Abstract
Perceptual learning refers to the phenomenon that practice or training in perceptual tasks often substantially improves perceptual performance. Often exhibiting stimulus or task specificities, perceptual learning differs from learning in the cognitive or motor domains. Research on perceptual learning reveals important plasticity in adult perceptual systems, and as well as the limitations in the information processing of the human observer. In this article, we review the behavioral results, mechanisms, physiological basis, computational models, and applications of visual perceptual learning.
1. Introduction
Although early Gestalt psychologists denied any role of learning in perception, (Helmholtz, 1911) made learning an essential component in his theories of perception. Taking an ecological approach, Eleanor J. Gibson (Gibson, 1967) reviewed development of perceptual expertise in early childhood and postulated that perceptual learning is a process of discovering how to transform previously overlooked potentials of sensory stimulation into effective information. The systematic documentation of various specificities of perceptual learning with implications of a early sensory site of learning re-charged the research on perceptual learning (Karni and Sagi, 1991). Since then, perceptual learning in adult human observers has been documented in a wide range of perceptual tasks in visual, auditory, and somatosensory domains (Fahle and Poggio, 2002). In this review, we focus on perceptual learning in the visual domain.
2. Perceptual Learning
Perceptual learning has been documented in virtually every visual task, including the detection or discrimination of visual gratings (DeValois, 1977; Fiorentini and Berardi, 1980; 1981; Mayer, 1983), stimulus orientation judgment (Dosher and Lu, 1998; Shiu and Pashler, 1992; Vogels and Orban, 1985), motion direction discrimination (Ball and Sekuler, 1982; 1987; Ball, Sekuler, and Machamer, 1983), texture discrimination (Ahissar and Hochstein, 1996; Karni and Sagi, 1991; Karni and Sagi, 1993), time to perceive random dot stereograms (Ramachandran and Braddick, 1973), stereoacuity (Fendick and Westheimer, 1983), hyperacuity and vernier tasks (Beard, Levi, and Reich, 1995; Bennett and Westheimer, 1991; Fahle and Edelman, 1993; Kumar and Glaser, 1993; McKee and Westheimer, 1978; Saarinen and Levi, 1995), and object recognition (Furmanski and Engel, 2000).
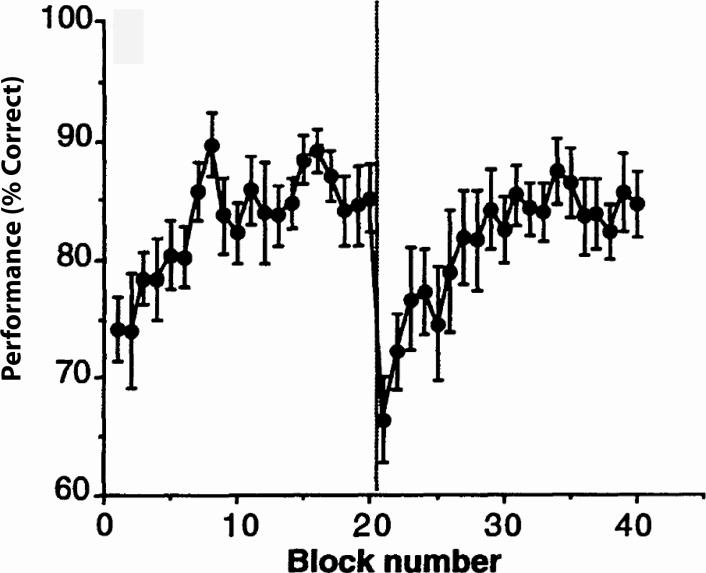
The trade-mark finding in perceptual learning is that some of what is learned is specific to stimulus or task factors such as retinal location (Karni and Sagi, 1991), spatial frequency (Fiorentini and Berardi, 1980), orientation (Poggio, Fahle, and Edelman, 1992) (Figure 1), or background texture (Ahissar and Hochstein, 1996). Perceptual learning that is highly specific to retinal location and stimulus has been claimed to reflect neural plasticity in basic visual processing mechanisms (Karni and Sagi, 1991).
Figure 1.
Effect of switching from vertical to horizontal verniers (or vice versa) after block 20. Averaged results of 12 naïve subjects; 6 started with horizontal verneiers, and the others stated with vertical verniers. There is no transfer of learning. (After Poggio, Fahle, & Edelman, 1992).
Several recent papers re-examined specificity of perceptual learning and found that a number of factors in the training procedures, some of that were not obviously related to specificity or transfer of learning, determine the degree of specificity, including task precision (Jeter, Dosher, Petrov, and Lu, 2009), task difficulty (Ahissar and Hochstein, 1997), number of trials (Censor and Sagi, 2009), and training schedule (Xiao, Zhang, Wang, Klein, Levi, and Yu, 2008). Xiao et al (2008) developed a novel double-training paradigm that employed conventional feature training (e.g., contrast) at one location, and additional training with an irrelevant feature/task (e.g., orientation) at a second location, either simultaneously or at a different time. They showed that this additional location training enabled a complete transfer of feature learning (e.g., contrast) to the second location. Understanding factors that determine specificity/transfer of perceptual learning is one of the most important challenges in the study of perceptual learning.
