chromosomal loci of interest.
Inositol 1,4,5-triphosphate 3-kinase C (ITPKC) as a susceptibility gene for KD
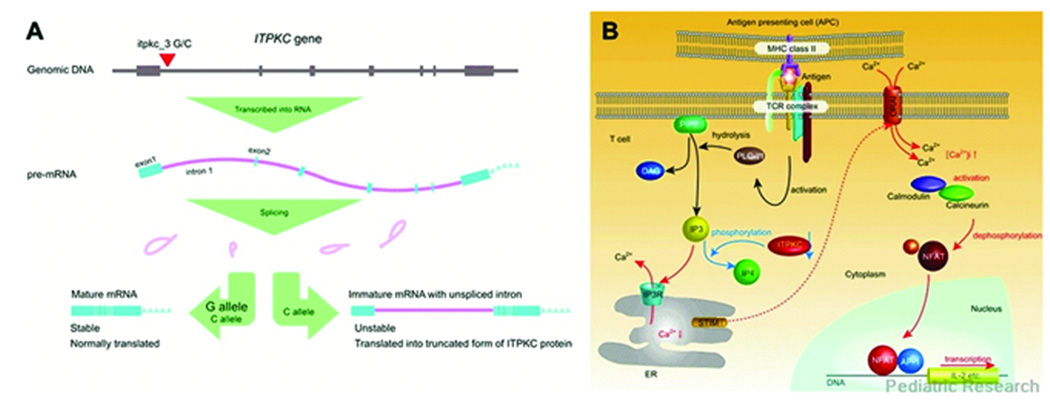
SNPs showing significant association with KD are beginning to be identified. One such SNP was found in the ITPKC gene, which plays an important role in signal transduction in T lymphocytes, particularly in the negative regulation of the Ca2+/nuclear factor of activated T-cells (NFAT) pathway (Figure 2B) (41). A polymorphism of itpkc_3 (C allele rather than G allele), conferring reduced expression of mature ITPKC mRNA by altering pre-mRNA splicing efficiency (Figure 2A), was associated with susceptibility to KD and with the development of coronary artery abnormalities in the Japanese population, and was associated with the development of coronary artery abnormalities in a U.S. cohort (42). Reduced activity of ITPKC in KD may result in increased T lymphocyte activation and an exaggerated inflammatory response in KD tissues. This gene was associated with an approximate doubling of KD risk. It is highly probable that other KD susceptibility genes will be identified in the near future from similar studies.
Figure 2.
Functional significance of itpkc_3 on ITPKC mRNA and Ca2+/NFAT pathway. (A) Effect of itpkc_3 C allele on splicing of ITPKC pre mRNA. The C allele of itpkc_3 reduces splicing efficiency of IPTKC premRNA. mRNAs harboring unspliced intron 1 cannot be translated properly and will be degraded early by nonsense-mediated decay mechanism. (B) Proposed role of ITPKC as a negative regulator of Ca2+/NFAT pathway. When the T-cell receptor (TCR) is bound by antigen/MHC complex on antigen presenting cells (APCs), adaptor molecules and kinases are recruited and phospholipse C-γ1 (PLC-γ1) is activated by phosphorylation of its tyrosine residue. IP3 and diacylglycerol (DAG), another second messenger molecule, are generated by hydrolysis of phosphatidylinositol 3,4-bisphosphate (PIP2) by activated PLC-γ1. IP3 binds to its receptor expressed on endoplasmic reticulum (ER) membrane and causes the release of Ca2+ into the cytoplasm. Then depletion of Ca2+ store in ER evokes a process termed as store operated Ca2+ entry in which extracellular Ca2+ enters through calcium release-activated Ca2+ channels on the plasma membrane. Recent advances in research identified the role of stromal interaction molecule (STIM) as a sensor of Ca2+ in ER and ORAI as a calcium release-activated Ca2+ channel. Cytoplasmic Ca2+ binds calmodulin, which in turn activates calcineurin, a calmodulin-dependent phosphatase. Activated calcineurin dephosphorylate NFAT in the cytoplasm and lead nuclear translocation of NFAT. NFAT in the nucleus drives transcription of genes important in T cell activation as a homodimer or heterodimer with other transcription factors. AP1 is one of the transcription partners of NFAT, which is activated by a signal from TCR mediated by DAG (72–74). Reactions and amounts of molecules increased by the effect of itpkc_3 C alleles were represented by red characters and arrows and those reduced by blue, respectively. [Ca2+]i: intracellular free Ca2+ concentration. Reprinted with permission from Onouchi Y. Molecular Genetics of Kawasaki Disease. Pediatric Research 65(5 Part 2):46R–54R, 2009.
