Abstract
Statins may have beneficial effects in atherogenesis given their antithrombotic properties involving non-lipid mechanisms that modify endothelial function of tissue factor induction by thrombin. In this study, we investigate the effect of atorvastatin on tissue factor (TF) activity in thrombin-stimulated endothelial cells and its regulation through mevalonate or its derivatives. First subculture of human umbilical endothelial cells was used for this study. Cells were treated with thrombin and atorvastatin for different time intervals and dosage. Tissue factor activity was measured as Factor Xa generation induced by Tissue Factor-Factor VIIa complex on confluent cells. Our results show that atorvastatin prevents the thrombin-induced up-regulation of tissue factor activity in a concentration-dependent manner. Mevalonate and geranylgeranyl pyrophosphate reversed this inhibitory effect of atorvastatin on tissue factor activity, while the presence of farnesyl pyrophosphate did not prevent the atorvastatin effect on thrombin-induced tissue factor activity. Rho-kinase inhibitor did not affect the thrombin stimulation of tissue factor activity. High amount of hydrophobic isoprenoid groups decreases the thrombin-induced TF activity and may promote endothelial cell anti-thrombotic action. Rho kinase pathways do not have a major role in the thrombin-mediated TF activity. The inhibitory effect of atorvastatin on thrombin-induced TF activity was partially reversed by MVA and GGPP but not FPP.
Keywords: Atorvastatin, Tissue factor, Thrombin, Endothelial cells, Geranylgeranyl pyrophosphate
Introduction
Besides their well-known lipid lowering effect, statins display other non-lipid-lowering pharmacological activities. Statins, by means of the inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, also inhibit the biosynthesis of mevalonate, precursor of farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP), which permits the attachment of signaling proteins to the cell membrane (Edwards and Ericsson 1999).
It has been suggested that simvastatin inhibits the rate of thrombin generation by directly interfering with monocyte expression of TF (Ferro et al. 2000). Furthermore, simvastatin inhibits thrombin-induced TF expression in aortic endothelial cells, effect associated with reduced RhoA kinase activation (Eto et al. 2002). Recently, it has been reported that atorvastatin attenuated TF antigen, activity and TF mRNA levels in adipocytes (Li et al. 2007).
It is known that thrombin induces a rapid geranylgeranylation of RhoA in the process of RhoA activation in endothelial cells (Ohkawara et al. 2005). As the effect of statins in the cholesterol pathway is carried out by inhibiting the reactions of protein prenylation involved in signal transduction mechanisms, we plan to further elucidate the contribution of atorvastatin in TF activity in thrombin-stimulated endothelial cells, and analyze the regulation of this effect through mevalonate or its derivatives.
Materials and methods
Human umbilical vein endothelial cells culture
Endothelial cells from human umbilical cord veins were prepared according to the method previously described (Martínez-Sales et al. 2007). Confluent endothelial cell monolayers were harvested from the culture flasks using 0.25% trypsin, 0.01% EDTA in 10 mM phosphate buffer, 150 mM NaCl, pH 7.4 (PBS), without added Ca2+ or Mg2+. Cells were plated in 96-well culture plates, at a density of approximately 1.5 × 104 cells/well, and they were grown to reach 80–100% confluence in the above-mentioned culture medium. The cells were identified as endothelial cells by their typical cobblestone appearance and by the presence of von Willebrand factor demonstrated through an immunocytochemistry method using rabbit immunoglobulin to human von Willebrand factor (Dako, Denmark).
The study protocol was approved by the Research Ethics Committee. All study procedures comply with the Declaration of Helsinki 1975.
Experimental design
Confluent subcultures of HUVEC at passages 1 and 2 were incubated in medium 199 supplemented with 2% FBS. Cells were treated with different concentrations of thrombin (0.05–1 U/mL) for different periods of time to establish the most appropriate experimental conditions. Cell cultures previously treated with atorvastatin (Calbiochem, Germany) dissolved in DMSO and culture cell media at concentrations ranging from 0.001 to 10 μM for 24 h were later stimulated with 0.5 U/ml thrombin for 4 h. Control cells were treated with the same concentration of DMSO, which never exceeded 0.1%.
