Abstract
Background
Excess caloric intake is strongly associated with the development of increased adiposity, glucose intolerance, insulin resistance, dyslipidemia, and hyperleptinemia (i.e., the cardiometabolic syndrome). Research efforts have focused attention primarily on the quality (i.e., nutritional content) and/or quantity of ingested calories as potential causes for diet-induced pathology. Despite growing acceptance that biological rhythms profoundly influence energy homeostasis, little is known regarding how the timing of nutrient ingestion influences development of common metabolic diseases.
Objective
To test the hypothesis that the time of day at which dietary fat is consumed significantly influences multiple cardiometabolic syndrome parameters.
Results
We report that mice fed either low or high fat diets in a contiguous fashion during the 12 hour awake/active period adjust both food intake and energy expenditure appropriately, such that metabolic parameters are maintained within a normal physiologic range. In contrast, fluctuation in dietary composition during the active period (as occurs in humans) markedly influences whole body metabolic homeostasis. Mice fed a high fat meal at the beginning of the active period retain metabolic flexibility in response to dietary challenges later in the active period (as revealed by indirect calorimetry). Conversely, consumption of high fat meal at the end of the active phase leads to increased weight gain, adiposity, glucose intolerance, hyperinsulinemia, hypertriglyceridemia, and hyperleptinemia (i.e., cardiometabolic syndrome) in mice. The latter perturbations in energy/metabolic homeostasis are independent of daily total or fat-derived calories.
Conclusions
The time-of-day at which carbohydrate versus fat is consumed markedly influences multiple cardiometabolic syndrome parameters.
Keywords: Adiposity, Chronobiology, Feeding, Glucose Tolerance, Metabolism
Introduction
Concurrent with the dramatic rise in obesity and related co-morbidities that has taken place in the last 25 years, a number of striking changes have also occurred in our environment during this time. In addition to an increase in the abundance of energy dense, low quality, highly palatable food and a decrease in physical activity duration, time spent awake has also increased dramatically. We now live in a 24-hour (hr) environment, in which patterns of sleeping, eating, and physical activity may be substantially out of synchrony with the daily cycles of light and dark. Several studies have demonstrated that both shift work and sleep deprivation are associated with increased risk for obesity, type 2 diabetes, and cardiovascular disease (1-3). Only a limited number of human studies have examined the influences that the timing of feeding has on features of the cardiometabolic syndrome (4, 5). Similarly, very few animal studies have been performed to address this highly relevant question. Recently, Arble et al. reported that limiting high fat intake to the 12-hr light phase (which represents the inactive/sleep phase for rodents) results in significantly greater weight gain in mice compared to animals restricted to high fat feeding during the 12-hr dark phase (the active/awake phase for rodents) (6). While this observation is important, limiting food availability to the time in which these nocturnal animals are normally sleeping is also associated with disturbed biological rhythms at multiple levels, ranging from behavior (e.g., sleep) to molecular (e.g., circadian clocks) (7).
The purpose of the present study was to examine how manipulations in the timing of feeding during the normal waking phase would influence physiologic measures related to the cardiometabolic syndrome. Here we demonstrate that when mice are fed contiguously (either high or low fat diets) during their normal waking period, they are able to respond to the caloric density of the food and adjust both food intake and energy expenditure to maintain normal growth and adiposity. In contrast, mice fed a variable diet that includes time-of-day-restricted “meals” of both low fat and high fat food demonstrate significant differences in their phenotypic response to the changes in food quality. We report that high fat feeding at the transition from sleeping to waking appears to be critically important in enabling metabolic flexibility and adaptation to high carbohydrate meals presented at later time points. Conversely, high carbohydrate feeding at the beginning of the waking period dramatically impairs the metabolic plasticity required for responding appropriately to high fat meals presented at the end of the waking period. Calorically dense high fat food ingestion at the end of the waking period is associated with excessive body weight gain, adiposity, impaired glucose tolerance, hyperinsulinemia, hypertriglyceridemia, and hyperleptinemia (i.e., cardiometabolic syndrome), despite no differences in daily total or fat-derived calories. These findings are directly relevant to humans, in which mixed meals and high caloric density at the end of the waking period have become the norm.
