Abstract
Background
Enhanced external counterpulsation (EECP) is associated with improvement in endothelial function, angina and quality of life in patients with symptomatic coronary artery disease, although the mechanisms underlying the observed clinical benefits are not completely clear. The purpose of this study was to examine the effects of EECP on circulating haematopoietic progenitor cells (HPCs) and endothelial progenitor cells (EPCs) in patients with refractory angina. We compared HPC and EPC counts between patients scheduled for EECP and patients with normal angiographic coronary arteries, with and without coronary endothelial dysfunction. We hypothesized that an increase in circulating bone-marrow derived progenitor cells in response to EECP may be part of the mechanism of action of EECP.
Methods
Thirteen consecutive patients scheduled to receive EECP treatment were prospectively enrolled. Clinical characteristics were recorded and venous blood (5ml) was drawn on day 1, day 17, day 35 (final session) and one month post completion of EECP therapy. Buffy coat was extracted and HPCs and EPCs were counted by flow cytometry.
Results
Median Canadian Cardiovascular Society (CCS) angina class decreased and Duke Activity Status Index (DASI) functional score increased significantly (both, p<0.05) in response to EECP, an effect that was maintained at one month after termination of treatment. Flow cytometric analysis revealed an accompanying significant increase in CD34+, CD133+ and CD34+, CD133+ CPC counts over the course of treatment (p<0.05). DASI scores correlated significantly with CD34+ (R=0.38 p=0.02), CD133+ (R=0.5, p=0.006) and CD34+, CD133+ (R= 0.47, p=0.01) CPC counts.
Conclusion
This study shows that HPCs, but not EPCs are significantly increased in response to EECP treatment and correlate with reproducible measures of clinical improvement. These findings are the first to link the functional improvement observed with EECP treatment with increased circulating progenitor cells.
Background
Enhanced external counterpulsation (EECP) therapy is associated with improvements in myocardial ischaemia, angina, nitrate use, 1 exercise tolerance, 1–3 and quality of life 4, 5 in refractory angina patients who fail to respond to conventional revascularization and aggressive anti-anginal medication. Furthermore, the report from the International EECP Patient Registry showed that EECP treatment decreased angina episodes and improved quality of life even in patients with severe left ventricular (LV) dysfunction (ejection fraction < or = 35%). 6 These beneficial effects occur early after initiation of therapy and are sustained in many patients up to 5 years later. 7, 8
EECP is a noninvasive treatment for angina that uses the sequential inflation and deflation of lower extremity pneumatic cuffs to reduce left ventricular afterload and augment diastolic flow and coronary perfusion pressure. While the haemodynamic effects are well-established, 9 the exact mechanisms by which EECP exerts its beneficial clinical effects are unresolved. Improvement in the peripheral endothelial function of intractable angina patients as measured by reactive hyperemia-peripheral arterial tonometry after EECP has been suggested as a mechanism of action of EECP. 10 Tao and colleagues have demonstrated that EECP improves endothelium-dependent vasorelaxation in the carotid arteries of hypercholesterolemic pigs. 11 The pathways by which EECP can improve endothelial function and improve symptoms of angina are poorly understood.
We have recently demonstrated a reduction in circulating haematopoietic progenitor cells (HPCs), but not in endothelial precursor cells (EPCs) as currently defined, in the syndrome of coronary endothelial dysfunction.12 We hypothesised that EECP, owing to its documented beneficial effects on coronary endothelial function, would have an effect on specific circulating CD34+ subsets. Therefore, circulating CD34+ cells expressing CD45 (dim) and without evidence of CD45 expression were analyzed. As discussed previously, these subtypes are in keeping with HPCs and putative EPCs respectively. 13, 14
Methods
Patients
Thirteen consecutive patients with chronic stable angina referred for EECP treatment were enrolled in the study. The study was approved by the Institutional Review Board of the Mayo Clinic, Rochester, Minnesota, USA, and written informed consent was obtained from all patients. All patients were symptomatic of refractory angina, had several prior cardiac events and interventions, including coronary artery bypass grafting and percutaneous coronary interventions, and were considered unsuitable for further conventional percutaneous or surgical revascularization by at least two senior interventional cardiologists (table 1).
