Abstract
The biological mechanisms of human social behavior are complex. Animal models may facilitate the understanding of these mechanisms and may help one to develop treatment strategies for abnormal human social behavior, a core symptom in numerous clinical conditions. The zebrafish is perhaps the most social vertebrate among commonly used laboratory species. Given its practical features and the numerous genetic tools developed for it, it should be a promising tool. Zebrafish shoal, i.e. form tight multimember groups, but the ontogenesis of this behavior has not been described. Analyzing the development of shoaling is a step towards discovering the mechanisms of this behavior. Here we study age-dependent changes of shoaling in zebrafish from day 7 post fertilization to over 5 months of age by measuring the distance between all pairs of fish in freely swimming groups of ten subjects. Our longitudinal (repeated measure within subject) and cross sectional (non-repeated measure between subject) analyses both demonstrated a significant increase of shoaling with age (decreased distance between shoal members). Given the sophisticated genetic and developmental biology methods already available for zebrafish, we argue that our behavioral results open a new avenue towards the understanding of the development of vertebrate social behavior and of its mechanisms and abnormalities.
Introduction
The zebrafish has been enjoying much popularity in embryology for the past three decades (for examples see Schweitzer & Driever, 2009; Holder & Xu, 2008) thanks to this species’ transparent embryo and its numerous other practical features that make it an ideal laboratory model organism (e.g. Lin et al., 2009). Indeed, the zebrafish has been employed as a model for a variety of human diseases including cancer (Stoletoy & Lemke, 2008), movement (Flinn et al., 2008) and sleep disorders (Zimmerman et al., 2008) among other conditions (for a review see Lieschke & Currie, 2007).
As increasing number of genetic tools has become available for zebrafish (Patton & Zon, 2001; Keller & Murtha, 2004) the popularity of this species has grown in a variety of disciplines including behavioral neuroscience (Sison et al., 2006). However, unlike the body of knowledge available on embryonic development and genetics of zebrafish, the behavior of this species is still poorly characterized. This is a crucial drawback because behavioral analysis has the potential to reveal a variety of functional changes in the brain and has been argued to be an important screening method in forward genetics as well as pharmacology (Gerlai, 2002). Nevertheless, most recently numerous studies have appeared that demonstrated the utility of zebrafish behavioral analysis in the investigation of vertebrate brain function (Gerlai, 2010; Egan et al., 2009). Some of these recent papers utilized the analysis of zebrafish’s social behavior (Gerlai et al., 2009; Miller & Gerlai, 2007; 2008;. Saverino & Gerlai, 2008; Speedie & Gerlai, 2008).
Social behavior is a complex phenomenon whose biological mechanisms and development are not well understood in vertebrates. Abnormal social behavior is a defining characteristic of a variety of human psychiatric and neurodevelopmental conditions including depression (Bell-Dolan & Peterson, 1993), anxiety disorders (Leibowitz et al., 1985), and the autism spectrum disorders (Reichow & Volkmar, 2010). The underlying mechanisms of the abnormal social behavior associated with these diseases are not well understood (Bartz & Hollander, 2006). Given the similarities between zebrafish and other vertebrates, including humans, in the layout of the brain (Tropepe & Sive, 2003), in the neurochemical properties of the brain (Zhdanova, 2006), and in characteristics of many other levels of biological organization (Egan et al., 2009), including nucleotide sequence of genes, the zebrafish is thought to be suitable as a model for investigating the biology and genetics of vertebrate brain functions (Gerlai, 2003). Briefly, findings from studies in zebrafish are expected to generalize well to humans and may shed insights on complex human conditions including the autism spectrum disorders (Tropepe & Sive, 2003).
The zebrafish is a shoaling fish, it aggregates, i.e. forms multimember groups in nature and in the laboratory. Shoaling is thought to provide the individual fish with multiple benefits, including access to mates, efficient foraging, and defense against predators (Griffiths et al., 2004; Ledesma & McRobert, 2008; Morrell & James, 2008). Although the adaptive nature of shoaling is well documented, the biological mechanisms and the development of this behavior are far from understood. In the current paper, we focus on the latter: we investigate, for the first time, whether shoaling develops, i.e. whether it changes with age, in zebrafish. Our prior personal observations suggested that newly hatched zebrafish disperse while adult zebrafish have been documented to exhibit robust shoaling, a strong preference for staying close to conspecifics (Al-Imari & Gerlai, 2008; Miller & Gerlai, 2007; 2008; Saverino & Gerlai, 2008). A previous study investigating the effects of kin exposure on preference for conspecifics in zebrafish revealed an imprinting-like effect of olfactory cues at an early age of the fish (6 days post fertilization, dpf) and suggested that some preference for conspecifics already exists at this stage of development (Gerlach et al., 2007). Preference for conspecifics was also demonstrated to be based solely upon visual cues in another study (Engeszer et al., 2007), which found measurable preference for conspecifics at postflexion stage (about 12 dpf) of zebrafish. However, it is not known whether the preference responses quantified in the above studies represented shoaling or other type of responses (e.g. agonistic) because the subjects were tested singly and could not interact with the stimulus fish. Furthermore, the question whether shoaling changes with age, i.e. the developmental trajectory of shoaling itself, has not been investigated up till now.