Materials and Methods
Animals
Male WT mice (on the FVB/N background) were housed at the Children's Nutrition Research Facility of the Children's Nutrition Research Center at Baylor College of Medicine (Houston, TX) under temperature-, humidity-, and light- controlled conditions. A strict 12-hour light/12-hour dark cycle regime was enforced (lights on at 6AM; zeitgeber time [ZT] 0). Mice received food and water ad libitum, unless otherwise specified. Mice were housed in standard micro-isolator cages, prior to initiation of feeding protocols (during which time mice were housed on wire-bottom cages to prevent consumption of bedding and feces). All animal experiments were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Rodent diets and feeding studies
A high fat diet (45% calories from fat, Research Diets, New Brunswick, NJ; catalogue number D12451) and a control diet for the high fat feeding studies (10% calories from fat, Research Diets, New Brunswick, NJ; catalogue number D12450B) were utilized for this study. Diets were matched for protein content, and fat and carbohydrate were derived from the same source for each diet. Mice were randomly assigned to one of four different experiments (see Figure 1). The first and second studies included extended (12-hr) periods of contiguous access to a specific diet. In the first study (Figure 1A), mice were fed either 12-hr high fat diet during the light phase (starting at ZT 0) followed by 12-hr control diet during the dark phase (starting at ZT 12), or 12-hr of control diet during the light phase followed by 12-hr high fat diet during the dark phase. In the second, third, and forth studies, food access was restricted to the 12-hr dark period, which represents the normal waking period for these nocturnal animals. In the second study (Figure 1B), mice were fed either a high fat or a control diet for the entire 12-hr dark phase. The third and forth studies were designed to simulate “meals” of different compositions presented at distinct times during the awake/active phase. The awake/active phase was divided into three distinct four-hour time periods (TPs; which could be considered periods of breakfast, lunch, and dinner, for TP1, TP2, and TP3 respectively). In the third study (Figure 1C), mice were either given 4 hours of high fat diet at the beginning of the dark phase (i.e., sleep/wake transition, TP1) followed by 8 hours of control diet (TP2 and TP3), or mice were given 8 h of control diet at the beginning of the dark phase (i.e., TP1 and TP2), followed by 4 hours of high fat diet during the last four hours of the dark phase (i.e., TP3). In the fourth experiment (Figure 1D), 4 hours meals at the beginning and end of the waking period were separated by 4 hours of food restriction. Mice were either given a high fat “meal” during the first four hours of the dark phase (i.e., sleep/wake transition, TP1) followed by no food for four hours (i.e., TP2) and a control “meal” during the last 4 hours of the waking period (i.e., TP3) or mice were given a control “meal” upon waking (i.e., first 4 hours of the dark phase, TP1) followed by no food for four hours (i.e., TP2) and a high fat “meal” during the last four hours of the dark phase (i.e., TP3). All feeding regimes were enforced for 12 weeks.
Figure 1.
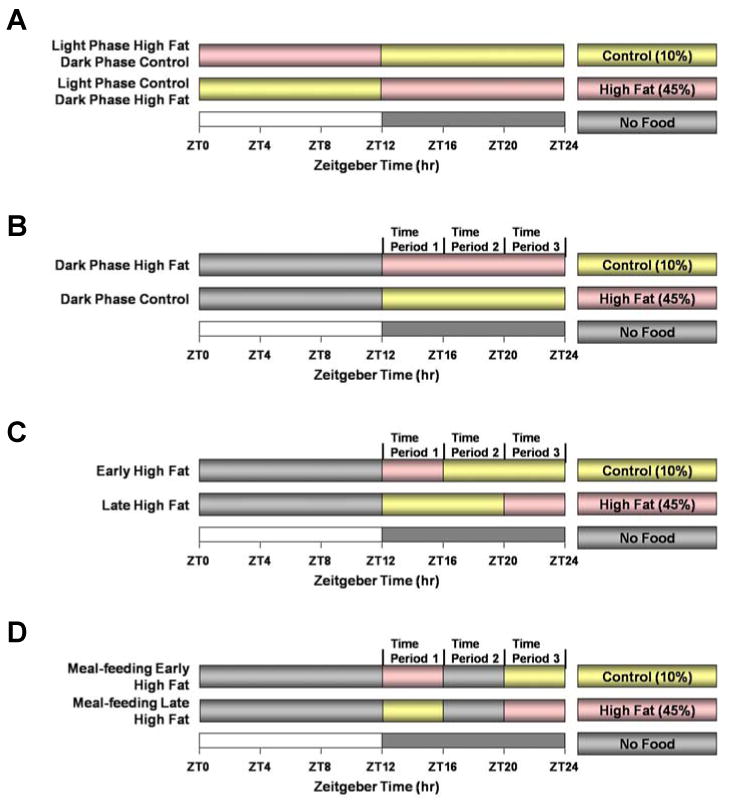
Feeding regimes employed in the current study.
Non-invasive mouse monitoring
Food intake was monitored daily during feeding protocols, using self-contained feeders designed to eliminate spillage (Research Products International Corp., Mt. Prospect, IL). Body weight was monitored in mice during feeding protocols at one-week intervals. Twenty-four hour patterns of food intake, energy expenditure (indirect calorimetry), and physical activity were measured using a Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments Inc., Columbus, OH). CLAMS-derived data are presented at 4-hour intervals; data at 30-minute intervals are located within Supplementary Figures, with the exception of Figure 6.
Figure 6.
Mice were divided into two distinct feeding groups, as depicted in Figure 1D. The effects of these feeding regimes on respiratory quotient were determined at 30-minute intervals. Data are shown as mean +/- SEM for 5 independent observations. * denotes p<0.05 main diet effect.
Body composition
Body composition was determined in mice using the Lunar PIXImus Densitometer (GE Medical Systems, Madison, WI). Mice were sedated with ketamine/xylaxine (80mg/kg and 16mg/kg respectively).
