Summary
Background
Hematological abnormalities are common manifestations of advanced HIV-1 infection that could affect the outcomes of highly-active antiretroviral therapy (HAART). Although most HIV-1-infected individuals live in resource-constrained countries, there is little information about the frequency of hematological abnormalities such as anemia, neutropenia, and thrombocytopenia among individuals with advanced HIV-1 disease.
Methods
This study compared the prevalence of pre-antiretroviral therapy hematological abnormalities among 1571 participants in a randomized trial of antiretroviral efficacy in Africa, Asia, South America, the Caribbean, and the USA. Potential covariates for anemia, neutropenia, and thrombocytopenia were identified in univariate analyses and evaluated in separate multivariable models for each hematological condition.
Results
The frequencies of neutropenia (absolute neutrophil count ≤ 1.3 × 109/l), anemia (hemoglobin ≤ 10 g/dl), and thrombocytopenia (platelets ≤ 125 × 109/l) at initiation of antiretroviral therapy were 14%, 12%, and 7%, respectively, and varied by country (p < 0.0001 for each). In multivariable models, anemia was associated with gender, platelet count, and country; neutropenia was associated with CD4+ lymphocyte and platelet counts; and thrombocytopenia was associated with country, gender, and chronic hepatitis B infection.
Conclusions
Differences in the frequency of pretreatment hematological abnormalities could have important implications for the choice of antiretroviral regimen in resource-constrained settings.
Keywords: HIV, Anemia, Neutropenia, Thrombocytopenia, Resource-limited countries
Introduction
Hematological abnormalities (neutropenia, anemia, and thrombocytopenia) are common manifestations of advanced HIV-1 infection that could potentially limit the use of some components of antiretroviral therapy (ART) regimens.1,2 It is not known if the prevalence of hematological abnormalities at the time of ART initiation in persons with advanced HIV-1 infection differs around the world. Knowledge of how pretreatment hematological parameters compare across diverse settings is needed to inform recommendations for the choice of initial antiretroviral regimens in developing countries. The present study estimated the prevalence of neutropenia, anemia, and thrombocytopenia at the initiation of ART, and investigated associations between each of pretreatment neutropenia, anemia, and thrombocytopenia and baseline covariates of race, geographic location, gender, HIV-1 disease status, and nutritional status.
Methods
Study population
The AIDS Clinical Trials Group (ACTG) Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS) study is an ongoing clinical trial (ClinicalTrials.gov identifier NCT00084136) that enrolled 1571 antiretroviral-naïve participants in nine countries (Brazil, Haiti, India, Malawi, Peru, South Africa, Thailand, the USA, and Zimbabwe) between May 2005 and August 2007. Entry criteria included CD4+ lymphocyte count <300 cells/mm3, ART-naïve, no recent acute illness (i.e., pneumonia, gastroenteritis, or pelvic inflammatory disease) or opportunistic infections (OI), absolute neutrophil count (ANC) >0.75 × 109/l, hemoglobin >7.5 g/dl, platelet count >50 × 109/l, calculated creatinine clearance >60 ml/min, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase less than five times the upper limit of normal, and total bilirubin less than 2.5-fold above the upper limit of normal. Women who received single-dose nevirapine or zidovudine for any period of time during pregnancy were included. Women with the use of two or more antiretroviral drugs to prevent vertical transmission within 6 months of enrollment for greater than 7 days were excluded. Patients were excluded from the study if they had chemotherapy or radiation therapy within 45 days of enrollment. Informed consent was obtained from all participants, and the human experimentation guidelines of the US Department of Health and Human Services and local site institutional review boards and ethics committees were followed.
Data analysis
Neutropenia was defined as an ANC ≤ 1.3 × 109/l and anemia was defined as a hemoglobin =10.0 g/dl. Anemia was further evaluated by the mean corpuscular volume (MCV) and defined as microcytic if the MCV was <82 fl, normal if between 82 and 100 fl, and macrocytic if >100 fl. Thrombocytopenia was defined as a platelet count <125 × 109/l.