To address the above questions, we analyzed age-dependent changes of shoaling behavior in freely moving groups of zebrafish. The main rationale for our study is as follows. If significant age-dependent (i.e. developmental) changes in shoaling behavior are identified, this discovery could open new research avenues for numerous investigations. For example, one could study the ecological/adaptive importance of developmental alteration of social behavior. Another important goal would be to investigate the mechanisms of the age-dependent changes in social behavior, a classical developmental biology question. Here we report behavioral findings showing a significant developmental change in shoaling in zebrafish and based on this we argue that zebrafish will be an excellent tool with which the mechanisms of vertebrate social behavior may be investigated.
Methods
Animals and Housing
In total, one thousand one hundred and ninety zebrafish (Danio rerio) of the AB strain were utilized for the three experiments outlined below. The fish were bred in-house and originated from progenitors obtained from the Zebrafish International Research Centre (ZIRC) (Eugene, Oregon). All experiments described below were approved by the University of Toronto Animal Care Committee. All fish used in this study were bred, raised and housed in the same environment. Gender could not visually be determined when testing commenced (at 7 days post fertilization). After completion of the experiments and after the subjects had reached maturity, the gender distribution within the shoals tested was determined to be 50% male 50% female.
Upon hatching, the animals were housed in groups of ten in 1l plastic aquaria. After five weeks post-fertilization the animals were transferred to 2.8l Plexiglas aquaria that were part of a recirculating filtration aquaculture rack system which had a mechanical, biological, and activated carbon (chemical) filter as well as a UV sterilizing unit (Aquaneering Inc. (San Diego, Ca, USA). Water was maintained at 27°C. The system water used on the rack as well as during the development and testing of the fish was reverse osmosis purified and was supplemented with 60mg/l Instant Ocean Sea Salt to achieve water chemistry appropriate for zebrafish.
Zebrafish were kept at a 12h light/12h dark cycle with lights on at 7am and off at 7pm. All fish were fed twice daily with Larval Artificial Plankton 100 (particle size below 100 μm, ZeiglerBros, Inc., Gardners, PA, USA) until two weeks post spawning, after which animals were fed twice daily with nauplii of brine shrimp (Artemia salina) until they were four weeks old. Older and adult fish were fed a 1:1 mixture of flake food (Tetramin Tropical fish flake food, Tetra Co, Melle, Germany) and powered spirulina (Jehmco Inc., Lambertville, NJ, USA).
Open Field Task
All zebrafish that were housed together were tested together, forming a given shoal. Each shoal was identified by an ID number and remained constant (same shoal members) throughout the experiments (the unit of statistical analysis here is the shoal, and the sample sizes (n) shown below represent the number of shoals tested). Each group (shoal) consisted of ten fish. The home tank was placed next to the testing arena for transfer. Fish were netted as a group (in most cases all 10 fish could be captured with one net, due to the size of the net and the holding tank) and immediately released in the center of the arena. Transfer (air time) from the holding tank to the arena was not more than 3 seconds. The fish were released simultaneously in the center of a square plexi-glass tank, the open field, and were allowed to explore the field freely. Each trial lasted six minutes and the behavior of fish during the trial was recorded with an overhead video camera (JVC Everio Hard Drive GZ-MG750BU). After the open field trial, the group was returned to its home tank. In experiments 1 and 2, the arena size was kept proportional to the body length of the growing fish (and thus constant for a particular age group), a practice recommended by others (Gallego & Heath, 1994; Masuda et al., 2003; Vogel, 2008). But in experiment 3, two separate age-groups of fish (30 and 60 day old) were tested in six different arena sizes each, and the order of use of different arena sizes was randomized. Behavioral testing was always conducted between 0900 and 1600 h.
Experiment 1: Longitudinal developmental analysis of shoaling
The purpose of this experiment was to investigate the trajectory of potential age-dependent changes of shoaling behavior in zebrafish. Nineteen groups, each consisting of ten fish, were utilized in this experiment. Each was tested at 7, 18, 26, 42, 49, 59, 66, 70, and 76 dpf. That is, the same groups of fish were followed throughout their development, a repeated measure design.
Apparatus
Distance traveled has been argued to be the function of the linear dimension of the fish, e.g. their body length, therefore arena sizes or movement parameters such as speed or total distance swum are usually normalized to the length of fish, i.e. expressed in body lengths (Hale, 1999). In zebrafish, speed has also been found to be positively and linearly correlated with body length (Kimmel et al., 1974) and thus we decided to keep the linear dimensions of our open field experimental tanks proportional to the body length of our developing fish. We employed open fields whose linear dimensions were 28× the average body length of the zebrafish tested in them. This ratio gave us sufficiently large tanks in which the fish were not physically forced to stay close to each other and thus any shoaling observed would be the result of social cohesion and not of the physical constraints of the environment. On the other hand, this tank to fish size ratio was sufficiently small so as to allow high quality video recording and analysis (large enough subjects on the video screen). For the youngest age group, the 3.2 mm long 5 dpf old fish, the youngest free swimming age, we constructed a 90 × 90 × 30 mm (width × length × depth) tank. All open field tanks employed for the older age groups reported here were proportional, i.e. scaled up versions of this tank. That is for the 7, 18, 26, 42, 49, 59, 66, 70 and 76 dpf fish we used open fields whose linear dimensions were 1.7, 2.4, 3.3, 4.5, 5.0, 5.5, 6.5, 6.9, and 7.5 times those of the smallest open field, respectively. The level of water in these open fields was kept at 90% of the depth of the tanks.