Glucose tolerance test
For glucose tolerance tests (GTT), 12-hr fasting glucose levels were measured, followed by administration of 10% D-glucose at a dose of 1 g/kg (I.P.). Plasma glucose was measured 15, 30, 60, and 120 minutes following glucose injection. During the GTT, glucose measurements were performed in triplicate for each mouse at a given time point (i.e., technical replicates) using a Freestyle Lite glucometer (Abbott Laboratories, Abbott Park, IL). This test was performed at ZT12 (i.e., the light-to-dark transition), such that the 12-hr fast was enforced during the sleep phase.
Humoral factor measurements
Non-fasted (i.e., fed state) plasma glucose, insulin, triglyceride, and leptin concentrations were measured using commercially available kits (Thermo Scientific, Waltham, MA; Wako Diagnostics, Richmond, VA; Crystal Chem Inc., Downers Grove, IL; Thermo Scientific, Waltham, MA).
Statistical analysis
All results are expressed as the mean ± standard error (SEM). Statistical analysis was performed using two-way or repeated-measure ANOVA. Stata version IC10.0 (Stata Corp., San Antonio, TX) was used to perform two-way ANOVA to investigate the main effects of diet and time. Repeated-measure ANOVA was used to determine the effects of different diets over a 24-hour period. A full model including second-order interactions was conducted for each experiment. Significant differences were determined using Type III sums of squares. The null hypothesis of no model effects was rejected at p < 0.05. Bonferroni post hoc analyses were performed for pair-wise comparisons.
Results
Contiguous high fat feeding throughout the waking phase does not result in cardiometabolic syndrome development
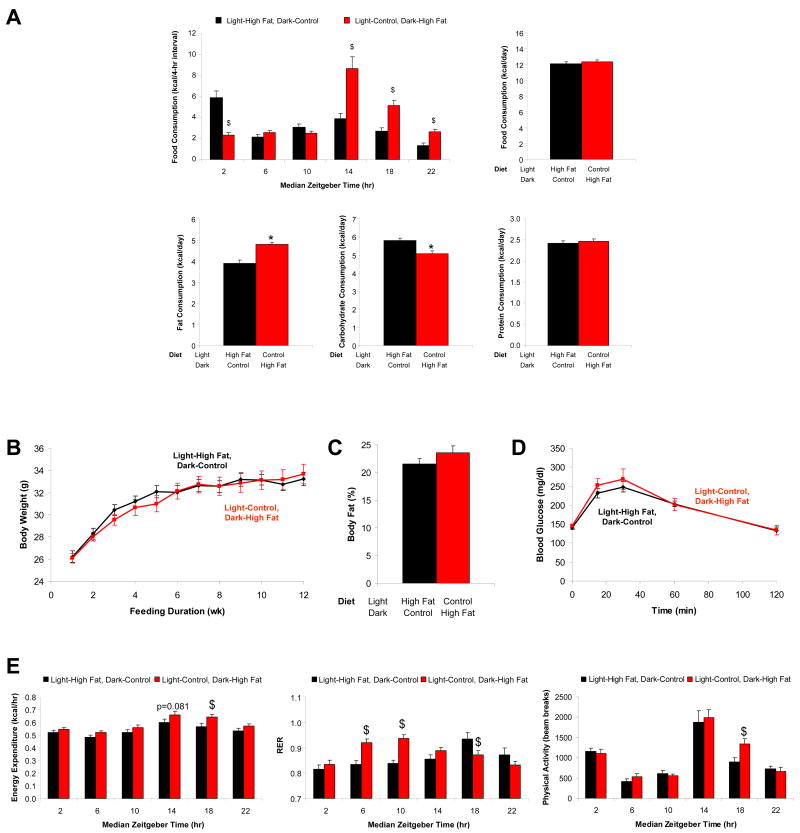
We and others have demonstrated that ad libitum high fat feeding (i.e., 45% calories from fat) for 12 weeks results in significantly increased body weight gain and adiposity in wild-type FVB/N mice, along with predicted alterations in whole body energy expenditure and metabolism, as well as decreased glucose tolerance (Supplementary Figures 1 and 2) (8-10). We subsequently investigated whether consumption of the high fat diet during the light versus the dark phase would influence these parameters, while taking care not to overtly disrupt circadian behavior. Mice were randomly assigned to one of two groups, as illustrated in Figure 1A. Mice in the light phase high fat (LPHF) group were provided the high fat diet during the light phase, followed by the control diet during the dark phase. Mice in the dark phase high fat (DPHF) group were provided the control diet during the light phase, followed by the high fat diet during the dark phase. Food was available ad libitum during both phases. Although daily total caloric intake did not differ between the two groups, DPHF mice consumed more fat-derived calories compared to LPHF mice (Figure 2A). A large proportion of the dietary fat consumed occurred at the initiation of dark phase and light phase for DPHF and LPHF mice respectively (Figure 2A and Supplementary Figure 3). No significant differences were observed for body weight or body composition between LPHF and DPHF mice after 12 weeks of the feeding regime (Figures 2B and 2C). In addition, no significant differences were observed for glucose tolerance between mice in these two feeding groups (Figure 2D). Indirect calorimetry exposed higher oxygen consumption and energy expenditure in DPHF mice, compared to LPHF mice (Figure 2E and Supplementary Figure 3). This did not appear to be due to differences in physical activity (Figure 2E and Supplementary Figure 3). These data suggest that, during extended periods of a contiguous diet, animals are able to adequately adjust whole body homeostasis, allowing for excess dietary lipid ingestion.