Multivariable logistic methods were used to explore the correlates of baseline anemia, neutropenia, and thrombocytopenia as individual outcome variables. Covariates explored included the following: age; screening CD4+ lymphocyte count; plasma HIV-1 RNA; body mass index (BMI); ANC (except for neutropenia); platelet count (except for thrombocytopenia); hemoglobin level (except for anemia); history of AIDS-related diagnoses; self-reported quality of life category (obtained by standardized interview); Karnofsky score group; self-identified race; self-identified ethnicity; sex; use of alternative therapies; current ongoing AIDS diagnosis; and hepatitis B surface antigen (HBsAg) positivity.
A purposeful variable selection method (as opposed to a mechanical method) was used to build multivariable logistic models for each of the three hematological outcomes of interest. An initial multivariable model included all covariates whose likelihood ratio p-value from the univariate model was <0.2. More parsimonious models were explored by removal of covariates one at a time, starting with the covariate with the largest p-value. The Akaike’s information criterion (AIC) adjustment to the log likelihood statistic (which penalizes for more complex models), was used to compare nested models. Even if removal of the covariate did not significantly affect the model fit by the AIC, if it was a confounder or effect modifier (e.g., resulted in >20% change in the parameter estimate of another covariate in the model) then the covariate was retained in the model. Confounding between country and race was initially handled by stratifying models by country. The resulting preliminary main effects model was then tested to see if continuous covariates were linear in the logit (and if not, groupings suggested by the data were chosen for best fit). Finally, each two-way interaction between covariates was introduced into the model one at a time. Goodness of fit for the unstratified model was considered by using the Hosmer and Lemeshow goodness-of-fit test. In final, non-stratified models, the country effect was parameterized as difference from overall countries (rather than using a reference group). Estimated (adjusted) odds ratios (AOR) and corresponding 95% confidence intervals (CIs) for covariates in the final model are presented here.
Results
Complete baseline hematological data were available for 1532 of 1571 participants (98%). The study population was 53% male, median age 34 years, with a median weight of 60.3 kg and BMI of 22.3 kg/m2. The median CD4 lymphocyte count was 172 cells/mm3.
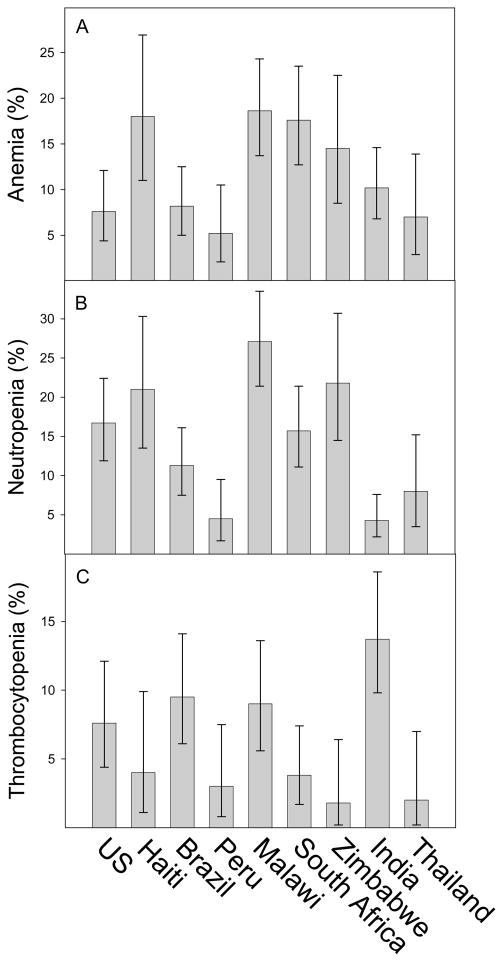
Anemia was present in 187 participants (11.9%; 95% CI 10.3–13.6%). Fifty percent of anemias were microcytic and 50% were normocytic; macrocytic anemia was uncommon (one case). The odds of anemia were associated with having a previous AIDS-defining diagnosis (AOR 1.9; 95% CI 1.1–3.3) and BMI (AOR for each 5-unit decrease in BMI 1.5; 95% CI 1.2–1.9). Females had higher odds of anemia than males, but the magnitude of the odds ratio depended on CD4+ cell count: 50 cells/mm3 (AOR 3.0; 95% CI 1.8–5.0); 150 cells/mm3 (AOR 5.0; 95% CI 3.2–7.8); 250 cells/mm3 (AOR 8.3; 95% CI 3.8–18.2). Anemia prevalence varied by country (Figure 1A; p < 0.0001) and the prevalence of anemia was highest in Malawi, Haiti, South Africa, and Zimbabwe. Significant heterogeneity in odds of anemia by country remained after controlling for BMI, gender, platelet count, history of AIDS OI, and screening CD4+ cell count (p = 0.009); odds for anemia were highest in South Africa (AOR 1.8; 95% CI 1.2–2.7), Haiti (AOR 1.6; 95% CI 0.9–2.7), and Malawi (AOR 1.5; 95% CI 1.1–2.2). Only 36 women took zidovudine for preventing mother-to-child transmission for an average of 50.9 days at different times of exposure prior to enrollment in the study.