A Genome-Wide Association Study in a Caucasian population
A genome-wide association study in Caucasian populations identified eight susceptibility loci (43), all different than those reported in the Japanese population (40). Five genes, calcium/calmodulin-dependent protein kinase (CaM Kinase) II delta, CAMK2D; CUB and Sushi multiple domains 1, CSMD1; ligand of numb-protein X1, LNX1; N-acetylated alpha-linked acidic dipeptidase-like 2, NAALADL2; and t-complex 1, TCP1; were shown to be associated with KD and had decreased transcript abundance in the acute phase of illness. Three of these genes have functional relationships that may be relevant for inflammation, apopotosis, and cardiovascular pathology (43). That this study did not identify ITPKC or other previously reported candidate gene associations such as interleukin 4 (IL4) (44), vascular endothelial growth factor A (VEGFA) (45), chemokine receptor 5 (CCR5) (46), or mannose-binding lectin (protein C) 2 (MBL2) (47) shows the difficulty of identifying multigenic disease susceptibility loci with confidence in the absence of extremely large sample sizes. Further study is necessary to confirm the associations of these genes with KD susceptibility in the Caucasian population.
Conclusions
This is an exciting time in KD research. An investigation of oligoclonal IgA responses in acute KD has led to production of synthetic KD antibodies that identify ICI in KD tissues. Further investigation of the proteins and nucleic acids in KD ICI is likely to yield information about the etiologic agent(s) of the disease. The completion of the human genome project and advances in molecular genetics have led to new tools allowing a resurgence of research in defining genetic factors associated with KD susceptibility. ITPKC, a gene involved in negative regulation of T lymphocyte responses, is the first gene to be associated with the development of KD and with coronary artery aneurysm formation in these new studies. It is likely that additional genes conferring susceptibility to KD will be identified in the near future; identification of such genes could be key to the development of novel therapies for this potentially fatal disorder of childhood. Identification of the etiologic agent(s) is the best single means to allow for development of a diagnostic test, and could also allow for improved therapy and ultimate prevention of the illness.
Summary points
Clinical and epidemiologic features of KD support an infectious cause, and the epidemiology indicates a likely genetic susceptibility to the disease
The innate immune response is activated in acute KD, as demonstrated by production of many cytokines
The adaptive immune response is also activated in acute KD, manifested by oligoclonal, antigen-driven CD8 T lymphocyte and IgA and IgM B lymphocyte responses
Synthetic antibodies derived from oligoclonal lgA gene sequences identify antigen in acute KD tissues
KD antigen is localized to intracytoplasmic inclusion bodies in acute KD ciliated bronchial epithelium, and in a subset of macrophages in KD tissues
Intracytoplasmic inclusion bodies in acute KD appear consistent with aggregates of viral proteins and RNA
New molecular genetic studies of KD are ongoing, and are likely to lead to the discovery of many susceptibility genes
One newly identified KD susceptibility gene is ITPKC, a negative regulator of T lymphocyte activation; patients with KD may have reduced activity of ITPKC with exaggerated inflammatory responses
Future Issues
Define the genes conferring susceptibility to KD, and the genes that are associated with an increased risk of developing coronary artery aneurysms
Identification of KD susceptibility genes could lead to novel therapies for the disorder
Define the proteins and nucleic acids in KD ICI
Following identification of the specific etiology of KD, a diagnostic test, improved therapy, and ultimately, a vaccine can be developed
List of abbreviations
- KD
Kawasaki Disease
- ICI
intracytoplasmic inclusion bodies
- ITPKC
Inositol 1,4,5-triphosphate 3-kinase C
- SNP
single nucleotide polymorphism
Footnotes
Posted with permission from the Annual Review of Medicine, Volume 62© 2011 by Annual Reviews, http://www.annualreviews.org
References
- 1.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–347. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Sleeper LA, McCrindle BW, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356:663–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967;16:178–222. [PubMed] [Google Scholar]
- 4.Patriarca PA, Rogers MF, Morens DM, et al. Kawasaki syndrome: association with the application of rug shampoo. Lancet. 1982;2:578–580. doi: 10.1016/s0140-6736(82)90660-2. [DOI] [PubMed] [Google Scholar]
- 5.Ohga K, Yamanaka R, Kinumaki H, et al. Kawasaki disease and rug shampoo. Lancet. 1983;1:930. doi: 10.1016/s0140-6736(83)91358-2. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura Y, Yashiro M, Uehara R, et al. Increasing incidence of Kawasaki disease in Japan: nationwide survey. Pediatr Int. 2008;50:287–290. doi: 10.1111/j.1442-200X.2008.02572.x. [DOI] [PubMed] [Google Scholar]
- 7.Bell DM, Brink EW, Nitzkin JL, et al. Kawasaki syndrome: description of two outbreaks in the United States. N Engl J Med. 1981;304:1568–1575. doi: 10.1056/NEJM198106253042603. [DOI] [PubMed] [Google Scholar]