Figure 2.
Mice were divided into two distinct feeding groups, as depicted in Figure 1A. The effects of these feeding regimes on caloric intake (A), body weight (B), percent body fat (C), glucose tolerance (D), as well as energy expenditure, respiratory quotient, and physical activity (E) were determined. Data are shown as mean +/- SEM for 5-8 independent observations. * denotes p<0.05 main diet effect and $ denotes p<0.05 diet effect at a specific ZT.
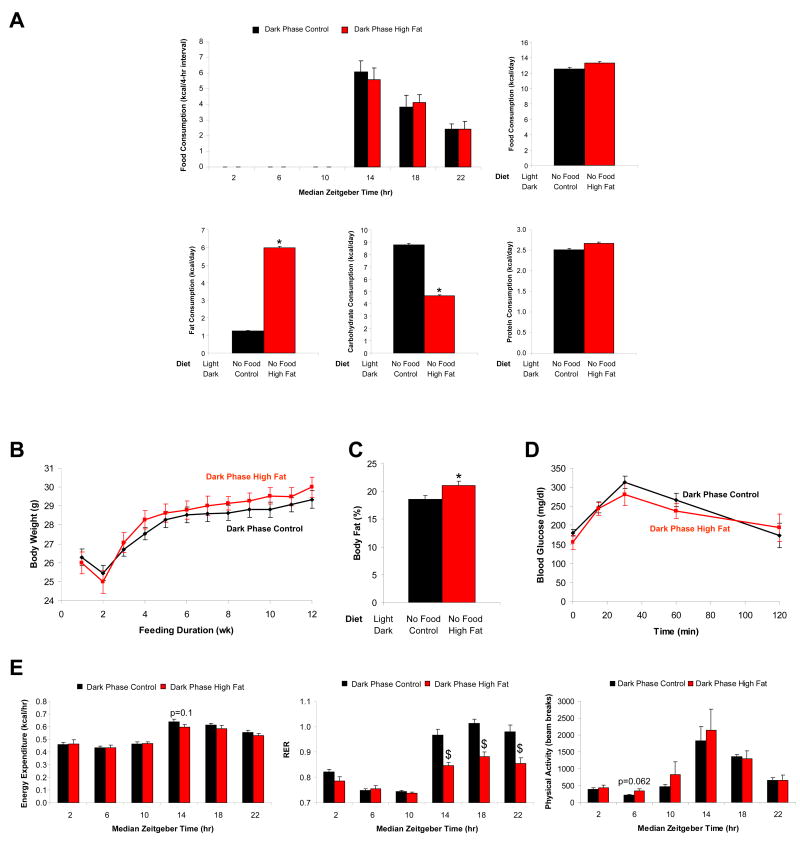
To investigate further whether high fat feeding during the waking phase influences cardiometabolic syndrome parameters, mice were randomly assigned into one of two groups, as shown in Figure 1B. Here, mice were allowed to consume either a control diet or a high fat diet only during the dark phase (normal waking period); during the light phase (the sleep/inactive phase for the rodent) mice were not allowed access to food (i.e., period of food withdrawal). Mice fed the high fat diet consumed more fat-derived calories compared to mice fed the control diet (Figure 3A). Again, the majority of calories were consumed at the beginning of the dark phase (Figure 3A and Supplementary Figure 4). Despite these differences in fat-derived calories, no differences were observed in body weight or glucose tolerance between the two feeding groups (although a slight, but significant increase in body fat composition was observed in the high fat feeding group; Figures 3B, 3C, and 3D). Indirect calorimetry showed no significant differences in oxygen consumption or energy expenditure in high fat fed mice versus control mice (Figure 3E and Supplementary Figure 4). No difference in physical activity was observed (Figure 3E and Supplementary Figure 4). However, RER was significantly lower in high fat fed mice, consistent with increased fatty acid oxidation (Figure 3E and Supplementary Figure 4). These data are again consistent with the hypothesis that mice fed a contiguous diet for extended periods during the waking phase are able to compensate and adjust energy expenditure to match the quality and quantity of calories consumed.
Figure 3.
Mice were divided into two distinct feeding groups, as depicted in Figure 1B. The effects of these feeding regimes on caloric intake (A), body weight (B), percent body fat (C), glucose tolerance (D), as well as energy expenditure, respiratory quotient, and physical activity (E) were determined. Data are shown as mean +/- SEM for 5-25 independent observations. * denotes p<0.05 main diet effect and $ denotes p<0.05 diet effect at a specific ZT.