3. Mechanisms of Learning
Mechanisms of perceptual learning, i.e., what is learned during perceptual learning, have been investigated in recent years in psychophysics (Dosher and Lu, 1998; 1999; Gold, Bennett, and Sekuler, 1999; Saarinen and Levi, 1995), neurophysiology (Crist, Li, and Gilbert, 2001; Ghose, Yang, and Maunsell, 2002; Schoups, Vogels, Qian, and Orban, 2001), brain imaging (Schiltz, Bodart, Dubois, Dejardin, Michel, Roucoux, Crommelinck, and Orban, 1999; Schwartz, Maquet, and Frith, 2002), and patients (Fahle and Daum, 2002; Xu, Lu, Wang, Dosher, Zhou, Yang, Zhang, and Zhou, 2010).
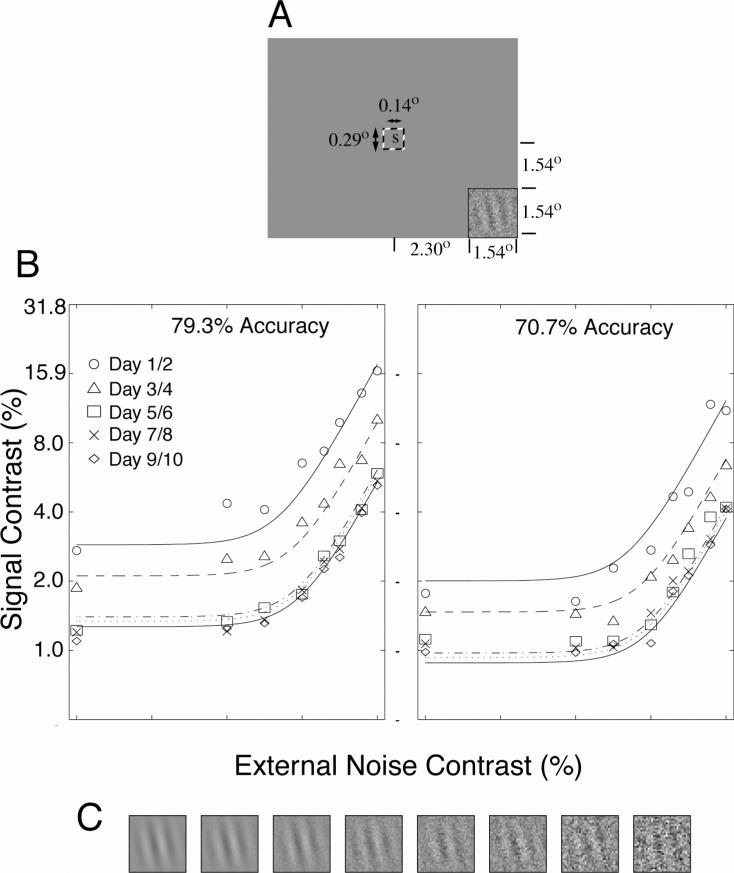
In psychophysical studies, Dosher and Lu (1998) introduced a theoretical framework and an external noise plus training paradigm to analyze how perceptual inefficiencies improve over the course of perceptual learning (Figure 2). Perceptual inefficiencies are attributed to three limitations in perceptual processes (Lu and Dosher, 2008): an imperfect perceptual template, internal additive noise, and multiplicative noise. Systematic measurements of human performance as a function of both the amount of external noise added to the signal stimulus and the length of training received by the observers make it possible to distinguish three mechanisms of perceptual learning: perceptual template retuning, stimulus enhancement, and contrast-gain control reduction. It has been consistently found that two independent mechanisms (Figure 3), stimulus enhancement and external noise exclusion, support perceptual learning in a range of tasks (Dosher and Lu, 2007; Dosher and Lu, 1998; 1999; 2005; Lu, Chu, and Dosher, 2006; Lu and Dosher, 2004).
Figure 2.
The perceptual template model (PTM) and the signatures of three mechanisms of perceptual learning. (After (Dosher and Lu, 1998)).
Figure 3.
A perceptual learning task using the external noise paradigm. (A) Spatial layout of the task, including the peripheral orientation discrimination Gabor stimulus, and a central letter stimulus for a secondary task. (B) Contrast threshold (Gabor signal contrast corresponding to the criterion accuracy) as a function of the external noise in the stimulus. Threshold is a systematic function of criterion, external noise, and practice (data from Dosher & Lu, 1998). (C) Examples of a signal of constant contrast embedded in increasing amounts of external noise.
Although practice-induced neuronal plasticity has been documented in auditory (Metherate and Weinberger, 1990; Weinberger, Javid, and Lepan, 1993) and somato-sensory cortices (Jenkins, Merzenich, Ochs, Allard, and Guic-Robles, 1990; Recanzone, Merzenich, and Schreiner, 1992), and in some visual fMRI studies (Schiltz et al., 1999; Schwartz et al., 2002; Vaina, Belliveau, des Roziers, and Zeffiro, 1998), evidence for practice-induced neuronal plasticity in early visual cortical areas is however modest (Crist et al., 2001; Ghose, Yang, and Maunsell, 2002; Schoups, Vogels, Qian, and Orban, 2001; Yang and Maunsell, 2004), although neurons in V1 may exhibit task specific tuning (Li, Piech, and Gilbert, 2004) that seem to reflect selection of task-relevant stimulus features for a particular task rather than persistent cross-task changes in neuronal tuning. Law and Gold (Law and Gold, 2008) found that perceptual learning in motion direction discrimination does not involve neuronal response changes in the middle temporal area (MT), but rather in the lateral intraparietal area (LIP), a brain area related to selective readout of MT neurons. They conclude, “...[our] results suggest that the perceptual improvements corresponded to an increasingly selective readout of highly sensitive MT neurons by a decision process, represented in LIP, that instructed the behavioral response.” In sum, these reports found that early visual representations showed either no change or modest changes in the slopes of tuning functions following perceptual learning.