Experiment 2: Cross sectional analysis of age differences in shoaling
In the above described longitudinal developmental study shoaling responses could be influenced by repeated handling and exposure to the open field task. Depending on the salience of the stimulus or the context in which it is delivered, repeated exposure to stimuli may lead to habituation or sensitization even in simple invertebrates (Carew et al., 1971; Pinsker et al., 1970). In mammals repeated postnatal handling has been shown to lead to habituation (reduction) of stress induced anxiety later in life (e.g. Meerlo et al., 1999). Similarly, repeated exposure to the same object (e.g. Dere et al., 2007 and references therein) or environment (e.g. Gerlai & Roder, 1993 and references therein) has been shown to lead to habituation in mice. Unfortunately, whether handling and repeated exposure to testing would lead to habituation or sensitization in zebrafish, and under what circumstances, has not been systematically investigated, but our personal observations suggested that inappropriate handling could easily lead to sensitization (increased fear responses) in zebrafish.. Similar observations have been published with other fish species suggesting fear or stress inducing effects of repeated handling (Pauker et al., 2005). Briefly, repeated handling induced elevation in fear could in principle lead to enhanced shoaling, i.e. tighter shoal cohesion. To address this potential confound, we conducted a non-repeated measure cross sectional experiment. We analyzed shoaling behavior of 7 different age groups of fish with “AGE” as the between subject factor, i.e. fish were tested in the open field only once. The order of testing fish of different ages was randomized. All other parameters (housing conditions, recording methods, size of open field, etc.) were identical to those of the longitudinal developmental study. The age groups corresponded closely to those tested in the longitudinal analysis but we also decided to include two older groups, a 121 dpf and a 173 dpf group. The open field sizes were as explained in the first experiment but for the two oldest groups we used larger tanks (to keep the linear dimensions of these tanks consistently 28 times the body length of the subjects), so the dimensions of the tanks were 8.4 and 10.6 times of the smallest open filed (described above).
Experiment 3: Randomization of open field size
In both the longitudinal developmental and the cross sectional age effect analyses the size of the open field was proportional to body size of the tested fish. However, one could argue that perhaps the absolute size of the open field itself could drive the observed behavioral changes. For example, a larger open area may induce elevated fear as fish in this environment may be more vulnerable to areal predation and the increased fear may lead to tighter shoals, a typical antipredatory response in zebrafish (Miller & Gerlai, 2007). The purpose of the third experiment was to address this question, i.e. to investigate whether exposure to different sizes of open fields itself alters shoaling within the same age-group of fish. Naive groups of fish were exposed to different tank sizes as described in experiment 1, but instead of increasing tank sizes over time, exposure to the different arena sizes was randomized. As in experiment 1, a repeated measure design was used to expose the same groups of fish to different tank sizes. Two age groups of fish were tested, 30 dpf (juvenile) and 60 dpf (adult).
Apparatus
For the fish that were 30 dpf at the time of testing, we used one arena that was identical in size to what was employed in experiment 1 (M: 150 × 150 × 50 mm, length × width × depth), referred to as the Medium sized tank. We also tested two smaller (XS=100 × 100 × 33 mm, and S=120 × 120 × 40 mm) and two larger (L=200 × 200 × 66 mm, and XL=250 × 250 × 83 mm) arenas. For the groups tested at their age of 60 dpf again we used an arena whose size corresponded to what was used in experiment 1 (M=450 × 450 × 83 mm) and we also tested two smaller (XS=300 × 300 × 100 mm, and S=400 × 400 × 133 mm) and two larger arena sizes (L=500 × 500 × 167 mm, and XL=600 × 600 × 200 mm). The linear dimensions of the medium sized arenas (M) for both age groups were 28 times the body length of the corresponding age group fish as employed before, however the extra small (XS) was 18 times, the small (S) 22 times, the large (L) was 36 times and the extra large (XL) was 45 times the body length of the corresponding age group fish.
Procedure
For each age cohort we tested ten groups of zebrafish with each group (shoal) containing ten individuals. Each of these shoals was housed separately, i.e. the holding density was ten fish per 3-liter tank. Once every two days each group was subjected to the open field task, a repeated measure design, as outlined below. The group of ten zebrafish was released from the center of the arena and the fish were allowed to freely explore the arena for six minutes. After the trial, the fish were returned to their home tank.
Behavioral recording and quantification
All sessions were recorded with a JVC HDD (JVC Everio Hard Drive GZ-MG750BU) overhead camera. Recorded digital files were converted to AVI format using Cyberlink Powerdirector. Still images were obtained for every 5 sec of the complete duration of the 6 minute recorded trials. From each of the still images, the distances between a given fish from all other fish were measured and averaged for that given fish. Thus for a ten member shoal we obtained ten averages (one for each fish) and calculated the mean of the averages (the inter-individual distance) and its standard error, values that characterized the shoal itself. The quantification was performed using a custom software application developed in-house described in detail elsewhere (Miller & Gerlai, 2007). The software loaded the video file recorded, and a human observer inputted the sampling rate (which was 0.2 Hz, i.e. one image every 5 sec as mentioned above). The software then provided the observer with a still image at the requested starting time point and once the recording of the location of fish was completed from that given still image it advanced to the next image by the preset amount of time (i.e. by 5 sec). Recording of the location of each fish on the image was achieved manually, i.e. the human observer located the fish on the image and clicked on it with the mouse. Although labor intensive, this method is very precise given that the human observer is unlikely to be confused as to what represents the fish on the image and thus recording errors were minimal. Prior to locating the fish, the observer calibrated the area of the testing arena by overlaying a square outline on the boundaries of the tank. By providing the software with the measurements of the testing arena, it could then calculate the distances between the fish from the coordinate points extracted from each location. These distances quantified were subsequently exported to a text file for statistical analysis as described above.