Dietary composition at the beginning versus the end of the active phase ‘programs’ metabolism
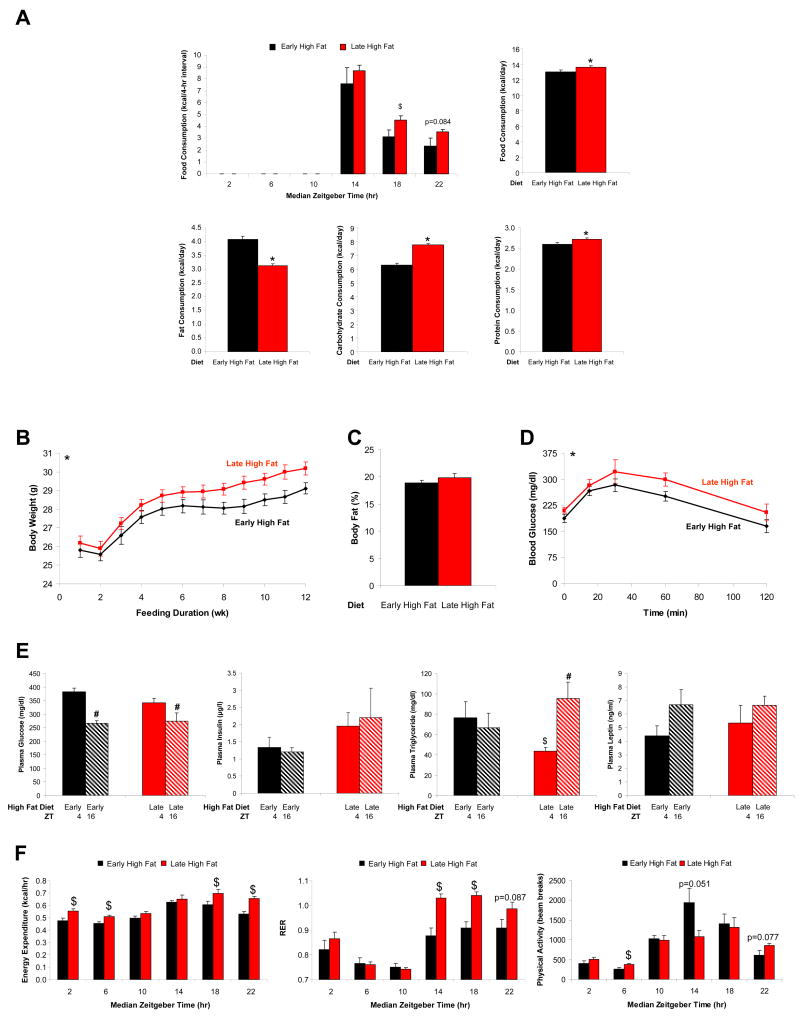
The above described feeding studies suggest that extended periods of a contiguous diet during the waking phase results in concomitant alterations in whole body energy metabolism, thereby preventing cardiometabolic syndrome development. In both feeding regimes investigated thus far, introduction of the high fat diet at the beginning of the waking phase resulted in an immediate consumption of the diet at that time. We therefore hypothesized that consumption of a high fat diet at the beginning of the waking phase may play an important role in energy homeostasis. In order to test this hypothesis, mice were randomly assigned to one of two distinct feeding groups, as depicted in Figure 1C. For both groups, mice were allowed access to food only during the waking (dark) phase, and feeding was divided into three distinct time periods, as described in the Methods section. Early high fat fed mice consumed more fat derived calories compared to late high fat fed mice (Figure 4A). In contrast, late high fat fed mice consumed significantly more total calories, particularly during TP2 and TP3 (Figure 4A and Supplementary Figure 5). Increased caloric intake in late high fat fed mice was associated with significantly higher body weights, as well as decreased glucose tolerance, compared to early high fat fed mice (Figures 4B and 4D). Consistent with insulin resistance in late high fat fed mice, hyperinsulinemia and hypertriglyceridemia were observed (Figure 4E). Indirect calorimetry studies showed greater energy expenditure in late high fat fed mice (Figure 4F and Supplementary Figure 5). Interestingly, RER values remained low in early high fat fed mice throughout the dark phase, despite consumption of a control (low fat) diet for 8 hours during this time period (Figure 4F and Supplementary Figure 5). Similarly, RER values remained elevated in the late high fat fed mice throughout the dark phase, despite consumption of a high fat diet for the last 4 hours during this waking period, suggesting that the waking meal programs metabolism for the remainder of the active/waking period.
Figure 4.
Mice were divided into two distinct feeding groups, as depicted in Figure 1C. The effects of these feeding regimes on caloric intake (A), body weight (B), percent body fat (C), glucose tolerance (D), humoral factors (E), as well as energy expenditure, respiratory quotient, and physical activity (F) were determined. Data are shown as mean +/- SEM for 6-24 independent observations. * denotes p<0.05 main diet effect, $ denotes p<0.05 diet effect at a specific ZT, and # denotes p<0.05 time effect within a specific feeding group.