One critical difference between existing neurophysiological investigations of perceptual learning contrasting cortical plasticity in auditory and somato-sensory domains, which exhibit strong plasticity, and that in the visual domain, where evidence for early visual plasticity is modest, lies in the animal specifies: non-primates are used in the auditory and somato-sensory domains, whereas primates are used in the visual domain. It's possible that primates exhibit less training-induced plasticity than non-primates in early visual areas (Karmarkar and Dan, 2006; Yao, Shi, Han, Gao, and Dan, 2007).
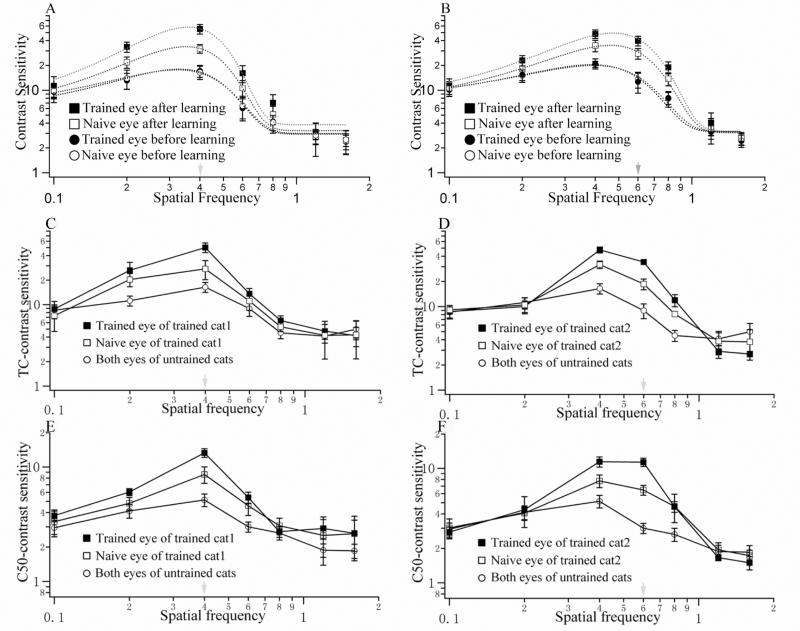
Some recent evidence might suggest greater plasticity in early visual areas occurs in non-primates. (Hua, Bao, Huang, Wang, Xu, Zhou, and Lu, 2010) examined the effects of training in grating orientation identification on both perceptual and neuronal contrast sensitivity functions of cats using combined psychophysical measurements with extracellular single-unit recording under anesthetized preparations. Conditioning was used to train cats to identify the orientation of a high contrast ±45° sinusoidal grating. Subsequently, the same procedure was used to measure monocular contrast sensitivity functions (CSF) in both eyes. The cats were then trained monocularly to perform a near-threshold orientation identification task. After approximately forty days of training, monocular CSFs were measured again, followed by extracellular recordings of single-unit activities from the primary visual cortex (V1) of anesthetized cats. Contrast response functions to the preferred stimuli were measured for isolated neurons. The combined contrast sensitivities of individual neurons were then used to construct the neuronal CSFs for neuronal populations that responded preferentially to the stimuli presented via trained or untrained eyes (Figure 4).
Figure 4.
Contrast sensitivity functions in the trained and untrained eyes before and after training for cat1 (A) and cat2 (B). Smooth curves represent the best fitting Gauss functions. The green arrows indicate the trained spatial frequency, and the error bars represent 1 SD. (CD) TC-contrast sensitivity functions of V1 neurons recorded from cat1 (C) and cat2 (D). (EF) C50-contrast sensitivity functions of V1 neurons recorded from cat1 (E) and cat2 (F). Green arrows indicate the trained spatial frequency. All values are displayed as mean ± SEM. (After Hua et al, 2010).
Hua et al (2010) found that (1) training improved perceptual contrast sensitivity, with some degree of specificity for the training spatial frequency and training eye, (2) training also improved the contrast sensitivity of V1 neurons responding preferentially to the trained spatial frequency, (3) perceptual and neuronal CSFs were highly correlated both before and after training, and (4) a systematic analysis of the parameters of the neuronal contrast response functions indicated that the learning-induced plasticity was caused by increased contrast-gain of the neurons associated with training. The increased contrast-gain resulted in a parallel leftward shift of the neuronal contrast response functions, consistent with decreased post-synaptic polarization (Carandini and Ferster, 1997; Ohzawa, Sclar, and Freeman, 1985; Sanchez-Vives, Nowak, and McCormick, 2000a; b; Sclar, Lennie, and DePriest, 1989).
In addition to the difference in animal species, the Hua et al (2010) study differs from previous neurophysiological studies on perceptual learning in two other ways: (1) previous electrophysiological studies exploring training-induced visual cortical plasticity generally used orientation threshold as the dependent measure. Hua et al (2010) used contrast thresholds as the dependent measure. It is possible that different neural networks might be involved in orientation discrimination and contrast detection. (2) Hua et al (2010) recorded the response of V1 neurons in anesthetized and paralysed cats, whereas previous studies made recordings in awake-behaving monkeys. Compared to studies on anesthetized cats, recordings from early visual cortical areas of wake monkeys may include substantial top-down influences from higher visual cortical areas (Gazzaley, Cooney, McEvoy, Knight, and D'Esposito, 2005; Li et al., 2004; Watanabe, Harner, Miyauchi, Sasaki, Nielsen, Palomo, and Mukai, 1998). New studies are necessary to further investigate all these factors.