Figure 1.
Prevalence of hematological abnormalities prior to initiation of antiretroviral therapy, by country. (A) Anemia, defined as a hemoglobin ≤10.0 g/dl. (B) Neutropenia, defined as an absolute neutrophil count ≤1.3 × 109/l. (C) Thrombocytopenia, defined as a platelet count less than 125 × 109/l.
Neutropenia was present in 224 participants (14.3%; 95% CI 12.6–16.1%). The odds of neutropenia were 0.85 (95% CI 0.77–0.94) for every 50 cells/mm3 increase in CD4 lymphocytes; 0.6 (95% CI 0.5–0.7) for every 100 × 109/l increase in platelets; and 0.7 (95% CI 0.4–1.1) for every increase of hemoglobin by 5 mg/dl. Compared to men, women had a higher odds of neutropenia (AOR 1.3; 95% CI 0.9–1.9), and compared to Hispanics, non-Hispanics had higher odds (AOR 1.5; 95% CI 0.7–3.4), whereas other ethnicities (small groups) had lower odds (AOR 0.4; 95% CI 0.2–0.8). The prevalence of neutropenia was associated with country (Figure 1B; p < 0.0001): neutropenia was highest in Malawi, Zimbabwe, Haiti, the USA, and South Africa. Significant heterogeneity in odds of neutropenia by country remained after controlling for CD4+ lymphocyte count, hemoglobin, platelets, sex, and ethnicity (p < 0.0001). The adjusted odds of neutropenia were greatest in Malawi (AOR 2.3; 95% CI 1.5–3.3), Haiti (AOR 2.2; 95% CI 1.3–3.6), and Zimbabwe (AOR 1.6; 95% CI 1.0–2.6).
Thrombocytopenia was present in 113 participants (7.2%; 95% CI 6–8.6%). Thrombocytopenia was associated with HBsAg (AOR 2.3; 95% CI 1.1–4.3) and decreased odds were associated with female sex (AOR 0.5; 95% CI 0.3–0.8) and ANC <1.75 × 109/l (AOR 4.3; 95% CI 2.0–10.7). Thrombocytopenia was associated with country (Figure 1C; p < 0.0001) with highest prevalences in India, Brazil, Malawi, and the USA. After controlling for HBsAg, sex, and ANC, significant heterogeneity remained across countries (p < 0.0001). The adjusted odds of thrombocytopenia were greatest in India (AOR 3.7; 95% CI 2.3–5.8), Brazil (AOR 2.2; 95% CI 1.3–3.6), and Malawi (AOR 1.8; 95% CI 1.1–3.0).
Out of the 1554 persons with all three measurements at baseline (out of 1571 randomized), 18 (or 1.2%) had both neutropenia and thrombocytopenia, 14 (or 0.9%) had both anemia and thrombocytopenia, 34 (or 2.2%) had both anemia and neutropenia, and five (or 0.3%) had pancytopenia. Overall then, a total of 71 (or 4.8%) participants with all three evaluations had more than one type of cytopenia.
Discussion
The PEARLS study provides the first comparative analysis of hematological abnormalities among people with advanced untreated HIV-1 infection around the world. Our study is unique because hematological data were collected prospectively from treatment-naïve individuals at the time of entry into a clinical trial of ART in nine countries in diverse geographic settings. Because participants were required to meet uniform inclusion and exclusion criteria prior to study entry, the study population was more homogeneous across geographic areas than independent cohorts.