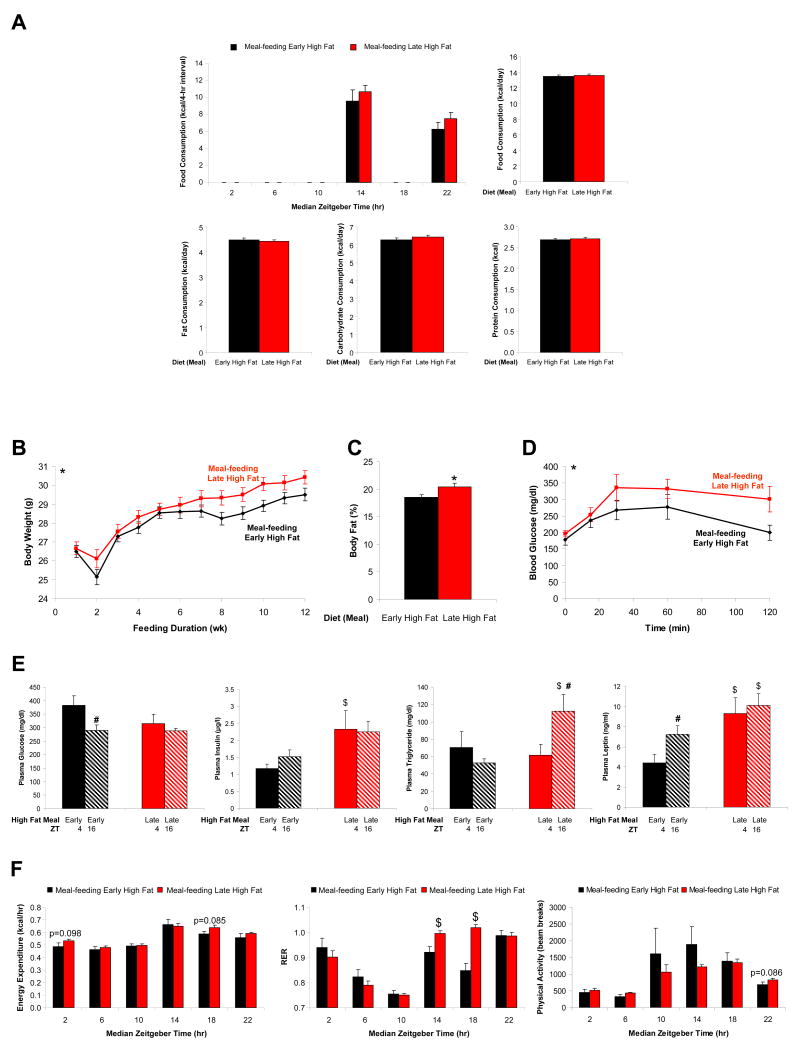
The results described above suggested that the composition of the diet consumed during the beginning versus the end of the dark (active) phase potentially plays an important role in dictating metabolism throughout the active period. However, differences in total and fat-derived calories, independent of the time of day, were observed between the feeding groups (Figure 4A). In an attempt to ensure that the only variable between two feeding groups was the time of day at which dietary fat was consumed (as opposed to total or fat-derived calories), in the absence of enforced paired feeding, two additional feeding groups were next employed, as illustrated in Figure 1D. Again, early high fat fed mice were allowed access to the high fat diet during the first 4 hours of the awake/dark phase (i.e., TP1), and were provided the control diet during the last 4 hours of the awake/dark phase (i.e., TP3), with food access withdrawn during both the middle 4 hours of the awake/dark phase (i.e., TP2), as well as during the sleep/light phase. A similar feeding strategy was employed for late high fat fed mice, with the exception that control diet was provided during TP1 and high fat diet was provided during TP3. Mice within the two feeding groups consumed identical total and fat-derived calories; the only variable was the time of day at which the dietary fat was consumed (Figure 5A and Supplementary Figure 6). Despite identical daily total caloric consumption, early high fat fed mice exhibited significantly lower body weights and body fat composition, as well as increased glucose tolerance, relative to late high fat fed mice (Figures 5B, 5C, and 5D). Along with insulin resistance and excess adiposity in late high fat fed mice, hyperinsulinemia, hypertriglyceridemia, and hyperleptinemia were also observed (Figure 5E). Indirect calorimetry revealed anticipated time-of-day-dependent changes in RER for the early high fat fed mice, with low RER values during TP1, that decrease further during the TP2 4-hour fast, and that approach a value close to 1 during carbohydrate consumption in TP3 (Figure 5F). Remarkably, metabolic plasticity is completely lost in the late high fat fed mice, as RER values remain close to a value of 1 throughout the entire awake/dark phase, despite a period of fasting followed by high fat diet consumption (Figure 5F); these diet-induced differences in metabolic plasticity are readily apparent upon inspection of the 30-minute interval data (Figure 6).