Table 1.
Baseline characteristics of study cohort.
| Patient Cohort | Percentage | Number (n=13) |
|---|---|---|
| Male | 100% | 13 |
| Age > 65 years | 85% | 11 |
| BMI > 25 | 85% | 11 |
| LVEF < 35% | 31% | 4 |
| Prior CABG | 85% | 11 |
| Prior Redo-CABG | 23% | 3 |
| Prior PCI | 62% | 8 |
| More than 1 Prior PCI | 46% | 6 |
| Prior EECP | 8% | 1 |
| Prior TMR | 8% | 1 |
| Type 1 Diabetes | 15% | 2 |
| Type 2 Diabetes | 31% | 4 |
| Hypertension | 92% | 12 |
| Hyperlipidemia | 100% | 13 |
| Current smoker | 0% | 0 |
| Ex smoker | 69% | 9 |
| CRF (on dialysis) | 15% | 2 |
| PAD | 62% | 8 |
| Cerebrovascular disease | 31% | 4 |
The patients were referred for EECP treatment because they had a chronic condition characterized by the presence of severe, Canadian Cardiovascular Society (CCS) class III or IV angina caused by myocardial ischaemia in the presence of angiographic multivessel native coronary artery disease (CAD) that could not be controlled by a combination of optimal tolerated medical therapy (table 2), angioplasty/stent, and/or coronary artery bypass surgery. Cardiovascular medications remained unchanged during the 7-week course of EECP treatment.
Table 2.
Cardiovascular pharmacotherapy for study cohort.
| Patient cohort | Percentage | Number (n=13) |
|---|---|---|
| Aspirin | 100% | 13 |
| Clopidogrel | 31% | 4 |
| Lipid lowering therapy | 100% | 13 |
| Long acting nitrate | 100% | 13 |
| Calcium channel antagonist | 85% | 11 |
| Diuretic | 62% | 8 |
| ACE Inhibitor | 46% | 6 |
| ARB | 46% | 6 |
| L-arginine | 23% | 3 |
| Beta blocker | 85% | 11 |
Exclusion criteria included acute unstable angina, large aortic aneurysm, severe aortic valvular insufficiency, markedly irregular heart rhythm, hypertrophic cardiomyopathy, overt cardiac failure, severe uncontrolled hypertension (systolic pressure >170 mm Hg or diastolic pressure >100 mm Hg), or severe peripheral vascular disease. Patients were instructed to abstain from eating, smoking, and drinking caffeinated beverages at least 2 h before each EECP session.
Patients were interviewed before each session to obtain information on severity and number of angina episodes, amount of nitroglycerin used, and general cardiovascular function. Each patient’s functional capacity was estimated at baseline, at treatment midpoint and after the full course of EECP using the Duke Activity Status Index (DASI). The DASI is a simple, easily administered, 12-item quality-of-life instrument that provides a patient’s self-assessment of their functional capabilities. Original development of the DASI was correlated and validated to estimate maximal oxygen consumption measurements at peak exercise. 15 Functional capacity is assessed based on the estimated peak oxygen uptake, as determined by the following equation:
This total score can be divided by 3.5 to estimate metabolic equivalent tasks (METs).
The control group consisted of 19 patients evaluated for chest pain by cardiac catheterization, but without findings of significant obstructive disease. These patients all underwent invasive assessment of coronary endothelial function by intracoronary acetylcholine challenge testing as described previously, 16, 17 and coronary endothelial function was found to be abnormal in 12 patients and normal in 7. Cell counts were analyzed from buffy coat in an identical manner to that described for the EECP patient cohort and were compared to circulating progenitor cell in this group.