Results
Experiment 1: Longitudinal developmental analysis of shoaling
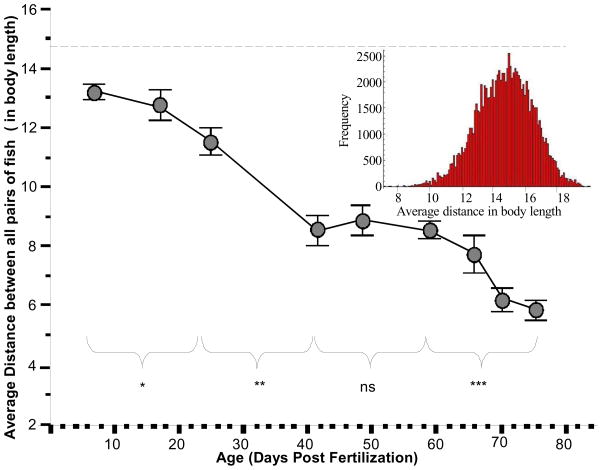
The longitudinal analysis of the development of shoaling in freely moving zebrafish suggested a robust change in social behavior. At the youngest age tested zebrafish appeared rather dispersed: at age 7 dpf they exhibited an average distance from each other (figure 1, first data point) that equaled 13.22 body lengths (SD = ± 1.009). Previously, the average distance among shoal members of zebrafish in 10 member shoals was estimated to be approximately 4 body lengths in adult fish (Miller & Gerlai, 2008). The value we obtained for the 7 dpf zebrafish was significantly above this value (t = 25.85, df = 7, p < 0.001). Does this large average distance observed in 7dpf fish represent random distribution, increased repulsion (inter-individual distances above random chance), or some cohesion (shoaling, i.e. inter-individual distances below random chance)?
Fig. 1. The average distance between all pairs of fish within the shoal significantly decreases with the age of the fish, a longitudinal analysis.
Mean ± SEM is shown. n = 19 shoals (each shoal consisting of 10 fish) were tested from 7dpf to 76dpf of age, i.e. on nine occasions. The arena size was kept proportional to the average body length of the fish (28X the body length) and the average distance between all different pairs of fish is also expressed in body lengths. The inset shows the results of a Monte Carlo simulation and shows the distribution of average distance between all pairs of fish in 10-fish shoals in case of random positioning of shoal members within the shoal. The mean of this distribution, i.e. random chance, is indicated on the main graph by the dashed line. Comparison between particular age points is indicated above the X-axis (ns = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001). For further details see Methods and Results.
Unfortunately, determining what inter-individual distance value (the average distance between all possible pairs of fish within a shoal) would correspond to random chance has been a controversial and a complicated problem (Clark & Evans, 1954). Thus to address this question we have run a Monte Carlo simulation with parameters identical to our set up (10 fish in an arena measuring 28 × 28 body length). We ran the simulation 10,000 times and plotted the results, which gave us a Gaussian (normal) distribution of average distance values with a mean equaling 14.6 body lengths and standard deviation of 1.7 (inset, figure 1). We compared our empirical results to the results obtained from this simulation (random chance) using the independent samples t-test (assuming unequal sample sizes and variance) and found that the empirical value we obtained for 7 day old zebrafish was significantly smaller than random chance (t = −5.784, df = 18.19, p < 0.001). This suggests that although 7 dpf zebrafish do not form as tight shoals as adults do, these young fish are already attracted, albeit modestly, to each other. As a cautionary note, however, we also need to point out that our Monte Carlo simulation had no assumptions about any “rules” zebrafish may follow in their environment. Fish must respond to numerous environmental stimuli other than those of their shoal mates. These stimuli may also influence the way fish distribute themselves. Systematic analysis of what environmental stimuli and how may influence the distribution of zebrafish in their environment (laboratory tanks or natural habitat) has not been conducted. Therefore, although the test tanks we employed presented a fairly homogeneous environment, it is possible that the distribution of fish in these tanks, even without any shoaling, could deviate from random chance.
Figure 1 shows that the distance among shoal members decreases with age and reaches 5.81 body-lengths at 76 dpf, a value very close to the one that was previously found optimal in adults in a study using high resolution temporal analysis of shoal cohesion (Miller & Gerlai, 2007). Repeated measure ANOVA confirmed this observation and revealed a significant increase of shoal cohesion with age (decrease of average distance among shoal members, F(8, 128) = 32.95, p < 0.0001). Post hoc multiple comparison tests including Tukey HSD are not appropriate for repeated measure designs. Furthermore, the periods between different age groups were not always consistent due to practical limitations. To avoid type one error and to make the age group comparisons more consistent, we conducted the following analysis. We identified four pairs of age groups for which the age difference between the groups was similar (7 vs. 26 dpf; 26 vs 42 dpf; 42 vs 59 dpf; and 59 vs. 76 dpf) and conducted four separate repeated measure ANOVAs (each with two levels) with a Bonferroni correction for multiple comparisons. The results showed a significant age effect for all (F7vs26 (1, 17) = 10.31, p < 0.05; F26vs42 (1, 17) = 15.92, p < 0.01; F59vs76 (1, 17) = 72.81, p < 0.001) but the comparison of 42 vs 59 dpf groups (F(1, 17) = 0.003, p > 0.05).