Anemia is an important hematological marker because it has been reported to be a significant independent risk factor for mortality in HIV-1-infected patients.3 The high rate of anemia in Africa and Haiti could be related to the levels of poverty, malnutrition, and the overall poor economic state in these areas of the world. The gross domestic product (GDP) purchasing power parity (PPP) per capita in Haiti, Malawi, and Zimbabwe are US$1317, US$836, and US$236, respectively, compared to Thailand, Peru, Brazil, and the USA, where the GDP PPP are US$8239, US$8594, US$10 466, and US$47 700, respectively.4
Fifty percent of the anemias observed at baseline were microcytic and the majority (specifically 360 or 67% of 541) of participants in Africa were women (of whom 93% reported reproductive potential, and only 4% were older than 50 years), therefore the significant percentage of microcytic anemia may presumably have been from menstrual-related blood loss and/or recent pregnancy. We cannot exclude the possibility that human genetics were a factor in the occurrence of anemia in this study. However, inheritable causes of anemia, such as sickle cell trait/disease and thalassemias, are not as prevalent in the southern African countries where this study was performed.5,6
Other studies have provided evidence that there are high risks of severe anemia after starting ART in resource-limited settings. In the DART study in Zimbabwe and Uganda there was a higher prevalence of severe anemia (hemoglobin <6.5 g/dl) in patients on zidovudine than expected from data from developed countries.7 Predictive factors for severe anemia after starting antiretroviral medication in DART were female sex, baseline anemia, low CD4, and low BMI. Our study also showed similar risk factors for anemia. Anemia has been shown to be associated with HIV disease progression. There are several proposed mechanisms of interruption of hematopoiesis through disruption of several cytokine mechanisms by HIV infection of bone marrow stromal cells and macrophages.8 Pretreatment anemia was also highly predictive of anemia after initiation of zidovudine in the TAHOD cohort from the Asia- Pacific region, indicating that in areas of the world where there are significant rates of baseline anemia, hematological parameters should be monitored closely after initiation of zidovudine.9 Previous zidovudine usage for pregnancy may have contributed to the baseline anemia in women, however the overall small exposure pre-enrolment to the study makes this unlikely. Our finding from the PEARLS study that the prevalence of pretreatment anemia differs across diverse resource-limited settings suggests that geographic location may be associated with the risk of anemia after initiation of ART.
Neutropenia is a common hematological abnormality in persons with untreated HIV-1 infection. Several mechanisms for neutropenia in HIV-1 infection have been proposed, including decreased production of granulocyte colony-stimulating factor (G-CSF), a soluble inhibitory substance that decreases neutrophil production,10–12 and autoimmunity.13 The Women’s Interagency HIV Study cohort found baseline neutropenia in 44% of this cohort and a longitudinal analysis found that worsening HIV-1 disease was associated with subsequent neutropenia.1 Neutropenia was observed in 21% of patients prior to starting co-trimoxazole prophylaxis in Côte d’Ivoire.14 Our finding that neutropenia in the PEARLS cohort was independently associated with CD4+ lymphocyte and platelet counts, hemoglobin level, sex, ethnicity, and country, suggests that the stage of HIV-1 infection is an important contributor to pre-treatment neutropenia.