EECP Therapy
All patients were treated with 35 hours of EECP divided into 1-hour daily treatments over a period of 7 to 8 weeks. The EECP device (Luminair, Vasomedical Inc., NY, USA) is composed of an air compressor, a computer module, a set of cuffs, and a treatment table. For each treatment, cuffs were wrapped around the calves and lower and upper thighs (including the buttocks) of the patient. Cuffs were connected by air hoses to the air-compressor unit. The EECP device inflates the cuffs with air and then deflates them in a sequence that is synchronized to the patient’s cardiac cycle. Pressure is applied sequentially from the calves to the buttocks, starting in early diastole. At the end of diastole, the compressed air is released rapidly from the cuffs to remove the externally applied pressure. EECP was performed at external cuff pressures of 0.35 to 0.40 kg/cm2. Assessment of acute diastolic pressure augmentation during EECP was monitored using conventional finger plethysmography.
Flow Cytometry
Mononuclear cells were extracted using density gradient centrifugation and then analysed by flow cytometry as previously described for CD34+ CD45dim VEGFR2− cells (HPCs) and CD34+ CD45− VEGFR+ cells (EPCs). 12
Statistical Methods
All data were stored and analyzed using JMP software. Data are presented as mean value and standard deviation for continuous variables. Comparisons between the baseline (before the first session) indices of functional capacity (DASI score, peak VO2 and total METS achieved) and those after EECP treatment (before the 35th session) were assessed using a paired 2-tailed Student t test for paired observations. A Kruskal-Wallis test was used to analyze progenitor cell counts at four time points. Correlations between cell counts and indices of functional capacity were performed using a sum of least squares regression analysis. A p value of <0.05 was considered to be significant.
Results
Thirteen patients with advanced symptomatic coronary artery disease were prospectively studied (table 1). The patient cohort was entirely of male gender with a mean age of 71 years (range 64–84 years). Patients had a long history of CAD, most had undergone multiple percutaneous coronary interventions, and the majority had undergone coronary artery bypass grafting. The patients had extensive comorbidites including hypertension, hyperlipidaemia, chronic renal impairment (15% were dialysis dependent), diabetes mellitus and peripheral arterial disease. All patients had refractory CCS class III and IV angina and were not felt to be candidates for further percutaneous or surgical revascularisation as determined by at least two senior operators. The majority of patients were taking a combination of up to 5 anti-anginal medications (table 2). All thirteen patients completed the entire EECP treatment, which lasted for 7 to 8 weeks (35 1-hour sessions) and experienced no serious cardiovascular events.
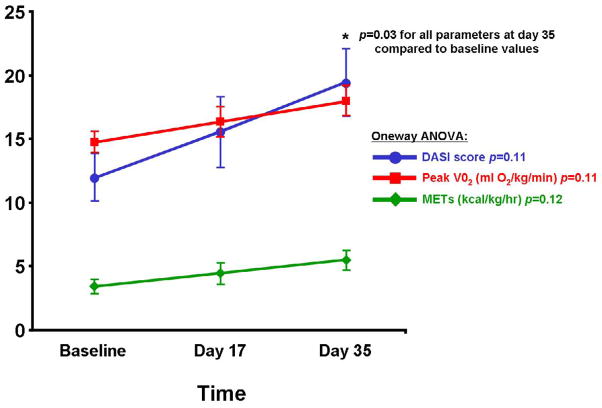
Thirty-five EECP sessions were associated with an improvement of one CCS class in 8 (62%) patients and by two CCS classes in 2 (15%) patients, whereas 3 (23%) patients demonstrated no change in CCS class at the end of EECP treatment. Median CCS class decreased significantly in response to EECP, an effect that was maintained at one month after termination of treatment. The mean DASI score, a measure of functional status, also increased significantly with EECP treatment (baseline 12±2 vs. 19±3 at 35 days), with a corresponding increase in derived maximum VO2 (14.7±0.8 vs. 18.0±1.1) and metabolic equivalents or METs (3.4±0.5 vs. 5.5± 0.7), p=0.03 comparing 35 day values to baseline for all parameters (figure 1).
Figure 1. DASI Scores, O2 Uptake estimate and METS in response to EECP.
Data are displayed at baseline, at 17 days and at 35 days of treatment. Statistical analysis was performed using one-way ANOVA analysis and also between baseline and 35day values by Student t-test.