Experiment 2: Cross sectional analysis of age differences in shoaling
The first experiment showed a significant increase of shoal density over the course of the development of zebrafish. It is possible however, that repeated handling of the subjects (longitudinal study) affected shoal cohesion, for example, by inducing increased antipredatory responses leading to tighter shoal cohesion as explained above. To exclude this temporal confound, we performed a cross sectional analysis in which each group of fish was tested (and handled) only once but multiple age groups were analyzed the same time in a randomized order. The developmental time points (age groups) corresponded approximately to those tested in the longitudinal analysis but we added two older groups to extend the age range.
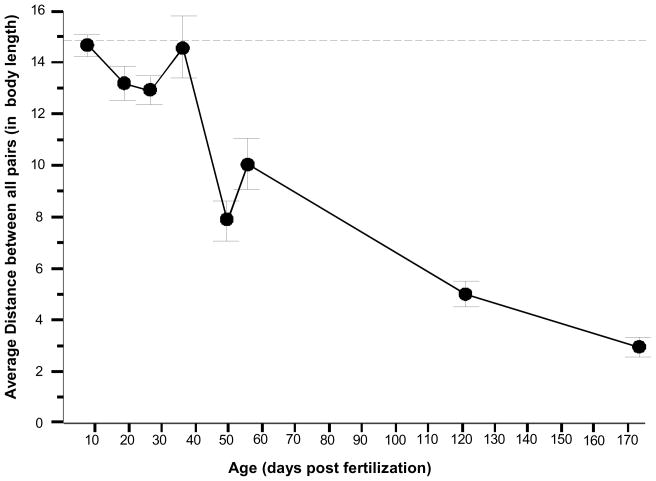
In general, the cross sectional analysis confirmed what we found before: a significantly increased shoal density (reduction of distance among shoal members) with age (Figure 2) ANOVA showed this age dependent change to be significant (F(1,7)=23.121, p<0.001) and post hoc Tukey Honestly Significant Difference (HSD) test revealed a significant difference in shoal density between various age groups. Fish tested at 7 dpf, 18 dpf, 26 dpf, and 36 dpf were not significantly different from each other (and they were also not different from random chance, |t| < 1.021, df > 3, p > 0.05) but these age groups were significantly different from age groups tested at 49 dpf, 121 dpf, and 173 dpf (p < 0.05) and these latter age groups were also significantly below random chance (|t| > 2.51, df > 5, p < 0.05). Age groups in the upper age range (121 dpf, and 173 dpf) were found not to differ significantly from each other (p > 0.05).
Fig. 2. The average distance between all pairs of fish within the shoal significantly decreases with the age of the fish, a cross sectional analysis.
Mean ± SEM is shown. n = 8 shoals with each shoal consisting of 10 fish. Each shoal was tested once. The arena size was kept proportional to the average body length of the fish (28X the body length) and the average distance between all different pairs of fish is also expressed in body lengths. For further details, including the results of post hoc Tukey HSD test, see Methods and Results.
Experiment 3: Randomization of open field size
Although the second experiment confirmed that shoal cohesion increases with the age of zebrafish, this increase could still be due to two separate factors: one, increased social cohesion as the fish develop, or two, increased tank size. Throughout our experiments the tank size was kept proportional to the body length of the tested subjects and thus increased with the age of the fish. The rationale for this was that the ability to traverse a set distance is believed to be proportional to the length of the fish and thus numerous investigators standardized according to body length of the subject (Gallego & Heath, 1994; Masuda et al., 2003; Vogel, 2008; Hale, 1999). However, as explained above, it is possible that altering the tank size itself may have driven the observed behavioral changes and that zebrafish respond to absolute rather than the relative tank size. To address the question whether increasing tank size increases shoaling (decreases the distance among fish), we conducted experiment 3 in which we tested two age groups of fish, a 30 dpf (juvenile) and a 60 dpf (adult) group.
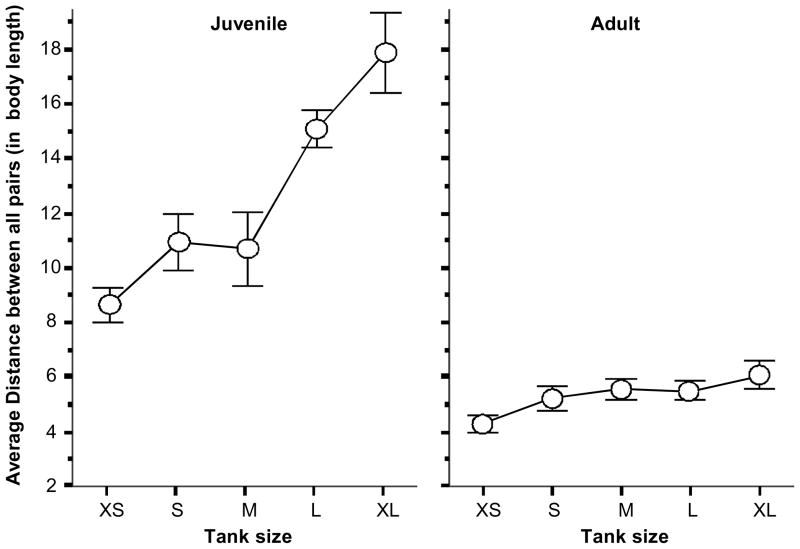
Neither age group analyzed showed significantly decreased average distances as the tank size increased (figure 3). In subjects aged 60 dpf the distances among subjects was not significantly different among the various tank sizes employed (F(1,4)=2.486, p>0.05), and the average distance was 8.47 body lengths, a value that corresponds well to what we have found both in the longitudinal (figure 1) and cross sectional (figure 2) analyses. The fish tested at 30 dpf exhibited a significant tank size dependent increase in average distance among shoal members (ANOVA, F(1,4) = 11.058, p < 0.05, also see Figure 3). Tukey HSD test showed that fish exposed to the largest tank (25×25cm), compared to those in the smallest tank (10×10cm) significantly (p < 0.05) differed from each other. It is notable, however, that this change is opposite in direction to what we have observed in the longitudinal and cross-sectional studies. Briefly, increasing tank sizes did not lead to decreasing average distance among shoal members in either age group studied. Why increasing tank sizes led to decreased shoal cohesion in the younger group of fish but not in the older is an interesting question from several viewpoints including a potential ecological aspect of this finding (perhaps younger fish need to disperse in larger volume of water to forage more efficiently for more homogeneously distributed food), and a potential fish husbandry viewpoint (what would be the ideal fish density and tank size one needs to use to achieve optimal foraging and growth rate in the laboratory?). Such questions will be addressed in future experimental analyses.