The influence of intermediate metabolites of cholesterol synthesis was tested by incubating the cells with either 100 μM mevalonate (Sigma-Aldrich Co, Germany) or 5 μM FPP (Sigma-Aldrich Co, Germany) or 5 μM GGPP (Sigma-Aldrich Co, Germany) in the presence and absence of 1 μM atorvastatin for 24 h. Mevalonate, FPP and GGPP were separately added to the culture medium at the same time as atorvastatin, and cells were later stimulated with 0.5 U/mL thrombin for 4 h. In other experiments, cells were previously treated with 1 μM atorvastatin or 10 μM Rho-kinase inhibitor (Calbiochem, Germany) for 24 h, and then, they were stimulated with 0.5 U/mL thrombin for 4 h. At the end of the incubation time periods, the cells were washed three times with PBS and tissue factor activity was measured immediately thereafter on the endothelial cell surface.
Measurement of tissue factor activity on HUVEC surface
TF activity was measured as Factor Xa generation induced by TF-Factor VIIa complex on confluent HUVEC in 96-well plates. The assay measured TF activity expressed at the luminal surface of intact living cells. HUVEC were washed twice with PBS and then incubated at room temperature for 30 min in a reaction mixture (150 μL) containing Factor VIIa (16 IE/mL, 5 nM) and Factor X (0.8 μg/mL). The reaction was stopped by adding glacial acetic acid (25 μL). Then, activity was determined with a chromogenic substrate, S-2222, for Factor Xa, the absorbance of the reaction solutions read at 405 nm on an ELISA plate reader (Thermo Labsystems). Recombinant human TF (RecombiPlasTin, Instrumentation Laboratory) was used to construct a calibration curve.
Data analysis
Results were expressed as mean values ± standard deviations of three to six independent experiments performed in triplicate. Comparisons among groups were evaluated by the ANOVA and Bonferroni post hoc test. Two-tailed p-values of 0.05 or less were considered statistically significant. All analyses were carried out using the Statistical Package of Social Sciences (SPSS, version 11.0 for Windows).
Results and discussion
The effect of thrombin on TF activity
Figure 1A shows the time course of TF activity in HUVEC treated with thrombin. The increased TF activity was maximal after 3–6 h of incubation, and went back near the baseline after 24 h of incubation. Figure 1B shows the dose-response effect of thrombin on endothelial cell TF activity. After incubation of HUVEC with thrombin for 4 h, TF activity increased, reaching a maximum between 0.3 and 1 U/mL. Thrombin increased endothelial cell surface TF activity in HUVEC in a time and concentration-dependent manner. There was only minimal TF activity on the surface of non-stimulated HUVEC.
Fig. 1.
Effect of thrombin on TF activity on the surface of HUVEC. A Time course of the effect of 0.5 U/mL thrombin on TF activity on the surface of HUVEC. Cells were treated with thrombin for the time indicated and then assayed for TF activity. B Dose-response of the effect of thrombin on the expression of TF activity on the surface of HUVEC. Cells were exposed to the indicated concentrations of thrombin for 4 h and then they were assayed for TF activity. Data represent means ± SD of three different experiments done in triplicate. TF tissue factor
One of the key steps in the thrombin-induced TF activity is the binding hydrophobic groups consisting of geranylgeranyl residues to the carboxyl terminal of Rho protein (Kunieda et al. 2003). In this regard, Fig. 2 shows that in HUVEC treated with 0.5 U/ml of thrombin, the induced TF activity did not change after adding 0.5 μM GGPP, while it significantly decreased after adding 10 or 20 μM GGPP. No significant changes were observed in TF activity by adding FPP and MVA at different concentrations. From these data it can be speculated that a high amount of hydrophobic isoprenoid groups decreases the thrombin-induced TF activity and may promote endothelial cell anti-thrombotic action. Recently it has been reported that thrombin-mediated TF activity was dependent on Rho kinase activity, phosphorylation of p38 (MAPK) and p85 and Akt dephosphorylation (Zhang et al. 2007). Simvastatin has been reported to prevent TF induction through inhibition of Rho/Rho-kinase and activation of Akt (Eto et al. 2002). Figure 2 shows that incubation of HUVEC with thrombin and inhibitor of Rho kinase does not change the amount of thrombin-mediated TF activity, with respect to HUVEC treated only with thrombin. From these data it can be speculated that the Rho kinase pathways do not have a major role, but the pathways involving the phosphorylation of p38 (MAPK) and p85 and Akt pathways dephosphorylation are the priorities in the thrombin-mediated TF activity. It is known that inhibition of mevalonate synthesis causes the down-regulation of signaling pathways mediated by geranylgeranylation and membrane localization of Rho GTP-binding proteins (Park et al. 2002; Tesfamariam 2006). In addition, in endothelial cells, rapid thrombin-induced geranylgeranylation was inhibited by pretreatment with atorvastatin (Ohkawara et al. 2005). Geranylgeranyl conjugation to Rho GTP-binding protein may be a rate-limiting step in statin inhibition of thrombin stimulation of cellular mechanisms.