4. The role of attention and feedback
Although earlier studies (Ahissar, Laiwand, Kozminsky, and Hochstein, 1998; Schoups et al., 2001; Shiu and Pashler, 1992) found that subjects can only learn the feature they paid attention to, recent studies by Watanabe and colleagues (Seitz and Watanabe, 2003; Watanabe, Nanez, Koyama, Mukai, Liederman, and Sasaki, 2002; Watanabe, Nanez, and Sasaki, 2001) found that attention to a feature is not necessary for perceptual learning of the feature to occur if the feature is irrelevant to the primary task performed by the subject. Recent studies suggest that the performance improvement from task-irrelevant learning can be enhanced by attending to the feature (Gutnisky, Hansen, Iliescu, and Dragoi, 2009).
Another important topic in perceptual learning concerns the role of feedback. The empirical pattern of results is quite complex (see Dosher & Lu, 2009, for a review). Whereas most perceptual learning studies employed trial-by-trial feedback, several studies documented significant perceptual learning with block, partial, or even no feedback, and no perceptual learning with false, random, manipulated block, and reversed feedback (Herzog and Fahle, 1997). Shibata, Yamagishi, Ishii, and Kawato (2009) showed that arbitrary block-feedback facilitated perceptual learning if it is more positive than the observer's actual performance. At high training accuracies, training with and without feedback generated essentially the same learning curves (Liu, Lu, and Dosher, 2010b), and significant learning was found in low training accuracy trials when they were mixed with high accuracy trials (Liu, Lu, and Dosher, 2009; Petrov, Dosher, and Lu, 2006). Liu, Lu, and Dosher (2010a) conducted a computational analysis of the complex pattern of empirical results on the role of feedback with the augmented Hebbian reweighting model (Petrov, Dosher & Lu, 2005), including a study that showed significant perceptual learning with block, partial, or even no feedback, and no perceptual learning with false, random, manipulated block, and reversed feedback (Herzog & Fahle, 1997), another study (Shibata et al, 2009) that showed that arbitrary block-feedback facilitated perceptual learning if it is more positive than the observer's actual performance, and the interaction between feedback and training accuracy (Liu, Lu & Dosher, 2010b). The simulation results are both qualitatively and quantitatively consistent with the data reported in the literature.
5. Computational Models
One major open question is whether perceptual learning reflects representation enhancement in early sensory areas or reweighting of sensory representation in the decision process. Petrov, Dosher, and Lu (2005) introduced a task analysis framework to evaluate the diagnostic value of experimental designs for discriminating reweighting and representational enhancement in perceptual learning. A systematic review of the literature suggests that the two potential forms of plasticity – reweighting versus representational change – make similar predictions about specificity in most of the existing studies that had previously been cited as evidence for representational enhancement.
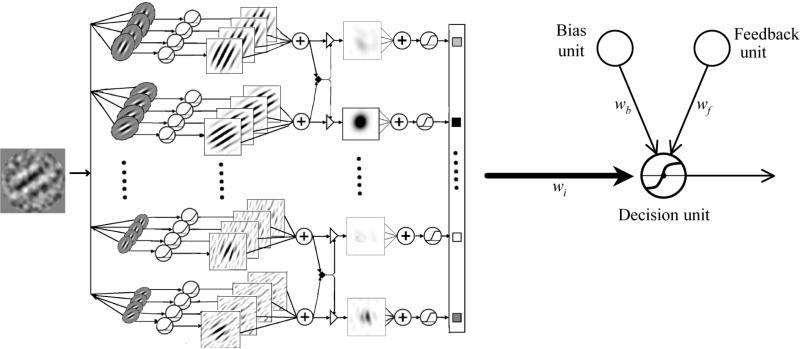
A number of models have been proposed in perceptual learning (Herzog and Fahle, 1998; Petrov, Dosher, and Lu, 2006; Petrov, Dosher, and Lu, 2005; Vaina, Sundareswaran, and Harris, 1995; Vallabha and McClelland, 2007; Weiss, Edelman, and Fahle, 1993; Zhaoping, Herzog, and Dayan, 2003) (see Tsodyks & Gilbert, 2004, for a review). All these models assume an appropriate stimulus representation and postulate incremental learning; none proposes systematic changes in representation. Based on the results from the task analysis and neurophysiology, Petrov et al (2005, 2006) implemented the reweighting hypothesis outlined in Dosher and Lu (1998) in a multi-channel Augmented Hebbian Reweighting Model (AHRM) (Figure 5). The AHRM consists of four units: representation units that encode input images as activation patterns, a task-specific decision unit that receives weighted inputs from the representation units, an adaptive bias unit that accumulates a running average of the response frequencies and works to balance the frequency of the two responses, and a feedback unit that makes use of external feedback when (and if) it is presented. Learning in the model occurs exclusively through incremental Hebbian modification of the weights between representation units and the decision unit; while the early processing pathway that constructs representations from the retinal image remains fixed throughout training. The AHRM has been very successful in modelling a wide range of phenomena in perceptual learning, including complex patterns of perceptual learning in an orientation discrimination experiment under destabilizing non-stationary manipulations both with and without trial-to-trial feedback (Petrov et al., 2005; Petrov et al., 2006), a large number of data patterns in external noise studies of perceptual learning (Lu, Liu, and Dosher, 2010), and the complex patterns of results on the role of feedback in perceptual learning (Liu et al., 2010a).
Figure 5.