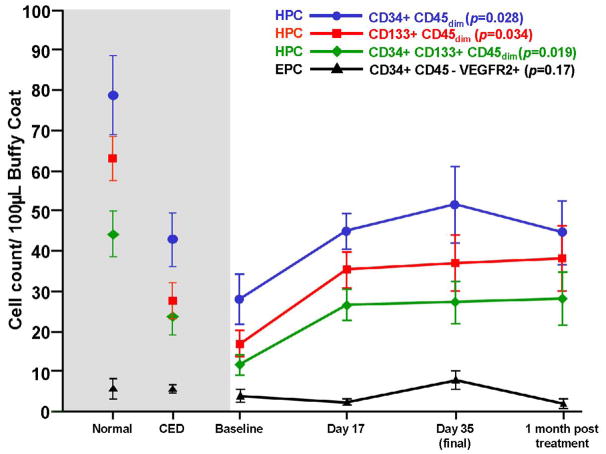
One-way ANOVA analysis of circulating progenitor cell counts measured by flow cytometry revealed a significant increase in CD34+45dim (p=0.03), CD133+45dim (p=0.03) and CD34+, CD133+, CD45dim cells (p=0.02) over the course of treatment (figure 2). These cells are in keeping with current definitions of circulating HPCs. [13] This finding was not observed in the case of CD34+ CD45− VEGFR2+ cells (figure 2) which are consistent with current definitions of EPCs.[14] Cell counts from the seven patients with normal coronary endothelial function, and twelve patients with abnormal coronary endothelial function at the time of invasive coronary hemodynamic assessment are also shown. The CD34+45dim, CD133+45dim and CD34+, CD133+, CD45dim cell counts (HPCs) of patients with both normal and abnormal coronary endothelial function, but no obstructive coronary disease differed significantly from patients recruited for EECP therapy before treatment was commenced (p=0.02). However by day 17 of EECP therapy, the differences in these cell counts were no longer apparent between EECP patients and patients with coronary endothelial dysfunction but no obstructive coronary disease. These findings were also observed at day 35 of EECP therapy and one month after EECP therapy was discontinued.
Figure 2. Effect of EECP on CPC counts.
CD34+, CD133+ and CD34, CD133 double positive cells at baseline, 17 days, 35 days and 1 month post treatment are shown (one-way ANOVA, Kruskal-Wallis test). Comparisons between baseline values and those at the other time points are also shown (Wilcoxon test). Historical control cell counts are shown to the left (shaded area) (Ref 12).
CD34+, CD45−, VEGFR2+ cell counts (putative EPCs) did not change significantly over the course of EECP therapy (p=0.17), and did not differ significantly from those found in patients with normal or abnormal coronary endothelial function and without significant obstructive coronary disease.
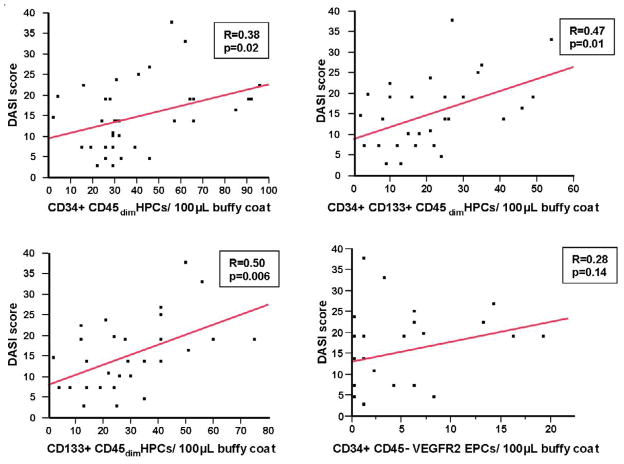
The DASI scores and the derived VO2 and METs values also correlated significantly with the CD34+45dim HPC cell count (R=0.38 p=0.02), CD133+45dim HPC cell count (R=0.5, p=0.006) and CD34+, CD133+, CD45dim HPC cell count (R= 0.47, p=0.01 ) on bivariate analysis. No significant correlation was observed between CD34+, CD45−,VEGFR2+ EPC count and DASI score (R=0.28, p=0.14) (Figure 3).