Figure 5.
Mice were divided into two distinct feeding groups, as depicted in Figure 1D. The effects of these feeding regimes on caloric intake (A), body weight (B), percent body fat (C), glucose tolerance (D), humoral factors (E), as well as energy expenditure, respiratory quotient, and physical activity (F) were determined. Data are shown as mean +/- SEM for 5-25 independent observations. * denotes p<0.05 main diet effect, $ denotes p<0.05 diet effect at a specific ZT, and # denotes p<0.05 time effect within a specific feeding group.
Discussion
The purpose of the present study was to determine whether the time of day at which a calorically dense high fat diet is consumed influences multiple cardiometabolic syndrome parameters in mice. At the same time, care was taken not to overtly disrupt normal biological rhythms (as observed when feeding is restricted to the inactive/sleep phase). We report that mice adequately adapt to high fat diets when presented in a contiguous manner throughout the active/waking period. However, when fed meals of different caloric quantity and quality at distinct periods during the waking phase, whole body metabolic maladaptation can ensue. For example, consumption of a high carbohydrate diet during the beginning of the active phase impairs metabolic plasticity. Furthermore, consumption of a calorically dense, high fat diet at the end of the active phase leads to accelerated weight gain, increased adiposity, glucose intolerance, hyperinsulinemia, hypertriglyceridemia, and hyperleptinemia (i.e., the cardiometabolic syndrome). The latter is independent of daily total or fat-derived calories. As such, the time-of-day at which high fat diets are consumed profoundly influences multiple cardiometabolic syndrome parameters.
Evidence exists in support of a relationship between time-of-day-dependent food consumption and body weight in humans. Individuals who do not eat breakfast tend to have an increased BMI, relative to those individuals that do eat breakfast (11). It has been suggested that greater total daily caloric intake in individuals that skip breakfast likely contributes towards increased BMI. Along the same lines, consumption of a carbohydrate-enriched breakfast has been associated with reduced caloric intake later in the day (12, 13). Conversely, night-eating syndrome is associated with a higher BMI (14). Shift workers also have increased BMI, as well as increased risk for diabetes mellitus and cardiovascular disease (2, 3). Despite a clear time-of-day-dependence for these reported observations, total caloric intake is typically considered the primary cause of elevated BMI in humans.
Recent animal studies suggest that an intrinsic, cell autonomous, molecular mechanism, known as the circadian clock, dramatically influences both appetite and energy expenditure over the course of the day (15, 16). Circadian clocks are defined as a self-sustained, transcriptionally-based series of positive and negative feedback loops, with a periodicity of approximately 24 hours (17). These clocks have been identified within virtually every mammalian cell, including critical organs/centers influencing metabolic homeostasis (18). Genetic disruption of this clock mechanism markedly influences multiple cardiometabolic syndrome parameters. For example, mutation of the transcription factor CLOCK is associated with disrupted feeding/fasting rhythms (hyperphagia during the inactive/sleep phase), increased weight gain, and hypertriglyceridemia (when mice are on a C57BL/6J background) (19). Conversely, genetic loss of the gene encoding for BMAL1 (the heterodimerization partner for CLOCK) is associated with leanness (20). Indeed, BMAL1 has been reported to play a critical role in adipogenesis (21). More recently, we have shown that cell autonomous clocks directly regulate triglyceride metabolism, promoting increased triglyceride synthesis at the end of the active/awake phase (8).
Given that time-of-day-dependent food intake is associated with BMI in humans, and that animal models of disrupted circadian behavior (i.e., CLOCK mutant BMAL1 null mice) exhibit marked alterations in adiposity, we investigated whether the time of day at which a caloric dense high fat diet is consumed influences various cardiometabolic syndrome parameters in mice. Consistent with such a concept, Arble et al recently reported that restricting high fat diet consumption to the inactive/sleep phase is associated with increased weight gain in mice (6). However, restricting food intake to this inappropriate time of the day has been shown to desynchronize peripheral clocks from the central, master clock, and to profoundly influence behavioral, physiological, and molecular rhythms (7). In attempts to avoid such a dyssynchony, and instead mimic more closely normal human behavior, the present studies presented diets either throughout the entire 24-hr period, or only during the waking period. These feeding regimes (Figure 1) had no overt periodicity effects on biological rhythms, such as physical activity or energy expenditure (Figures 2E, 3E, 4F, and 5F). In addition, given our recent findings that circadian clock mediated triglyceride synthesis is greatest at the end of the active phase (8), we anticipated that consumption of a high fat diet at this time would promote adiposity.