Fig. 3. Increasing tank size does not decrease the average distance between all pairs of fish within the shoal.
Mean ± SEM is shown. Two age groups, 30 dpf juveniles (J) and 60 dpf adults (A) were exposed to different tank sizes in a randomized manner. n = 10 shoals (with 10 fish in each shoal) were tested for each age group. Each shoal was tested in five differently sized tanks (XS, S, M, L, and XL). The medium (M) sized tank was identical to what was used in previous experiments, i.e. it was 28X the average body length of the fish tested. The arena sizes for juveniles were as follows: XS = 10 × 10cm, S= 12 × 12 cm, M = 15 × 15 cm, L=20 × 20cm, XL = 25 × 25 cm. The arena sizes for adults were as follows: XS=30 × 30 cm, S= 40 × 40 cm, M= 45 × 45 cm, L=50 × 50 cm, and XL=60 × 60 cm. Note that increasing tank sizes led to significantly increased (but not decreased) average distance between all pairs of fish in the juveniles and increasing tank sizes had no significant effect on adults. For additional details see Methods and Results.
Discussion
Developmental changes in shoaling have not been demonstrated in zebrafish. Zebrafish have previously been found to exhibit strong preference for conspecifics (Saverino & Gerlai, 2008; Gerlach et al., 2007). For example, the sight of conspecifics has been shown to support associative learning and thus this stimulus was considered rewarding (Al-Imari & Gerlai, 2008). Social behavior (preference for particular conspecific color variant) was also shown to be influenced by early exposure to the given color variant (Engeszer et al., 2007). Here we describe, for the first time, maturation of social behavior, i.e. changes in shoaling, in developing zebrafish. Our results suggest that newly hatched zebrafish form only loose aggregates, groups in which the positioning of the individual fish is close to random chance. However, as the fish develop, shoal cohesion significantly increases. Importantly, our data also demonstrate that the increase of shoal cohesion is independent of repeated exposure to the test environment, i.e. could be observed both in a longitudinal as well as in a cross sectional study. Last, the age-dependent increase of shoal cohesion cannot be explained by differences in the size of the test chambers employed for the different age-groups. In the younger (30 dpf) fish we found a negative correlation between shoal cohesion and tank size, and in the older age group tested (60 dpf) we found no correlation. Thus the increasing tank sizes we employed as the fish matured could not explain the age-dependent increase we observed in shoal cohesion. Therefore, we conclude that shoal cohesion significantly increases in zebrafish from the first few days of free swimming stage to adulthood.
What may be the ecological and evolutionary relevance of the age-dependent change in shoal cohesion of zebrafish is not known. It is possible that differential selection pressures may operate and differential ecological niches open as the fish mature. For example, larger piscivores may attack single prey when the prey reaches a particular size but may attempt to scoop up a group of prey when the prey is small Anderson, 2010; Barnes et al., 2010; Holmes & McCormick, 2009; Ioannou et al., 2009). Thus being in a tight shoal may only have advantages for larger prey fish, such as adult zebrafish, but not for smaller individuals. Similarly, being able to be close to potential mates has an obvious fitness advantage but only for adult, reproductively mature fish (Pitcher & Parrish, 1993). Furthermore, it is also likely that small microorganisms are more evenly distributed than larger insects or swarms of plankton and thus being dispersed may be more optimal from a foraging perspective for younger smaller fish and less so for older larger fish (Anderson, 2010). Which of these, or perhaps what other, selection forces may drive the age-dependent increase of shoaling in zebrafish is an experimental question that empirical studies will address in the future.
Another important question concerns the mechanisms underlying the developmental change in shoal cohesion. These mechanisms are not known at this point. Developmental analyses, including anatomical and molecular characterization of changes usually focus on embryonic stages of zebrafish (Schweitzer & Driever, 2009; Holder & Xu, 2008), i.e. the period of development up to 5 dpf, the free swimming stage. Our behavioral results, however, suggests that potential changes beyond this stage of development may also be important to investigate. To address this question one may need to conduct a thorough neuroanatomy analysis looking for structural changes, or analysis of changes in gene expression using microarrays, or perhaps more targeted methods including RT-PCR and/or immunostaining for particular proteins such as neurotransmitter receptors. Analysis of neurochemicals (levels of neurotransmitters and their metabolites) may also be conducted. Clearly, identification of the mechanisms underlying the changes in shoaling behavior will not be easy and may require a number of multidisciplinary analyses. It may also be noted that higher temporal resolution sampling of the different age groups may allow one to detect particular periods during which development of shoaling is accelerated or decelerated. Focus on such periods, if exist, may also aid mechanistic analyses.