Pre-antiretroviral thrombocytopenia was less commonly observed than neutropenia or anemia in the PEARLS study. Interestingly, the geographic distribution of thrombocytopenia was different than the distributions of anemia and neutropenia in PEARLS. The highest prevalences of anemia and neutropenia were observed in southern Africa, while the highest prevalences of thrombocytopenia were observed in India and Brazil. Higher odds of thrombocytopenia were independently associated with chronic active hepatitis B virus infection (HBV, as indicated by HBsAg positivity), male gender, and lower ANC. Thrombocytopenia in individuals with HBV can result from increased platelet sequestration from hypersplenism, effects of chronic illness on platelet production and maturation, and autoimmunity.15–17 HIV is also associated with thrombocytopenia and may be caused by a direct effect on megakaryocytes by the HIV virus. Anti-gp120 antibodies and platelet-specific antibodies have also been reported on platelets in HIV-seropositive patients.13,18
While the PEARLS study provides a unique opportunity to compare the factors associated with mild to moderate hematological abnormalities across diverse populations, this study has several important limitations. Since persons with grade 3 or 4 hematological abnormalities19 were excluded from participation in PEARLS, it is likely that the true prevalences of anemia, neutropenia, and thrombocytopenia among persons initiating antiretroviral therapy in these areas of the world are higher. The PEARLS entry criteria likely resulted in the selection of a study group that is overall healthier than the population of people who are initiating ART in these areas of the world (also known as the clinical trials or research selection bias). Despite these limitations, the PEARLS study provides evidence that the prevalence of hematological abnormalities in untreated HIV-1 infection is independently related to factors such as geographic location, gender, and chronic co-infections. These findings have implications for the optimal choice of initial antiretroviral agents, monitoring of antiretroviral toxicities, and improvement of antiretroviral treatment programs, especially in resource-limited countries.
Acknowledgments
This study was supported by grants from the National Institutes of Health AIDS Clinical Trials Group (ACTG), University of Witwatersrand 1U01 AI069463-01, ACTG Statistical and Data Analysis Center U01 AI068634, University of North Carolina Lilongwe CRS 1U01 AI069518-011, Partners/Harvard AIDS CTU 1U01-AI069472-01, IMPACTA Peru 1U01 AI069438-01 NARI-CTU, Pune India 1 U01AI069417-01, University of Colorado 1U01 AI069497-01, UCLA AIDS Prevention and Treatment CTU 1U01 AI069424-01, Johns Hopkins Adult AIDS CTU 1U01AI069465-01, Vanderbilt HIV CTUI 1U02 AI069439-01, University of Zimbabwe–University of California CTU U01A1069436-01.
The authors would first and foremost like to thank the Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS) study participants who volunteered their time and efforts.
The authors acknowledge the contributions of the following investigators: Edith Swann, HIV Research Branch, TRP, DAIDS, NIAD, NIH, Bethesda; Karin Klingman, HIV Research Branch, TRP, DAIDS, NIAID, NIH, Bethesda; Ronald L. Barnett, ACTG Operations Centre, Social and Scientific Systems, Inc., Silver Spring; Barbara Brizz, ACTG Operations Center, Social and Scientific Systems, Inc., Silver Spring; Victor De Gruttola, Statistical and Data Analysis Center, Harvard School of Public Health, Boston; Yvette Delph, ACTG Operations Center, Social and Scientific Systems, Inc., Silver Spring; Nikki Gettinger, ACTG Operations Center, Social and Scientific Systems Inc., Silver Spring; Ann Walawander, Frontier Science and Technology Research Foundation, Amherst; Apsara Nair, Frontier Science and Technology Research Foundation, Amherst; Ana I. Martinez, Pharmaceutical Affairs Branch DAIDS, NIAID, NIH, Bethesda; Ronald T. Mitsuyasu, UCLA CARE Center, Los Angeles; Susan H. Eshleman, The Johns Hopkins Medical Institution, Baltimore; Susan A. Fiscus, University of North Carolina, School of Medicine, Chapel Hill; Adriana Andrade, Division of Infectious Diseases, John Hopkins University, Baltimore; David