Fig. 2.
Effect of mevalonate, FPP, GGPP and Rho-kinase inhibitor on TF activity on the surface of HUVEC. Cells treated with the indicated concentrations of MVA, FPP, GGPP or Rho-kinase inhibitor for 24 h were additionally stimulated with thrombin (0.5 U/mL, 4 h). Cells were analyzed for TF activity. Data represent means ± SD of three different experiments done in triplicate. TF tissue factor; MVA mevalonate; FPP farnesyl pyrophosphate; GGPP geranylgeranyl pyrophosphate; Inh-Rho Rho-kinase inhibitor; Th thrombin
The effect of atorvastatin on thrombin-stimulated HUVEC
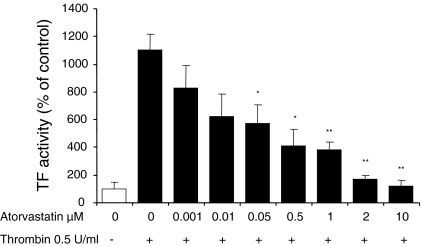
HUVEC were treated with atorvastatin for 24 h, and then the cells were incubated with 0.5 U/mL thrombin for 4 h. As expected, the results revealed that atorvastatin (0.001–10 μM) prevented the thrombin-induced up-regulation of TF activity in a concentration-dependent manner (Fig. 3). This effect was statistically significant for atorvastatin concentrations equal to or higher than 0.05 μM. The effect of atorvastatin was observed at concentrations that can be reached in circulating blood during chronic atorvastatin therapy (Cilla et al. 1996), suggesting that the effect of atorvastatin observes in this study are clinically relevant.
Fig. 3.
Effect of atorvastatin on TF activity on the surface of thrombin-stimulated HUVEC. Confluent monolayer of HUVEC treated for 24 h with the indicated concentrations of atorvastatin were later stimulated with 0.5 U/mL thrombin for 4 h. Confluent monolayers of HUVEC were assayed for TF activity. Data represent means ± SD of three different experiments done in triplicate. * p < 0.05, ** p < 0.01 significantly different between atorvastatin untreated and atorvastatin treated cells. TF tissue factor
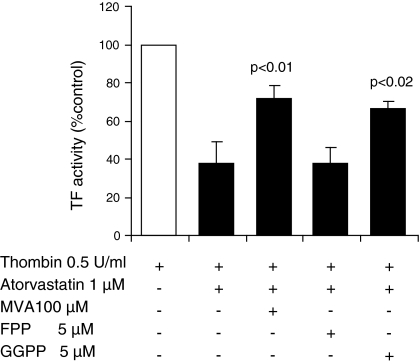
To confirm that the inhibitory effect of atorvastatin on thrombin-induced TF activity was due to deprivation of mevalonate, FPP or GGPP, HUVEC were incubated with either 100 μM mevalonate, or 5 μM FPP, or 5 μM GGPP in the presence of 1 μM atorvastatin for 24 h and then cells were stimulated with thrombin. Figure 4 shows that mevalonate and GGPP prevented the inhibitory effect of atorvastatin. The TF activity induced by thrombin was restored to more than 70%. However, FPP did not prevent the atorvastatin effect on TF activity induced by thrombin (Fig. 4). This metabolite does not contain hydrophobic residues that are necessary to anchor the Rho to intracellular membranes so that they can be translocated to the plasma membrane and be activated (Adamson et al. 1992). This result shows that the inhibitory effect of the thrombin-induced TF activity on HUVEC was partially reversed by MVA. This result is in agreement with those reported by Eto et al. (2002) in a study performed with simvastatin in human aortic endothelial cells. Additionally, GGPP caused the complete restoration of TF activity; however, FPP did not restore it. This observation agrees with those of Ishibashi et al. (2003) who found that GGPP but not FPP reversed the suppressive effect of cerivastatin on the expression of TF.
Fig. 4.