Figure 3. Bivariate analysis of CPC counts and DASI scores.
Correlations between CD34+, CD133+ and CD34+,CD133+ circulating progenitor cells are shown between DASI scores for all 13 patients at all timepoints. Correlations for the derived VO2 and METs estimates were identical to that for each cell type with the DASI score.
Discussion
Since the results of the first double-blind randomized placebo-controlled multicenter trial were published (MUST-EECP 18), EECP therapy has emerged as an effective, noninvasive, and durable therapeutic option for patients with angina and the American Heart Association recommends it as a Class IIb (Level of Evidence: B) intervention for treatment of refractory angina pectoris. 19, 20 The mechanism of action and the overall effects of EECP therapy have not been fully elucidated.
Development of new functional collateral vessels by increasing plasma nitric oxide (NO) and decreasing endothelin-1 levels to the ischemic myocardium has been postulated as a mechanism of action for EECP therapy. EECP has been shown to augment plasma nitrate/nitrite production, an indirect measure of plasma NO production, to cause down-regulation of endothelin-1 levels and indeed these findings can be sustained through the course of EECP therapy. 18
EECP therapy has demonstrated improvement in endothelial release of nitric oxide resulting in improved endothelial function 18. The mechanism of how endothelial improvement is brought about in these patients is uncertain and various theories include involvement of the NF-kappa signaling pathways, an increase in cyclic guanosine monophosphate (cGMP) 21 or an increase in vascular endothelial growth factor levels. 22
In our study we found that in patients with refractory angina undergoing EECP therapy, FACS (Flow cytometric analysis) revealed an increase in HPC counts over the course of treatment, which was statistically significant for all subtypes analyzed i.e. CD34+ CD45dim, CD133+ CD45dim and CD34 CD133+ CD45dim HPCs by one-way ANOVA (p<0.05). This increase in HPC counts remained sustained for one month after the final EECP session, and the associated clinical benefits in these patients is analogous to findings of Bonetti and colleagues, who showed that clinical and endothelial function improvement persisted at one month after EECP treatment. 10
Previous studies have shown that patients with refractory angina and multiple risk factors, as in our study cohort, have very low circulating levels of endothelial progenitor cells. 23 Patients with low circulating progenitor cell counts have a higher incidence of cardiovascular events compared to patients with higher counts. Recent studies have shown that the number of circulating progenitor cells predict severe endothelial dysfunction independent of classical cardiovascular risk factors.12 24 Our patient cohort also demonstrated a significant and sustained improvement in DASI scores during the EECP treatment period which correlated significantly with the HPC counts. Exercise alone has been shown to increase circulating circulating progenitor cell counts in healthy young patients but interestingly, older patients with peripheral arterial disease appear to be unable to mount a significant increase in circulating progenitors. 25 It is logical to surmise that EECP and an associated improvement in functional status leading to greater exercise capacity, together may have resulted in the significant increase in HPCs demonstrated in our patient population.
This study again demonstrates a difference in effect on circulating CD34+ subsets, in this case as a response to EECP therapy. HPC counts (CD45dim subset) became significantly elevated from baseline early in the course of treatment and this effect was maintained for the remainder of the treatment course and even one month afterwards. HPC counts also correlated with DASI scores in these patients, which also improved significantly from baseline and from which estimates of METs and peak VO2 may be derived. 15 This was not the case with cells fitting the description of the putative EPC (CD45−).