Augmented Hebbian Reweighting Model (AHRM, Petrov, Dosher & Lu, 2005, 2006) passes stimulus images through a representational system of orientation and spatial-frequency tuned units, with non-linearities and spatial pooling. These activations, along with inputs to a bias and feedback unit are weighted by the task-specific weighting system to yield a decision. The AHRM has predicted the dynamics of learning in non-stationary training, the various roles of feedback in learning, and performance in external noise paradigms. (After Petrov et al, 2005).
6. Applications
The remarkable levels of neural plasticity and neurogenesis in the adult nervous system (Bruel-Jungerman, Davis, and Laroche, 2007; Gould, 2007; Johansson, 2007; Kramer and Erickson, 2007) have led to the test and development of visual rehabilitation programs based on perceptual learning. Here we discuss one example application of perceptual learning in treating amblyopia.
Amblyopia is a developmental spatial vision impairment that cannot be corrected by refractive means. It affects about 3% of the population (Ciuffreda, Levi, and Selenow, 1991; McKee, Levi, and Movshon, 2003; Simmers, Ledgeway, Hess, and McGraw, 2003). Conventional wisdom on visual development suggests that spatial vision becomes hard-wired after a critical period, usually around 6-8 years of age (Berardi, Pizzorusso, Ratto, and Maffei, 2003); The amblyopic visual system is generally thought to be fully (though erroneously) developed by age eight and therefore no longer subject to therapeutic modifications. In clinical practice, only infant and young child amblyopes are treated, while patients older than eight years are left untreated (Greenwald and Parks, 1999).
Exploiting neural plasticity in the adult visual system, several laboratories have demonstrated that perceptual training can be used in the adult amblyopic visual system for visual rehabilitation (Chung, Li, and Levi, 2006; Levi and Polat, 1996; Li and Levi, 2004; Li, Levi, and Klein, 2004a; Li, Provost, and Levi, 2007; Polat, Ma-Naim, Belkin, and Sagi, 2004; Zhou, Huang, Xu, Tao, Qiu, Li, and Lu, 2006). One critical concern is the efficiency of such treatment. Because the hallmark of perceptual learning in the normal visual system is its specificity to the characteristics of the training stimulus (Fahle, 2002), there is a question about generalizability of such training. If perceptual learning in the amblyopic visual system were also highly specific to the characteristics of the training stimuli and task, perceptual learning as a therapy for amblyopia would not be very effective in improving general spatial vision. At a minimum, multiple training stimuli and tasks would need to be used to cover the range of stimuli and tasks important for daily visual functions.
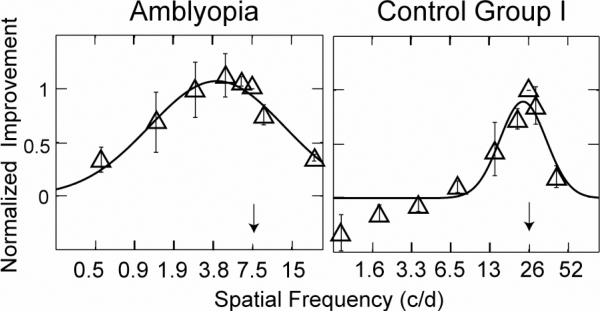
To evaluate and compare the generalizability of perceptual learning in amblyopic and normal vision, (Huang, Zhou, and Lu, 2008) estimated the bandwidth of perceptual learning in both normals and amblyopes. They found that the bandwidth of perceptual learning was drastically different (p<0.01): For the amblyopic observers, the average full bandwidth was 4.04 0.63 octaves; the average full bandwidth was only 1.40 0.30 octaves for the normal observers (Figure 6). The estimated 4.04 octaves bandwidth of perceptual learning implies that the impact of perceptual learning generalizes across spatial frequency channels in amblyopic eyes. Such a broad bandwidth of perceptual learning may underlie the improved visual acuity in the amblyopic eyes following training, a task that involves a wide range of spatial frequencies.
Figure 6.
Average contrast sensitivity improvements as functions of spatial frequency for the amblyopic (a) and first control groups (b). The magnitudes of contrast sensitivity improvements were normalized to that at the training spatial frequency; Spatial frequencies were normalized to the training frequency. Blue arrows indicate the average training spatial frequency. Data were weighted by their standard deviation. Only observers with significant contrast sensitivity improvements during training are included. Error bars indicate SEM. (After Huang et al, 2008).
7. Conclusion
The susceptibility of the adult visual system to training suggests that the perceptual system is not static even in adulthood. We cannot fully understand perception without understanding perceptual learning. Research on perceptual learning is of theoretical significance in illuminating plasticity in adult perceptual systems, and in understanding the limitations in the information processing of the human observer. It is of practical significance as a potential method for the development of perceptual expertise in normal populations and for the non-invasive amelioration of deficits in challenged populations by training.