The present study reports that feeding mice a high fat diet throughout the waking phase does not significantly influence body weight, adiposity or glucose tolerance (Figures 2 and 3). This is despite increased daily fat consumption (Figures 2A and 3A). The lack of weight gain appears to be due to a compensatory increase in energy expenditure and/or a balancing of total caloric intake (Figures 2 and 3). In contrast, when mice are fed carbohydrate- or fat- rich meals at distinct windows of time during the active/awake, cardiometabolic syndrome parameters are dramatically influenced. Feeding mice a calorically dense high fat diet at the end of the active period is associated with increased weight gain, adiposity, glucose intolerance, hyperinsulinemia, hypertriglyceridemia, and hyperleptinemia (Figures 4 and 5). This is independent of daily total or fat-derived calories (Figure 5A). In addition, consumption of a high carbohydrate meal at the beginning of the active/awake phase results in a profound metabolic inflexibility, again independent of daily total or fat-derived calories (Figures 5F and 6).
The implications of the present research are important for human dietary recommendations. Humans seldom eat a uniform diet throughout the day, thus requiring the ability to respond to alterations in diet quality. Currently, a typical human diet consists of a high carbohydrate morning meal followed by higher fat and/or more calorie-dense meals later in the day. Our studies provide evidence that the capacity to adjust to the dietary composition of a given meal or bout of feeding is an important component in energy balance and that such capacity appears to depend on the meal ingested upon waking. Consumption of a high fat waking meal is associated with increased ability to respond appropriately to carbohydrate meals ingested later in the waking period, while a high carbohydrate morning meal appears to “fix” metabolism toward carbohydrate utilization and impair the ability to adjust metabolism toward fat utilization later in the waking period. In addition, consumption of a calorically-dense high fat meal at the end of the active period promotes cardiometabolic syndrome development in mice. The findings of this study suggest that dietary recommendations for weight reduction and/or maintenance should include information about the timing of dietary intake, as well as the quality and quantity of intake.
Supplementary Material
Acknowledgments
This work was supported by Kraft Foods Inc., the USDA/ARS (6250-51000-046 and 6250-51000-044) and the National Heart, Lung, and Blood Institute (HL-074259). Ju-Yun Tsai was supported by the DeBakey Heart Fund at Baylor College of Medicine.
Footnotes
Conflict of Interest: The authors do not have any competing financial conflicts in relation to this work.
References
- 1.Harma M, Ilmarinen J. Towards the 24-hour society--new approaches for aging shift workers? Scand J Work Envion Health. 1999;25:610–615. doi: 10.5271/sjweh.488. [DOI] [PubMed] [Google Scholar]
- 2.Knutsson A, Akerstedt T, Jonsson B, Orth-Gomer K. Increased risk of ischaemic heart disease in shift workers. Lancet. 1986;12:89–92. doi: 10.1016/s0140-6736(86)91619-3. [DOI] [PubMed] [Google Scholar]
- 3.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Castro JM. The time of day of food intake influences overall intake in humans. J Nutr. 2004;134:104–111. doi: 10.1093/jn/134.1.104. [DOI] [PubMed] [Google Scholar]
- 5.de Castro JM. The time of day and the proportions of macronutrients eaten are related to total daily food intake. Br J Nutr. 2007;98:1077–1083. doi: 10.1017/S0007114507754296. [DOI] [PubMed] [Google Scholar]
- 6.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schibler U, Ripperger J, Brown S. Peripheral circadian oscillations in mammals: time and food. J Biol Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 8.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, et al. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 2009 doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 10.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini M, Verduci E, Scaglioni S, Salvatici E, Bonza M, Riva E, et al. Breakfast: a good habit, not a repetitive custom. J Int Med Res. 2008;36:613–624. doi: 10.1177/147323000803600401. [DOI] [PubMed] [Google Scholar]
- 12.Cho S, Dietrich M, Brown CJ, Clark CA, Block G. The effect of breakfast type on total daily energy intake and body mass index: results from the Third National Health and Nutrition Examination Survey (NHANES III) J Am Coll Nutr. 2003;22:296–302. doi: 10.1080/07315724.2003.10719307. [DOI] [PubMed] [Google Scholar]
- 13.Warren JM, Henry CJ, Simonite V. Low glycemic index breakfasts and reduced food intake in preadolescent children. Pediatrics. 2003;112:e414. doi: 10.1542/peds.112.5.e414. [DOI] [PubMed] [Google Scholar]
- 14.Stunkard AJ, Allison KC, Lundgren JD, O'Reardon JP. A biobehavioural model of the night eating syndrome. Obes Rev. 2009;10(2):69–77. doi: 10.1111/j.1467-789X.2009.00668.x. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Lin JD. Molecular Control of Circadian Metabolic Rhythms. J Appl Physiol. 2009 doi: 10.1152/japplphysiol.00467.2009. [DOI] [PubMed] [Google Scholar]
- 16.Eckel-Mahan K, Sassone-Corsi P. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol. 2009;16:462–467. doi: 10.1038/nsmb.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000;3:59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 18.Marcheva B, Ramsey KM, Affinati A, Bass J. Clock genes and metabolic disease. J Appl Physiol. 2009;107:1638–1646. doi: 10.1152/japplphysiol.00698.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turek F, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, et al. Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis. 2005;41:122–132. doi: 10.1002/gene.20102. [DOI] [PubMed] [Google Scholar]
- 21.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.