The restriction of this association to circulating haematopoeitic progenitor cell (HPC) is intruiging as these CD45dim cells do not form endothelial cells in culture - they are distinctly haematopoietic in nature. 13, 14 Previous evidence has demonstrated the production of numerous growth factors including angiopoietins and VEGF by HPCs in vitro, growth factors integral in angiogenesis.26–28 Co-culture experiments have shown enhanced in-vitro angioblast cell growth in the presence of CD34+ haematopoietic progenitor cells. 29 In addition, there is a key interplay between endothelial cells and hematopoietic progenitors in the bone marrow. 30–32 It is therefore likely that circulating hematopoietic progenitors play a key role in endogenous vascular and cardiac repair. Their depletion in numbers could conceivably result in downstream deleterious effects on endogenous self-renewal in cardiovascular disease. The paracrine effects of HPCs on resident endothelial cells and progenitors via production of angiopoietins and other cytokines may represent a potential mechanism for the relationship between circulating progenitor cells of haematopoietic lineage and observed cardioprotective effects. 27
We therefore propose that EECP, through mechanisms as yet unclear, induces increased production of HPCs by the bone marrow or decreased senescence of HPCs in the circulation, with a resultant enhanced paracrine effect on resident progenitor cells in the vascular beds and promotion of endogenous self repair.
Study limitations
This was a single centre study in a small cohort of patients without a preliminary sample size calculation which limits statistical power. However, complete follow-up was available in all patients in this prospective study and the findings of our study are indeed novel with respect to providing an insight into the mechanisms by which EECP achieves its effects.
Conclusions
This is the first prospective study of progenitor cells in vascular biology to suggest an association between functional measures of improvement post EECP therapy and circulating progenitor cell counts in patients with refractory angina pectoris. These findings are additive to our emerging understanding of the underlying mechanisms of the pro-angiogenic effects of EECP.
Acknowledgments
Special thanks to James E. Tarara and staff at the Mayo Clinic Flow Cytometry Core Facility personnel for their assistance with processing the samples and to Megan E. Crouch for her secretarial support.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology (25).
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arora RR, Chou TM, Jain D, et al. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–40. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 2.Lawson WE, Hui JC, Zheng ZS, et al. Improved exercise tolerance following enhanced external counterpulsation: cardiac or peripheral effect? Cardiology. 1996;87:271–5. doi: 10.1159/000177103. [DOI] [PubMed] [Google Scholar]
- 3.Urano H, Ikeda H, Ueno T, et al. Enhanced external counterpulsation improves exercise tolerance, reduces exercise-induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. J Am Coll Cardiol. 2001;37:93–9. doi: 10.1016/s0735-1097(00)01095-0. [DOI] [PubMed] [Google Scholar]
- 4.Springer S, Fife A, Lawson W, et al. Psychosocial effects of enhanced external counterpulsation in the angina patient: a second study. Psychosomatics. 2001;42:124–32. doi: 10.1176/appi.psy.42.2.124. [DOI] [PubMed] [Google Scholar]
- 5.Arora RR, Chou TM, Jain D, et al. Effects of enhanced external counterpulsation on Health-Related Quality of Life continue 12 months after treatment: a substudy of the Multicenter Study of Enhanced External Counterpulsation. J Investig Med. 2002;50:25–32. doi: 10.2310/6650.2002.33514. [DOI] [PubMed] [Google Scholar]
- 6.Soran O, Kennard ED, Kfoury AG, et al. Two-year clinical outcomes after enhanced external counterpulsation (EECP) therapy in patients with refractory angina pectoris and left ventricular dysfunction (report from The International EECP Patient Registry) Am J Cardiol. 2006;97:17–20. doi: 10.1016/j.amjcard.2005.07.122. [DOI] [PubMed] [Google Scholar]
- 7.Lawson WE, Hui JC, Cohn PF. Long-term prognosis of patients with angina treated with enhanced external counterpulsation: five-year follow-up study. Clin Cardiol. 2000;23:254–8. doi: 10.1002/clc.4960230406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson WE, Hui JC, Kennard ED, et al. Predictors of benefit in angina patients one year after completing enhanced external counterpulsation: initial responders to treatment versus nonresponders. Cardiology. 2005;103:201–6. doi: 10.1159/000085170. [DOI] [PubMed] [Google Scholar]