8.Acknowledgements
The research was supported by grants from the National Natural Science Foundation of China (30630027), Chinese Academy of Sciences (KSCX2-YW-R-255), Natural Science Foundation of Anhui Province (No. 070413138), National Basic Research Program of China (2009CB941303), and the US National Eye Institute (EY017491).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. References
- Ahissar M, Hochstein S. Learning pop-out detection: Specificities to stimulus characteristics. Vision Research. 1996;36:3487–3500. doi: 10.1016/0042-6989(96)00036-3. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S. Task difficulty and the specificity of perceptual learning. Nature. 1997;387:401–406. doi: 10.1038/387401a0. [DOI] [PubMed] [Google Scholar]
- Ahissar M, Laiwand R, Kozminsky G, Hochstein S. Learning pop-out detection: Building representations for conflicting target-distractor relationships. Vision Research. 1998;38:3095–3107. doi: 10.1016/s0042-6989(97)00449-5. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. A specific and enduring improvement in visual motion discrimination. Science. 1982;218:697–698. doi: 10.1126/science.7134968. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R. Direction-specific improvement in motion discrimination. Vision Research. 1987;27:953–965. doi: 10.1016/0042-6989(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Ball K, Sekuler R, Machamer J. Detection and identification of moving targets. Vision Research. 1983;23:229–238. doi: 10.1016/0042-6989(83)90111-6. [DOI] [PubMed] [Google Scholar]
- Beard BL, Levi DM, Reich LN. Perceptual learning in parafoveal vision. Vision Research. 1995;35:1679–1690. doi: 10.1016/0042-6989(94)00267-p. [DOI] [PubMed] [Google Scholar]
- Bennett RG, Westheimer G. The effect of training on visual alignment discrimination and grating resolution. Perception & Psychophysics. 1991;49:541–546. doi: 10.3758/bf03212188. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Laroche S. Brain plasticity mechanisms and memory: a party of four. Neuroscientist. 2007;13:492–505. doi: 10.1177/1073858407302725. [DOI] [PubMed] [Google Scholar]
- Carandini M, Ferster D. A tonic hyperpolarization underlying contrast adaptation in cat visual cortex. Science. 1997;276:949–952. doi: 10.1126/science.276.5314.949. [DOI] [PubMed] [Google Scholar]
- Censor N, Sagi D. Global resistance to local perceptual adaptation in texture discrimination. Vision Res. 2009;49:2550–2556. doi: 10.1016/j.visres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Chung ST, Li RW, Levi DM. Identification of contrast-defined letters benefits from perceptual learning in adults with amblyopia. Vision Res. 2006;46:3853–3861. doi: 10.1016/j.visres.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciuffreda KJ, Levi DM, Selenow A. Amblyopia: Basic and Clinical Aspects. Butterworth-Heinemann; Boston: 1991. [Google Scholar]
- Crist RE, Li W, Gilbert CD. Learning to see: Experience and attention in primary visual cortex. Nature Neuroscience. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- DeValois K. Spatial frequency adaptation can enhance contrast sensitivity. Vision Research. 1977;17:1057–1065. doi: 10.1016/0042-6989(77)90010-4. [DOI] [PubMed] [Google Scholar]
- Dosher B, Lu Z-L. Level and mechanisms of perceptual learning: Learning in luminance and texture objects. Vision Research. 2007;46:1996–2007. doi: 10.1016/j.visres.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu Z-L. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13988–13993. doi: 10.1073/pnas.95.23.13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosher BA, Lu Z-L. Mechanisms of perceptual learning. Vision Research. 1999;39:3197–3221. doi: 10.1016/s0042-6989(99)00059-0. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu Z-L. Perceptual learning in clear displays optimizes perceptual expertise: Learning the limiting process. Proc Natl Acad Sci U S A. 2005;102:5286–5290. doi: 10.1073/pnas.0500492102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M. Perceptual Learning. The MIT Press; Cambridge: 2002. [Google Scholar]
- Fahle M, Daum I. Perceptual learning in amnesia. Neuropsychologia. 2002;40:1167–1172. doi: 10.1016/s0028-3932(01)00231-7. [DOI] [PubMed] [Google Scholar]
- Fahle M, Edelman S. Long-term learning in vernier acuity: Effects of stimulus orientation, range and of feedback. Vision Research. 1993;33:397–412. doi: 10.1016/0042-6989(93)90094-d. [DOI] [PubMed] [Google Scholar]
- Fahle M, Poggio T. Perceptual learning. MIT Press; Cambridge, MA: 2002. [Google Scholar]
- Fendick M, Westheimer G. Effects of practice and the separation of test targets on foveal and peripheral stereoacuity. Vision Research. 1983;23:145–150. doi: 10.1016/0042-6989(83)90137-2. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Perceptual learning specific for orientation and spatial frequency. Nature. 1980;287:43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Berardi N. Learning in grating waveform discrimination: Specificity for orientation and spatial frequency. Vision Research. 1981;21:1149–1158. doi: 10.1016/0042-6989(81)90017-1. [DOI] [PubMed] [Google Scholar]
- Furmanski CS, Engel SA. Perceptual learning in object recognition: Object specificity and size invariance. Vision Research. 2000;40:473–484. doi: 10.1016/s0042-6989(99)00134-0. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, McEvoy K, Knight RT, D'Esposito M. Top-down enhancement and suppression of the magnitude and speed of neural activity. J Cogn Neurosci. 2005;17:507–517. doi: 10.1162/0898929053279522. [DOI] [PubMed] [Google Scholar]
- Ghose GM, Yang T, Maunsell JH. Physiological correlates of perceptual learning in monkey V1 and V2. J Neurophysiol. 2002a;87:1867–1888. doi: 10.1152/jn.00690.2001. [DOI] [PubMed] [Google Scholar]
- Gibson EJ. Principles of perceptual learning and development. Appleton Century Crofts; New York: 1967. [Google Scholar]