W. Haas, Infectious Diseases, Vanderbilt University, Nashville; Farida Amod, Nelson R Mandela School of Medicine, Durban; Vladimir Berthaud, Infectious Diseases, Vanderbilt University Medical Centre, Nashville; Robert C. Bollinger, Infectious Diseases, Vanderbilt University Medical Centre, Nashville; Yvonne Bryson, UCLA School of Medicine, Los Angeles; David Celentano, Johns Hopkins School of Hygiene and Public Health, Baltimore; David Chilongozi, Lilongwe, Malawi; Myron Cohen, University of North Carolina, Chapel Hill; Ann C. Collier, University of Washington, Harborview Medical Centre, Seattle; Judith Silverstein Currier, University of California, Los Angeles; Susan Cu-Uvin, The Miriam Hospital, Brown University, Providence; Joseph Eron, Division of Infectious Diseases, University of North Carolina; Charles Flexner, Johns Hopkins University Hospital, Baltimore; Joel E. Gallant, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore; Beatriz Grinsztejn, Evandro Chagas Clinical Research Institute, Brazil; Roy M. Gulick, The Cornell Clinical Trials Unit, New York; Scott M. Hammer, Division of Infectious Diseases, Columbia Presbyterian Medical Centre; Irving Hoffman, University of North Carolina, Chapel Hill; Mina Hosseinipour, Division of Infectious Diseases, University of North Carolina, Chapel Hill; Peter Kazembe, Lilongwe Central Hospital, Lilongwe, Malawi; Newton Kumwenda, Johns Hopkins Project, Malawi; Umesh Gangaram Lalloo, Family HIV Clinic, Nelson R Mandela School of Medicine, Durban; Javier R. Lama, Investigaciones Medicas en Salud (INMENSA), Peru; Jody Lawrence, University of California, San Francisco, Adult AIDS Clinical Trials Unit; Chiedza Maponga, Medical University of Zimbabwe, Zimbabwe; Francis Martinson, UNC Project, Lilongwe; Kenneth Mayer, Division of Infectious Diseases, Brown University School of Medicine, Pawtucket; Karin Nielsen, UCLA School of Medicine, Los Angeles; Jean William Pape, Cornell-GHESKIO, Haiti; Richard B. Pendame, Malawi; Bharat Ramratnam, Laboratory of Retrovirology, Brown University Medical School, Providence; Steve Safren, Department of Psychiatry, Massachusetts General Hospital, Boston; Jorge Sanchez, IMPACTA Peru Clinical Trials Unit; Ian M. Sanne, University of Witwatersrand, Infection Diseases Syndicate, Johannesburg; Breno Riegel Santos, Hospital Nossa Senhora da Conceicao-GHC, Brazil; Robert T. Schooley, University of California, San Diego; Patrice Severe, Infectious Diseases, Institut de Laboratoires et de Recherches, Haiti; Thira Sirisanthana, Research Institute for Health Sciences, Chiang Mai University, Thailand; Suniti Solomon, YRG Centre for AIDS Research and Education, India; Khuanchai Supparatpinyo, Department of Medicine, Chiang Mai University, Thailand; Steve Tabet, University of Washington, Harborview Medical Centre, Seattle; Taha Taha, Johns Hopkins University, School of Hygiene and Public Health, Baltimore; Srikanth Tripathy, National AIDS Research Institute, India; Charles van der Horst, Department of Medicine, University of North Carolina, Chapel Hill; Christine Wanke, Tufts University School of Medicine, Boston; Joan Gormley, The Miriam Hospital, Immunology Centre, Providence; Cheryl J. Marcus, University of North Carolina, Chapel Hill; Beverly Putnam, University of Colorado Health Sciences, Denver; Smanga Ntshele, Community Advisory Board Member, Durban; Carolyn Conner, Clinical and Scientific Affairs, Boehringer Ingelheim Pharmaceuticals, Ridgefield; Marita D. McDonough, Clinical and Scientific Affairs, Boehringer Ingelheim Pharmaceuticals, Ridgefield; Jonathon Uy, Bristol-Myers Squibb, Plainsboro; Gary Thal, Virology Medical Affairs, Bristol-Myers Squibb, Princeton; James F. Rooney, Clinical Research, Gilead Sciences, Inc., Foster City; Audrey L. Shaw, Gilead Sciences Inc., Clinical Research/Medical Affairs, Durham; Edde Loeliger, Clinical Development and Medical Affairs, Greenford, Middlesex; Keith A. Pappa, GlaxoSmithKline, Infectious Diseases Medicine, Triangle Park; Nancy Webb, Frontier Science and Technology Research Foundation, Inc., Amherst; David L. Shugarts, University of Colorado Health Sciences, Denver; Mark A. Winters, Stanford University Medical Center, Division of Infectious Diseases, Stanford.