- 9.Michaels AD, Accad M, Ports TA, et al. Left ventricular systolic unloading and augmentation of intracoronary pressure and Doppler flow during enhanced external counterpulsation. Circulation. 2002;106:1237–42. doi: 10.1161/01.cir.0000028336.95629.b0. [DOI] [PubMed] [Google Scholar]
- 10.Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–8. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 11.Tao J, Tu C, Yang Z, et al. Enhanced external counterpulsation improves endothelium-dependent vasorelaxation in the carotid arteries of hypercholesterolemic pigs. Int J Cardiol. 2006;112:269–74. doi: 10.1016/j.ijcard.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 12.Boilson BA, Kiernan TJ, Harbuzariu A, et al. Circulating CD34+ cell subsets in patients with coronary endothelial dysfunction. Nat Clin Pract Cardiovasc Med. 2008;5:489–96. doi: 10.1038/ncpcardio1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutherland DR, Anderson L, Keeney M, et al. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5:213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 14.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27:1572–9. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 15.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989;64:651–4. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 16.Hasdai D, Gibbons RJ, Holmes DR, Jr, et al. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–5. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 17.Suwaidi JA, Hamasaki S, Higano ST, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 18.Tseng H, Peterson TE, Berk BC. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ Res. 1995;77:869–78. doi: 10.1161/01.res.77.5.869. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons RJ, Abrams J, Chatterjee K, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Chronic Stable Angina) Circulation. 2003;107:149–58. doi: 10.1161/01.cir.0000047041.66447.29. [DOI] [PubMed] [Google Scholar]
- 20.Yang EH, Barsness GW. Evolving treatment strategies for chronic refractory angina. Expert Opin Pharmacother. 2006;7:259–66. doi: 10.1517/14656566.7.3.259. [DOI] [PubMed] [Google Scholar]
- 21.Levenson J, Pernollet MG, Iliou MC, et al. Cyclic GMP release by acute enhanced external counterpulsation. Am J Hypertens. 2006;19:867–72. doi: 10.1016/j.amjhyper.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Arora R, Chen HJ, Rabbani L. Effects of enhanced counterpulsation on vascular cell release of coagulation factors. Heart Lung. 2005;34:252–6. doi: 10.1016/j.hrtlng.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 24.Werner N, Wassmann S, Ahlers P, et al. Endothelial progenitor cells correlate with endothelial function in patients with coronary artery disease. Basic Res Cardiol. 2007;102:565–71. doi: 10.1007/s00395-007-0680-1. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer RG, Greene S, Arshi A, et al. Effect of acute exercise on endothelial progenitor cells in patients with peripheral arterial disease. Vasc Med. 2006;11:219–26. doi: 10.1177/1358863x06072213. [DOI] [PubMed] [Google Scholar]
- 26.Bautz F, Rafii S, Kanz L, et al. Expression and secretion of vascular endothelial growth factor-A by cytokine-stimulated hematopoietic progenitor cells. Possible role in the hematopoietic microenvironment. Exp Hematol. 2000;28:700–6. doi: 10.1016/s0301-472x(00)00168-5. [DOI] [PubMed] [Google Scholar]
- 27.Hildbrand P, Cirulli V, Prinsen RC, et al. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;104:2010–9. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- 28.Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–85. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 29.Harraz M, Jiao C, Hanlon HD, et al. CD34-blood -derived human endothelial cell progenitors. Stem Cells. 2001;19:304–12. doi: 10.1634/stemcells.19-4-304. [DOI] [PubMed] [Google Scholar]
- 30.Mohle R, Moore MA, Nachman RL, et al. Transendothelial migration of CD34+ and mature hematopoietic cells: an in vitro study using a human bone marrow endothelial cell line. Blood. 1997;89:72–80. [PubMed] [Google Scholar]
- 31.Rafii S, Shapiro F, Pettengell R, et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood. 1995;86:3353–63. [PubMed] [Google Scholar]
- 32.Rafii S, Shapiro F, Rimarachin J, et al. Isolation and characterization of human bone marrow microvascular endothelial cells: hematopoietic progenitor cell adhesion. Blood. 1994;84:10–9. [PubMed] [Google Scholar]