- 8.Dean AG, Melish ME, Hicks R, Palumbo NE. An epidemic of Kawasaki syndrome in Hawaii. J Pediatr. 1982;100:552–557. doi: 10.1016/S0022-3476(82)80751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason W, Jordan S, Sakai R, Takahashi M. Lack of effect of gamma-globulin infusion on circulating immune complexes in patients with Kawasaki syndrome. Pediatr Infect Dis J. 1988;7:94–99. doi: 10.1097/00006454-198802000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Rowley AH, Eckerley CA, Jack HM, et al. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997;159:5946–5955. [PubMed] [Google Scholar]
- 11.Holman RC, Belay ED, Christensen KY, et al. Hospitalizations for Kawasaki Syndrome Among Children in the United States, 1997–2007. Pediatr Infect Dis J. 2010 doi: 10.1097/INF.0b013e3181cf8705. [DOI] [PubMed] [Google Scholar]
- 12.Yanagawa H, Nakamura Y, Kawasaki T, Shigematsu I. Nationwide epidemic of Kawasaki disease in Japan during winter of 1985-86. Lancet. 1986;2:1138–1139. doi: 10.1016/s0140-6736(86)90541-6. [DOI] [PubMed] [Google Scholar]
- 13.Rowley AH, Baker SC, Orenstein JM, Shulman ST. Searching for the cause of Kawasaki disease--cytoplasmic inclusion bodies provide new insight. Nat Rev Microbiol. 2008;6:394–401. doi: 10.1038/nrmicro1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992;121:924–926. doi: 10.1016/s0022-3476(05)80343-9. [DOI] [PubMed] [Google Scholar]
- 15.Choi IH, Chwae YJ, Shim WS, et al. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997;159:481–486. [PubMed] [Google Scholar]
- 16.Rowley AH, Shulman ST, Spike BT, et al. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol. 2001;166:1334–1343. doi: 10.4049/jimmunol.166.2.1334. [DOI] [PubMed] [Google Scholar]
- 17.Lee HH, Shin JS, Kim DS. Immunoglobulin V(H) chain gene analysis of peripheral blood IgM-producing B cells in patients with Kawasaki disease. Yonsei Med J. 2009;50:493–504. doi: 10.3349/ymj.2009.50.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowley AH, Shulman ST, Garcia FL, et al. Cloning the arterial IgA antibody response during acute Kawasaki disease. J Immunol. 2005;175:8386–8391. doi: 10.4049/jimmunol.175.12.8386. [DOI] [PubMed] [Google Scholar]
- 19.Abe J, Kotzin BL, Jujo K, et al. Selective expansion of T cells expressing T-cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc Natl Acad Sci U S A. 1992;89:4066–4070. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abe J, Kotzin BL, Meissner C, et al. Characterization of T cell repertoire changes in acute Kawasaki disease. J Exp Med. 1993;177:791–796. doi: 10.1084/jem.177.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis N, Zheng R, Lamb JR, Levin M. Evidence for a superantigen mediated process in Kawasaki disease. Arch Dis Child. 1995;72:308–311. doi: 10.1136/adc.72.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietra BA, De Inocencio J, Giannini EH, Hirsch R. TCR V beta family repertoire and T cell activation markers in Kawasaki disease. J Immunol. 1994;153:1881–1888. [PubMed] [Google Scholar]
- 23.Sakaguchi M, Kato H, Nishiyori A, et al. Characterization of CD4+ T helper cells in patients with Kawasaki disease (KD): preferential production of tumour necrosis factor-alpha (TNF-alpha) by V beta 2- or V beta 8- CD4+ T helper cells. Clin Exp Immunol. 1995;99:276–282. doi: 10.1111/j.1365-2249.1995.tb05545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancia L, Wahlstrom J, Schiller B, et al. Characterization of the T-cell receptor V-beta repertoire in Kawasaki disease. Scand J Immunol. 1998;48:443–449. doi: 10.1046/j.1365-3083.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 25.Leung DY, Meissner HC, Fulton DR, et al. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet. 1993;342:1385–1388. doi: 10.1016/0140-6736(93)92752-f. [DOI] [PubMed] [Google Scholar]
- 26.Leung DY, Meissner HC, Shulman ST, et al. Prevalence of superantigen-secreting bacteria in patients with Kawasaki disease. J Pediatr. 2002;140:742–746. doi: 10.1067/mpd.2002.123664. [DOI] [PubMed] [Google Scholar]
- 27.Nagata S, Yamashiro Y, Ohtsuka Y, et al. Heat shock proteins and superantigenic properties of bacteria from the gastrointestinal tract of patients with Kawasaki disease. Immunology. 2009;128:511–520. doi: 10.1111/j.1365-2567.2009.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suenaga T, Suzuki H, Shibuta S, et al. Detection of multiple superantigen genes in stools of patients with Kawasaki disease. J Pediatr. 2009;155:266–270. doi: 10.1016/j.jpeds.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Rowley AH, Shulman ST, Mask CA, et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis. 2000;182:1183–1191. doi: 10.1086/315832. [DOI] [PubMed] [Google Scholar]
- 30.Brown TJ, Crawford SE, Cornwall ML, et al. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001;184:940–943. doi: 10.1086/323155. [DOI] [PubMed] [Google Scholar]
- 31.Rowley AH, Baker SC, Shulman ST, et al. Detection of antigen in bronchial epithelium and macrophages in acute Kawasaki disease by use of synthetic antibody. J Infect Dis. 2004;190:856–865. doi: 10.1086/422648. [DOI] [PubMed] [Google Scholar]
- 32. Rowley AH, Baker SC, Shulman ST, et al. Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis. 2005;192:1757–1766. doi: 10.1086/497171. This study demonstrates that synthetic antibodies prepared using prevalent IgA gene sequences in acute KD arterial tissue identify intracytoplasmic inclusion bodies that are consistent with aggregates of viral proteins and nucleic acids.