Footnotes
Conflict of interest: None of the authors have any conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levine A, Karim R, Mack W, et al. Neutropenia in human immunodeficiency virus infection: data from the Women’s Interagency HIV Study. Arch Intern Med. 2006;166:405–10. doi: 10.1001/archinte.166.4.405. [DOI] [PubMed] [Google Scholar]
- 2.Levine A, Berhane K, Karim R, et al. Impact of highly active antiretroviral therapy on anemia and relationship between anemia and survival in a large cohort of HIV-infected women: Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2004;37:1245–52. doi: 10.1097/01.qai.0000134759.01684.27. [DOI] [PubMed] [Google Scholar]
- 3.Moore R, Keruly J, Chaisson R. Anemia and survival in HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Wikipedia. List of countries by GDP (PPP) per capita. [accessed November 18, 2009]; Available at: http://en.wikipedia.org/wiki/List_of_countries_by_GDP_(PPP)_per_capita.
- 5.Serjeant G. Mortality from sickle cell anemia disease in Africa. BMJ. 2005;330:432–3. doi: 10.1136/bmj.330.7489.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Sickle cell anemia: report by the Secretariat. Secretariat Board Session 117. Geneva: WHO; 2005. pp. 1–5. [Google Scholar]
- 7.Ssali F, Stohr W, Munderi P, et al. Prevalence, incidence and predictors of severe anemia with zidovudine-containing regimens in African adults with HIV infections within the DART trial. Antivir Ther. 2006;11:741–9. doi: 10.1177/135965350601100612. [DOI] [PubMed] [Google Scholar]
- 8.Semba RD, Gray GE. Pathogenesis of anemia during human immunodeficiency virus infection. J Investig Med. 2001;49:225–39. doi: 10.2310/6650.2001.33967. [DOI] [PubMed] [Google Scholar]
- 9.Huffam S, Srasuebkul P, Zhou J, et al. Prior antiretroviral therapy experience protects against zidovudine-related anemia. HIV Med. 2007;8:465–71. doi: 10.1111/j.1468-1293.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 10.Leiderman IZ, Greenberg ML, Adelsberg BR, et al. A glycoprotein inhibitor of in vitro granuloporesis associated with AIDS. Blood. 1987;70:1267–72. [PubMed] [Google Scholar]
- 11.Mauss S, Steinmetz HT, Willers R, et al. Induction of granulocyte colony-stimulating factor by acute febrile infection but not by neutropenia in HIV seropositive individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:430–4. doi: 10.1097/00042560-199704150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Moses AV, Williams S, Heneveld ML, et al. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood. 1996;87:919–25. [PubMed] [Google Scholar]
- 13.Murphy MF, Metcalfe P, Waters HA, et al. Incidence and mechanism of neutropenia and thrombocytopenia in patients with human immunodeficiency virus infection. Br J Haematol. 1987;66:337–40. doi: 10.1111/j.1365-2141.1987.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 14.Toure S, Gabillard D, Inwoley A, et al. Incidence of neutropenia in HIV-infected African adults receiving co-trimoxazole prophylaxis: a 6 year cohort study in Abidjan, Côte D’Ivoire. Trans R Soc Trop Med Hyg. 2006;100:785–90. doi: 10.1016/j.trstmh.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Panasiuk A, Zak J. Autoimmune thrombocytopenia in chronic liver disease. Pol Merkur Lekarsk. 2001;11:487–90. [PubMed] [Google Scholar]
- 16.Michalska Z, Stalke P, Witczak-Malinowska K, et al. Autoimmune reactions in HBV and HCV. Med Sci Monit. 2001;7(Suppl 1):175–80. [PubMed] [Google Scholar]
- 17.Ciernik IF, Cone RW, Fehr J, et al. Impaired liver function and retroviral activity are risk factors contribution to HIV associated thrombocytopenia. Swiss HIV Cohort Study. AIDS. 1999;13:1913–20. doi: 10.1097/00002030-199910010-00014. [DOI] [PubMed] [Google Scholar]
- 18.Fauci A, Lane C. Harrison’s principles of internal medicine. 17. McGraw-Hill; 2008. Human immunodeficiency virus disease: AIDS and related disorders; p. 1180. [Google Scholar]
- 19.Division of AIDS (DAIDS) Table for grading the severity of adult and pediatric adverse events. DAIDS RSC. 2004 December; Available at: http://rcc.tech-res.com/DAIDS%20RCC%20Forms/TB_ToxicityTables_DAIDS_AE_GradingTable_FinalDec2004.pdf (accessed )