Effect of mevalonate, FPP and GGPP on TF activity of atorvastatin treated HUVEC. HUVEC treated with atorvastatin and MVA, FPP or GGPP for 24 h were additionally stimulated with thrombin (0.5 U/mL, 4 h). Cells were analyzed for TF activity. Data represent means ± SD of three different experiments done in triplicate. TF tissue factor; MVA mevalonate; FPP farnesyl pyrophosphate; GGPP geranylgeranyl pyrophosphate; Th thrombin
Conclusion
High amount of hydrophobic isoprenoid groups decreases the thrombin-induced TF activity and may promote endothelial cell anti-thrombotic action. Rho kinase pathways do not have a major role in the thrombin-mediated TF activity. The inhibitory effect of atorvastatin on thrombin-induced TF activity was partially reversed by MVA and GGPP but not FPP.
Acknowledgments
The authors thank Guadalupe Manzano and Josefa Llorens for their technical assistance in the performance of the experiments.
References
- Adamson P, Marshall CJ, Hall A, Tilbrook PA. Post-translational modifications of p21rho proteins. J Biol Chem. 1992;267:20033–20038. [PubMed] [Google Scholar]
- Cilla DD, Jr, Whitfield LR, Gibson DM, Sedman AJ, Posvar EL. Multiple-dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin Pharmacol Ther. 1996;60:687–695. doi: 10.1016/S0009-9236(96)90218-0. [DOI] [PubMed] [Google Scholar]
- Edwards PA, Ericsson J. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–185. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- Eto M, Kozai T, Cosentino F, Joch H, Lüscher TF. Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation. 2002;105:1729–1756. doi: 10.1161/01.CIR.0000015465.73933.3B. [DOI] [PubMed] [Google Scholar]
- Ferro D, Basili S, Alessandri C, Cara D, Violi F. Inhibition of tissue-factor-mediated thrombin generation by simvastatin. Atherosclerosis. 2000;149:111–116. doi: 10.1016/S0021-9150(99)00291-9. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Sakamoto T, Ohkawara H, Nagata K, Sugimoto K, Sakurada S, Sugimoto N, Watanabe A, Yokoyama K, Sakamoto N, Kurabayashi M, Takuwa Y, Maruyama Y. Integral role of RhoA activation in monocyte adhesion-triggered tissue factor expression in endothelial cells. Ateroscler Thromb Vasc Biol. 2003;23:681–687. doi: 10.1161/01.ATV.0000065194.00822.C7. [DOI] [PubMed] [Google Scholar]
- Kunieda Y, Nakagawa K, Nishimura H, Kato H, Ukimura N, Yano S, Kawano H, Kimura S, Nakagawa M, Tsuji H. HMG CoA reductase inhibitor suppresses the expression of tissue factor and plasminogen activator inhibitor-1 induced by angiotensin II in cultured rat aortic endothelial cells. Thromb Res. 2003;110:227–234. doi: 10.1016/S0049-3848(03)00346-3. [DOI] [PubMed] [Google Scholar]
- Li JQ, Zhao SP, Li QZ, Cai YC, Wu LR, Fang Y, Li P. Atorvastatin reduces tissue factor expression in adipose tissue of atherosclerotic rabbits. Int J Cardiol. 2007;115:229–234. doi: 10.1016/j.ijcard.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Martínez-Sales V, Vila V, Ferrando M, Reganon E. Atorvastatin neutralizes the up-regulation of thrombospondin-1 induced by thrombin in human umbilical vein endothelial cells. Endothelium. 2007;14:233–238. doi: 10.1080/10623320701617209. [DOI] [PubMed] [Google Scholar]
- Ohkawara H, Ishibashi T, Sakamoto T, Sugimoto K, Nagata K, Yokoyama K, Sakamoto N, Kamioka M, Matsuoka I, Fukuhara S, Sugimoto N, Takuwa Y, Maruyama Y. Thrombin-induced rapid geranyl geranylation of RhoA as an essential process for RhoA activation in endothelial cells. J Biol Chem. 2005;280:10182–10188. doi: 10.1074/jbc.M409547200. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kong D, Iruela-Arispe ML, Begley U, Tang D, Galper JB. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Cir Res. 2002;91:143–150. doi: 10.1161/01.RES.0000028149.15986.4C. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B. The effects of HMG-CoA reductase inhibitors on endothelial cells. Am J Cardiovasc Drugs. 2006;6:115–120. doi: 10.2165/00129784-200606020-00005. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Kelm RJ, Biswas P, Kashgarian M, Madri JA. PECAM-1 modulates thrombin-induced tissue factor expression on endothelial cells. J Cell Physiol. 2007;210:527–537. doi: 10.1002/jcp.20908. [DOI] [PubMed] [Google Scholar]