- Gold J, Bennett PJ, Sekuler AB. Signal but not noise changes with perceptual learning. Nature. 1999;402:176–178. doi: 10.1038/46027. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Greenwald MJ, Parks MM. Clinical Ophthalmology. Harper and Row; Hagerstown, MD: 1999. [Google Scholar]
- Gutnisky DA, Hansen BJ, Iliescu BF, Dragoi V. Attention alters visual plasticity during exposurebased learning. Curr Biol. 2009:555–560. doi: 10.1016/j.cub.2009.01.063. [DOI] [PubMed] [Google Scholar]
- Helmholtz HV. Treatise on physiological optics. 3rd ed. Optical Society of America; Rochester, NY: 1911. [Google Scholar]
- Herzog MH, Fahle M. The role of feedback in learning a vernier discrimination task. Vision Research. 1997;37:2133–2141. doi: 10.1016/s0042-6989(97)00043-6. [DOI] [PubMed] [Google Scholar]
- Herzog MH, Fahle M. Modeling perceptual learning: difficulties and how they can be overcome. Biological Cybernatics. 1998;78:107–117. doi: 10.1007/s004220050418. [DOI] [PubMed] [Google Scholar]
- Hua T, Bao P, Huang CB, Wang Z, Xu J, Zhou Y, Lu Z-L. Perceptual Learning Improves Contrast Sensitivity of V1 Neurons in Cats. Current Biology. 2010;20:887–894. doi: 10.1016/j.cub.2010.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Zhou Y, Lu ZL. Broad bandwidth of perceptual learning in the visual system of adults with anisometropic amblyopia. Proc Natl Acad Sci U S A. 2008;105:4068–4073. doi: 10.1073/pnas.0800824105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Jeter PE, Dosher B, Petrov A, Lu Z-L. Task precision at transfer determines specificity of perceptual learning. Journal of Vision. 2009;9:1–13. doi: 10.1167/9.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BB. Regeneration and plasticity in the brain and spinal cord. J Cereb Blood Flow Metab. 2007;27:1417–1430. doi: 10.1038/sj.jcbfm.9600486. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Dan Y. Experience-Dependent Plasticity in Adult Visual Cortex. Neuron. 2006;52:577–585. doi: 10.1016/j.neuron.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D. Where Practice Makes Perfect in Texture-Discrimination - Evidence for Primary Visual-Cortex Plasticity. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4966–4970. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Kumar T, Glaser DA. Initial perforamnce, learning and observer variability for hyperacuity tasks. Vision Research. 1993;33:2287–2300. doi: 10.1016/0042-6989(93)90106-7. [DOI] [PubMed] [Google Scholar]
- Law C-T, Gold JI. Neural correlates of perceptual learning in a sensorymotor, but not a sensory, cortical area. Nature Neuroscience. 2008;11:505–513. doi: 10.1038/nn2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Polat U. Neural plasticity in adults with amblyopia. Proc Natl Acad Sci U S A. 1996;93:6830–6834. doi: 10.1073/pnas.93.13.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RW, Levi DM. Characterizing the mechanisms of improvement for position discrimination in adult amblyopia. J Vis. 2004;4:476–487. doi: 10.1167/4.6.7. [DOI] [PubMed] [Google Scholar]
- Li RW, Levi DM, Klein SA. Perceptual learning improves efficiency by re-tuning the decision ‘template’ for position discrimination. Nat Neurosci. 2004a;7:178–183. doi: 10.1038/nn1183. [DOI] [PubMed] [Google Scholar]
- Li RW, Provost A, Levi DM. Extended perceptual learning results in substantial recovery of positional acuity and visual acuity in juvenile amblyopia. Invest Ophthalmol Vis Sci. 2007;48:5046–5051. doi: 10.1167/iovs.07-0324. [DOI] [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lu Z-L, Dosher B. Augmented Hebbian Learning Accounts for the Complex Pattern of Effects of Feedback in Perceptual Learning. Journal of Vision. 2010a;10:1115–1115. [Google Scholar]
- Liu J, Lu Z-L, Dosher B. Augmented Hebbian reweighting: Interactions between feedback and training accuracy in perceptual learning. Journal of Vision. 2010b;10:1–14. doi: 10.1167/10.10.29. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu Z-L, Dosher BA. Augmented Hebbian learning accounts for the Eureka effect in perceptual learning. Journal of Vision. 2009;9:851. [Google Scholar]
- Lu Z-L, Chu W, Dosher BA. Perceptual learning of motion direction discrimination in fovea: Separable mechanisms. Vision Research. 2006;45:2500–2510. doi: 10.1016/j.visres.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Dosher B. Characterizing observer states using external noise and observer models: Assessing internal representations with external noise. Psychological Review. 2008;115:44–82. doi: 10.1037/0033-295X.115.1.44. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Dosher BA. Perceptual learning retunes the perceptual template in foveal orientation identification. Journal of Vision. 2004;4:44–56. doi: 10.1167/4.1.5. [DOI] [PubMed] [Google Scholar]
- Lu Z-L, Liu J, Dosher B. Modeling Mechanisms of Perceptual Learning with Augmented Hebbian Reweighting. Vision Res. 2010;50:375–390. doi: 10.1016/j.visres.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. Practice improves adults’ sensitivity to diagonals. Vision Research. 1983;23:547–550. doi: 10.1016/0042-6989(83)90130-x. [DOI] [PubMed] [Google Scholar]
- McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- McKee SP, Westheimer G. Improvement in vernier acuity with practice. Perception & Psychophysics. 1978;24:258–262. doi: 10.3758/bf03206097. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Cholinergic modulation of responses to single tones produces tone-specific receptive field alterations in cat auditory cortex. Synapse. 1990;6:133–145. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- Ohzawa I, Sclar G, Freeman RD. Contrast gain control in the cat's visual system. J Neurophysiol. 1985;54:651–667. doi: 10.1152/jn.1985.54.3.651. [DOI] [PubMed] [Google Scholar]