The last question we wish to discuss concerns forward genetics and drug screening. The zebrafish has been utilized particularly successfully in high-throughput mutation screens (forward genetics) (Patton & Zon, 2001) but more recently also in drug screens (Chakraborty et al., 2009). This is partly due to the prolific nature and easy and cost effective of maintenance of this species in the laboratory. The problem for behavioral brain research related investigations, however, has been the paucity of behavioral tasks (Sison et al., 2006), i.e. the limited availability of appropriate screening tools. The current work suggests that analysis of shoaling may be an important way one can test complex functional changes in the brain of zebrafish. Alteration of shoaling has been achieved using different environmental stimuli including the presence or absence of food, the presentation of a model of an areal predator (Miller & Gerlai, 2007) as well as the delivery of the natural alarm substance of zebrafish (Speedie & Gerlai, 2008) suggesting that this behavioral response is modifiable and may be an appropriate readout for drug screens and perhaps mutation screens as well. Although the currently employed method to quantify shoaling is labor intensive, development of automated quantification of shoaling is underway. These new methods will enable the investigator to characterize the behavior of the shoal as a whole and also its individual members in a sophisticated manner. Once commercially available, these methods will allow medium to high throughput mutation and drug screens and thus will greatly facilitate the analysis of social behavior and its mechanisms.
Research Highlights.
Social behaviour is a complex phenomenon whose mechanisms may be best investigated with the use of animal models in the laboratory. Zebrafish is one of the most social laboratory species. However, the number of publications, and with it the amount of information, available on the behaviour of zebrafish is orders of magnitude less compared to traditional laboratory model organisms including the rat, the mouse or even the fruit fly. Characterization of social behaviour and development of social behavioural test paradigms together with the currently available genetic and pharmacological tools may make zebrafish the most powerful tool for the biological analysis of vertebrate social behaviour. In the current paper we characterize the ontogenesis (age dependent development) of social behaviour in zebrafish. We employ a sophisticated shoaling behaviour analysis software application and measure the distances among shoal members in a freely swimming zebrafish groups. Our results demonsrate, for the first time, significant age-dependent development of social behaviour. The results open several new lines of research into the biological mechanisms of social behaviour, into the analysis of its development and its ecological and evolutionary aspects.
Ethical statement.
The research reported in our manuscript has been conducted in accordance with local University, Provincial and Federal (Canadian Council for Animal Care) guidelines and has been reviewed and approved by the Local Animal Care Committee.
Acknowledgments
This research was supported by NIH/NIAAA (1R01AA015325-01A2) grant to RG. The authors would like to thank Diptendu Chatterjee, Tanya Scerbina, and Noam Miller, for their technical assistance.
List of abbreviations
- Dpf
days post fertilization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Imari L, Gerlai R. Sight of conspecifics as reward in associative learning in zebrafish (Danio rerio) Behav Brain Res. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Anderson JJ. Ratio- and predator-dependent functional forms for predators optimally foraging in patches. Am Nat. 2010;175:240–249. doi: 10.1086/649606. [DOI] [PubMed] [Google Scholar]
- Barnes C, Maxwell D, Reuman DC, Jennings S. Global patterns in predator-prey size relationships reveal size dependency of trophic transfer efficiency. Ecology. 2010;91:222–232. doi: 10.1890/08-2061.1. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Hollander E. The neuroscience of affiliation: Forging links between basic and clinical research on neuropeptides and social behavior. Hormones and Behavior. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Bell-Dolan DJ, Reaven NM, Peterson L. Depression and Social Functioning: A Multidimensional Study of the Linkages. J Clin Child Adol Psych. 1993;22:306–315. [Google Scholar]
- Carew TJ, Castellucci VF, Kandel ER. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int J Neurosci. 1971 Aug;2(2):79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10:116–124. doi: 10.2174/138920009787522197. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Evans FC. Distance to nearest neighbor as a measure of spatial relationships in populations. Ecology. 1954;35:445–453. [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeszer RE, Alberici Da Barbiano L, Ryan MJ, Parichy DM. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Animal Behaviour. 2007;74:1269–1275. doi: 10.1016/j.anbehav.2007.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn L, Bretaud S, Lo C, Ingham PW, Bandmann O. Zebrafish as a new animal model for movement disorders. Journal of Neurochemistry. 2008;106:1991–1997. doi: 10.1111/j.1471-4159.2008.05463.x. [DOI] [PubMed] [Google Scholar]
- Gallego A, Heath MR. The development of schooling behaviour in Atlantic herring Clupea harengus. Journal of Fish Biology. 1994;45:569–588. [Google Scholar]
- Gerlach G, Hodgins-Davis A, MacDonald B, Hannah RC. Benefits of kin association: related and familiar zebrafish larvae (Danio rerio) show improved growth. Behavioral Ecology and Sociobiology. 2007;61:1765–1770. [Google Scholar]