- 33.Yang ZP, Cummings PK, Patton DL, Kuo CC. Ultrastructural lung pathology of experimental Chlamydia pneumoniae pneumonitis in mice. J Infect Dis. 1994;170:464–467. doi: 10.1093/infdis/170.2.464. [DOI] [PubMed] [Google Scholar]
- 34. Rowley AH, Baker SC, Shulman ST, et al. RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS One. 2008;3:e1582. doi: 10.1371/journal.pone.0001582. In this study, KD ICI are demonstrated in late-stage KD fatalities, and DNA and RNA light microscopy stains reveal RNA in ICI, leading to the hypothesis of a persistent RNA virus as the etiologic agent of KD.
- 35. Onouchi Y. Molecular genetics of Kawasaki disease. Pediatr Res. 2009;65:46R–54R. doi: 10.1203/PDR.0b013e31819dba60. This review summarizes candidate gene and genome-wide association studies in KD.
- 36.Nakamura Y, Yashiro M, Uehara R, et al. Epidemiologic features of Kawasaki disease in Japan: results from the nationwide survey in 2005–2006. J Epidemiol. 2008;18:167–172. doi: 10.2188/jea.JE2008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holman RC, Curns AT, Belay ED, et al. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003;112:495–501. doi: 10.1542/peds.112.3.495. [DOI] [PubMed] [Google Scholar]
- 38.Fujita Y, Nakamura Y, Sakata K, et al. Kawasaki disease in families. Pediatrics. 1989;84:666–669. [PubMed] [Google Scholar]
- 39.Uehara R, Yashiro M, Nakamura Y, Yanagawa H. Kawasaki disease in parents and children. Acta Paediatr. 2003;92:694–697. doi: 10.1080/08035320310002768. [DOI] [PubMed] [Google Scholar]
- 40.Onouchi Y, Tamari M, Takahashi A, et al. A genomewide linkage analysis of Kawasaki disease: evidence for linkage to chromosome 12. J Hum Genet. 2007;52:179–190. doi: 10.1007/s10038-006-0092-3. [DOI] [PubMed] [Google Scholar]
- 41.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 42. Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. This study identifies the gene ITPKC as being associated with the risk of Kawasaki Disease in Japanese children, and with the risk of developing coronary artery abnormalities in U.S and Japanese children.
- 43.Burgner D, Davila S, Breunis WB, et al. A genome-wide association study identifies novel and functionally related susceptibility Loci for Kawasaki disease. PLoS Genet. 2009;5:e1000319. doi: 10.1371/journal.pgen.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns JC, Shimizu C, Shike H, et al. Family-based association analysis implicates IL-4 in susceptibility to Kawasaki disease. Genes Immun. 2005;6:438–444. doi: 10.1038/sj.gene.6364225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breunis WB, Biezeveld MH, Geissler J, et al. Vascular endothelial growth factor gene haplotypes in Kawasaki disease. Arthritis Rheum. 2006;54:1588–1594. doi: 10.1002/art.21811. [DOI] [PubMed] [Google Scholar]
- 46.Burns JC, Shimizu C, Gonzalez E, et al. Genetic variations in the receptor-ligand pair CCR5 and CCL3L1 are important determinants of susceptibility to Kawasaki disease. J Infect Dis. 2005;192:344–349. doi: 10.1086/430953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biezeveld MH, Kuipers IM, Geissler J, et al. Association of mannose-binding lectin genotype with cardiovascular abnormalities in Kawasaki disease. Lancet. 2003;361:1268–1270. doi: 10.1016/S0140-6736(03)12985-6. [DOI] [PubMed] [Google Scholar]