- Petrov A, Dosher BA, Lu Z-L. Perceptual learning through incremental channel reweighting. Psychological Review. 2005;112:715–743. doi: 10.1037/0033-295X.112.4.715. [DOI] [PubMed] [Google Scholar]
- Petrov AA, Dosher BA, Lu ZL. Perceptual learning without feedback in non-stationary contexts: Data and model. Vision Research. 2006;46:3177–3197. doi: 10.1016/j.visres.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Poggio T, Fahle M, Edelman S. Fast perceptual learning in visual hyperacuity. Science. 1992;256:1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- Polat U, Ma-Naim T, Belkin M, Sagi D. Improving vision in adult amblyopia by perceptual learning. Proc Natl Acad Sci U S A. 2004;101:6692–6697. doi: 10.1073/pnas.0401200101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Braddick O. Orientation-specific learning in stereopsis. Perception. 1973;2:371–376. doi: 10.1068/p020371. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Schreiner CE. Changes in the distributed temporal response properties of SI cortical neurons reflect improvements in performance on a temporally based tactile discrimination task. J Neurophysiol. 1992;67:1071–1091. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Saarinen J, Levi DM. Perceptual learning in vernier acuity: What is learned? Vision Research. 1995;35:519–527. doi: 10.1016/0042-6989(94)00141-8. [DOI] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro. J Neurosci. 2000a;20:4286–4299. doi: 10.1523/JNEUROSCI.20-11-04286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, Nowak LG, McCormick DA. Membrane mechanisms underlying contrast adaptation in cat area 17 in vivo. J Neurosci. 2000b;20:4267–4285. doi: 10.1523/JNEUROSCI.20-11-04267.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiltz C, Bodart JM, Dubois S, Dejardin S, Michel C, Roucoux A, Crommelinck M, Orban G. Neuronal mechanisms of perceptual learning: Changes in human brain activity with training in orientation discrimination. NeuroImage. 1999;9:46–62. doi: 10.1006/nimg.1998.0394. [DOI] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: A functional MRI study of visual texture discrimination. Proceedings of the National Academy of Science. 2002;99:17137–17142. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclar G, Lennie P, DePriest DD. Contrast adaptation in striate cortex of macaque. Vision Res. 1989;29:747–755. doi: 10.1016/0042-6989(89)90087-4. [DOI] [PubMed] [Google Scholar]
- Seitz AR, Watanabe T. Is subliminal learning really passive? Nature. 2003;422:36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- Shibata K, Yamagishi N, Ishii S, Kawato M. Boosting perceptual learning by fake feedback. Vision Res. 2009;49:2574–2585. doi: 10.1016/j.visres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Shiu L.-p., Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Perception & Psychophysics. 1992;52:582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- Simmers AJ, Ledgeway T, Hess RF, McGraw PV. Deficits to global motion processing in human amblyopia. Vision Res. 2003;43:729–738. doi: 10.1016/s0042-6989(02)00684-3. [DOI] [PubMed] [Google Scholar]
- Vaina LM, Belliveau JW, des Roziers EB, Zeffiro TA. Neural systems underlying learning and representation of global motion. Proc Natl Acad Sci U S A. 1998;95:12657–12662. doi: 10.1073/pnas.95.21.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaina LM, Sundareswaran V, Harris JG. Learning to ignore: Psychophysics and computational modeling of fast learning of direction in noisy motion stimuli. Cognitive Brain Research. 1995;2:155–163. doi: 10.1016/0926-6410(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Vallabha GK, McClelland JL. Success and failure of new speech category learning in adulthood: Consequences of learned Hebbian attractors in topographic maps. Cognitive, Affective and Behavioral Neuroscience. 2007;7:53–73. doi: 10.3758/cabn.7.1.53. [DOI] [PubMed] [Google Scholar]
- Vogels R, Orban GA. The effect of practice on the oblique effect in line orientation judgments. Vision Research. 1985;25:1679–1687. doi: 10.1016/0042-6989(85)90140-3. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Harner AM, Miyauchi S, Sasaki Y, Nielsen M, Palomo D, Mukai I. Task-dependent influences of attention on the activation of human primary visual cortex. Proc Natl Acad Sci U S A. 1998;95:11489–11492. doi: 10.1073/pnas.95.19.11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Nanez JE, Sr., Koyama S, Mukai I, Liederman J, Sasaki Y. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nature Neuroscience. 2002;5:1003–1009. doi: 10.1038/nn915. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Nanez JES, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci U S A. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Y, Edelman S, Fahle M. Models of perceptual learning in vernier hyperacuity. Neural Computation. 1993;5:695–718. [Google Scholar]
- Xiao L, Zhang J, Wang R, Klein S, Levi DM, Yu C. Complete Transfer of Perceptual Learning across Retinal Locations Enabled by Double Training. Current Biology. 2008;18:1922–1926. doi: 10.1016/j.cub.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Lu Z-L, Wang X, Dosher B, Zhou J, Yang R, Zhang D, Zhou Y. Category and Perceptual Learning in Subjects with Treated Wilson's Disease. PLoS ONE. 2010;5:9635–9643. doi: 10.1371/journal.pone.0009635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Shi L, Han F, Gao H, Dan Y. Rapid learning in cortical coding of visual scenes. Nat Neurosci. 2007;10:772–778. doi: 10.1038/nn1895. [DOI] [PubMed] [Google Scholar]
- Zhaoping L, Herzog MH, Dayan P. Quadratic ideal observation and recurrent preprocessing in perceptual learning. Network: Computation in Neural Systems. 2003;14:223–247. [PubMed] [Google Scholar]
- Zhou YF, Huang CB, Xu PJ, Tao LM, Qiu ZP, Li X, Lu ZL. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Research. 2006;46:739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]