- Gerlai R. High-throughput Behavioral Screens: the First Step towards Finding Genes Involved in Vertebrate Brain Function Using Zebrafish. Molecules. 2010;15:2609–2622. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. Phenomics: Fiction or the Future? Trends Neurosci. 2002;25:506–509. doi: 10.1016/s0166-2236(02)02250-6. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Zebra Fish: An Uncharted Behavior Genetic Model. Behavior Genetics. 2003;33:461–468. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Roder J. Female specific hyperactivity in S100 beta transgenic mice does not habituate in open-field. Behav Brain Res. 1993;59:119–124. doi: 10.1016/0166-4328(93)90157-l. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav Brain Res. 2009;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S, Brockmark S, Höjesjö J, Johnsson J. Coping with divided attention: the advantage of familiarity. Proc R Soc Lond B. 2004;271:695–699. doi: 10.1098/rspb.2003.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale ME. Locomotor mechanics during early life history: effects of size and ontogeny on fast-start performance of salmonid fishes. The Journal of Experimental Biology. 1999;202:1465–1479. doi: 10.1242/jeb.202.11.1465. [DOI] [PubMed] [Google Scholar]
- Holder N, Xu Q. The zebrafish: an overview of its early development. Methods Mol Biol. 2008;461:483–491. doi: 10.1007/978-1-60327-483-8_33. [DOI] [PubMed] [Google Scholar]
- Holmes TH, McCormick MI. Influence of prey body characteristics and performance on predator selection. Oecologia. 2009;159:401–413. doi: 10.1007/s00442-008-1220-x. [DOI] [PubMed] [Google Scholar]
- Ioannou CC, Morrell LJ, Ruxton GD, Krause J. The effect of prey density on predators: conspicuousness and attack success are sensitive to spatial scale. Am Nat. 2009;173:499–506. doi: 10.1086/597219. [DOI] [PubMed] [Google Scholar]
- Kato S, et al. A computer image processing system for quantification of zebrafish behavior. Journal of Neuroscience Methods. 2004;134:1–7. doi: 10.1016/j.jneumeth.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Keller ET, Murtha JM. The use of mature zebrafish (Danio rerio) as a model for human aging and disease. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2004;138:335–341. doi: 10.1016/j.cca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Patterson J, Kimmel RO. The development and behavioral characteristics of the startle response in the zebrafish. Developmental Psychobiology. 1974;7:47–60. doi: 10.1002/dev.420070109. [DOI] [PubMed] [Google Scholar]
- Ledesma JM, McRobert SP. Innate and Learned Shoaling Preferences Based on Body Coloration in Juvenile Mollies, Poecilia latipinna. Ethology. 2008;114:1044–1048. [Google Scholar]
- Liebowitz Michael R, MD, Gorman Jack M, MD, Fyer Abby J, MD, Klein Donald F., MD Social Phobia. Review of a Neglected Anxiety Disorder. Arch Gen Psychiatry. 1985;42:729–736. doi: 10.1001/archpsyc.1985.01790300097013. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lin Y, Chen Y, Yang X, Xu D, Liang S. Proteome analysis of a single zebrafish embryo using three different digestion strategies coupled with liquid chromatography-tandem mass spectrometry. Analytical Biochemistry. 2009;394:177–185. doi: 10.1016/j.ab.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Masuda R, Shoji J, Nakayama S, Tanaka M. Development of schooling behavior in Spanish mackerel Scomberomorus niphonius during early ontogeny. Fisheries Science. 2003;69:772–776. [Google Scholar]
- Meerlo P, Horvath KM, Nagy GM, Bohus B, Koolhaas JM. The influence of postnatal handling on adult neuroendocrine and behavioural stress reactivity. J Neuroendocrinol. 1999;11:925–933. doi: 10.1046/j.1365-2826.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Miller NY, Gerlai R. Oscillations in shoal cohesion in zebrafish (Danio rerio) Behav Brain Res. 2008;193:148–151. doi: 10.1016/j.bbr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell LJ, James R. Mechanisms for aggregation in animals: rule success depends on ecological variables. Behavioral Ecology. 2008;19:193–201. [Google Scholar]
- Patton EE, Zon LI. The art and design of genetic screens: zebrafish. Nat Rev Genet. 2001;2:956–966. doi: 10.1038/35103567. [DOI] [PubMed] [Google Scholar]
- Pauker CP, Ward DL, Sponholtz PJ, Hilwig KD. Effects of repeated hoopnetting and handling on bonytail chub. J Freshwater Ecol. 20:649–654. [Google Scholar]
- Pinsker H, Kupfermann I, Castellucci V, Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- Pitcher TJ, Parrish JK. Functions of shoaling behaviour in teleosts. In: Pitcher TJ, editor. Behaviour of Teleost Fishes. London: Chapman & Hall; 1993. pp. 363–439. [Google Scholar]
- Reichow B, Volkmar FR. Social skills interventions for individuals with autism: evaluation for evidence-based practices within a best evidence synthesis framework. J Autism Dev Disord. 2010;40:149–166. doi: 10.1007/s10803-009-0842-0. [DOI] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer J, Driever W. Development of the dopamine systems in zebrafish. Adv Exp Med Biol. 2009;651:1–14. doi: 10.1007/978-1-4419-0322-8_1. [DOI] [PubMed] [Google Scholar]
- Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes influencing vertebrate behavior: zebrafish making headway. Lab Animal. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav. Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoletov K, Klemke R. Catch of the day: zebrafish as a human cancer model. Oncogene. 2008;27:4509–4520. doi: 10.1038/onc.2008.95. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2:268–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Vogel S. Modes and scaling in aquatic locomotion. Integrative and Comparative Biology. 2008;48:702–712. doi: 10.1093/icb/icn014. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV. Sleep in Zebrafish. Zebrafish. 2006;3:215–226. doi: 10.1089/zeb.2006.3.215. [DOI] [PubMed] [Google Scholar]
- Zimmerman JE, Naidoo N, Raizen DM, Pack AI. Conservation of sleep: insights from non-mammalian model systems. Trends in Neurosciences. 2008;31:371–376. doi: 10.1016/j.tins.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]