Abstract
Cells stimulated with physiological stimuli usually exhibit oscillations in cytosolic Ca2+ concentration ([Ca2+]i), a signal playing central roles in regulation of various cellular processes. For explicating their unknown mechanisms, studies are commonly conducted in single cells from several cell lines, in particular the human epithelial kidney (HEK293) cell line. However, [Ca2+]i oscillating responses to agonists in vitro are found difficult to be induced and varied with different types of cells and agonists. This study shows that treatment of the wild type HEK293 cells with low concentrations of carbachol (1–10 μM), an agonist of the muscarinic receptor, resulted in non-oscillated but sustained [Ca2+]i increase by loading the cells with 1 μM fura2/AM. However, repetitive and long lasting [Ca2+]i oscillations could be induced in 31.1% of the tested cells loaded with 0.1 μM fura2/AM. Additionally, the occurrence of the typical Ca2+ spikes further increased to 47.2% and 60.7% when the Ca2+ concentration in the bathing medium was decreased from 1.8 mM to 1.5 mM and the medium temperature was set to 35 ± 1°C from 22 ± 2°C. Therefore, this study provides a useful approach for measuring [Ca2+]i oscillatory response to relevant physiological stimulation in a wild type cell line through the adjustments of the concentrations adopted for the Ca2+ indicator and extracellular medium Ca2+ and of the temperature set for the experiment.
Keywords: Ca2+ oscillations, Fura2/AM, Human epithelial kidney cells, Carbachol
Introduction
The increase in intracellular Ca2+ concentration ([Ca2+]i) is a ubiquitous intracellular signal pathway for controlling diverse cellular processes such as proliferation, development, contraction, secretion, apoptosis and necrosis (Clapham 1995; Berridge et al. 2000; Frey et al. 2000). Increases in [Ca2+]i have a wide range of spatial and temporal arrangement adapted to various stimuli from intra- and extra-cellular space, thereby providing a fine tuning for a precise regulation of different cellular functions. In both excitable and nonexcitable cells, it has been realized that local and global Ca2+ signals inside cells are usually regulated distinctly and contribute to different physiological phenomena (Clapham 1995; Hardingham et al. 1997; Frey et al. 2000; Berridge et al. 2003). Local Ca2+ signals (e.g., sparks and puffs), the spontaneous elementary events, occur at resting state and can contribute to the resting level of Ca2+ and certain physiological processes such as activation of ion channels (Meyer and Stryer 1991; Clapham 1995; Berridge et al. 2003). Global Ca2+ signaling, demonstrated as simply sustained elevation in [Ca2+] i or complex temporal Ca2+ fluctuations like repetitive oscillations or waves, is usually arising from excitation stimulations and contributes to various cellular processes such as excitation–contraction, excitation-secretion and excitation-expression couplings (Meyer and Stryer 1991; Clapham 1995; Hardingham et al. 1997; Berridge et al. 2003). It is widely accepted that the type of repetitive Ca2+ transients or oscillations reflects a normal Ca2+ signaling pattern in cells as they are generated at more physiologically-relevant concentrations for an agonist, whilst the sustained [Ca2+]i plateau signals are often seen following potent stimulation or maximal agonist concentrations (Bootman et al. 1997; Shuttleworth 1999; Berridge et al. 2000). In addition, more recent studies indicate that some specific intracellular second messengers and a variety of physiological processes are finely modulated by the information encoded in the frequency, amplitude and duration of [Ca2+]i transients (Thomas et al. 1996; Watt et al. 2000; Reither et al. 2006; Willoughby and Cooper 2006; Zhu et al. 2008). Therefore, it is important to investigate the dynamic characteristics and underling mechanism(s) hidden in this particular Ca2+ signaling for a better understanding of their roles in the regulation of cell functions. However, physiological Ca2+ oscillatory responses are difficult to be induced in individual cells in vitro, especially in wild type cell lines. This is likely due to various factors like the status of endoplasmic reticulum load (Bird and Putney 2005), the degree of Ca2+ influx across cell membrane (Luo et al. 2001; Sneyd et al. 2004) and the buffering ability of the mitochondria (Vay et al. 2007), all of which dramatically influence the initiation and patterns of Ca2+ transients independently. In addition to the factors inherited in the cells, we found, in the current study, that several experimental factors also greatly influenced or even prevented the endogenous [Ca2+]i oscillating genesis in the wild type human epithelial kidney (HEK293) cells, a cell line commonly used for investigating [Ca2+]i oscillation complexity and functional properties (Shuttleworth and Thompson 1998; Luo et al. 2001; Sneyd et al. 2004; Gerbino et al. 2005; Rey et al. 2005).
Materials and methods
Cell culture
HEK293 cells obtained from the ATCC were grown at 37°C in Dulbecco’s Eagle’s medium supplemented with 10% heat-inactivated fetal bovine serum and 2 mM glutamine in a humidified 95% air, 5% CO2 incubator. For Ca2+ measurements, cells were plated onto glass coverslips, cultured for 24 h to about 60% confluence, and used within 24–30 h after plating.
Fluorescence measurements
Fluorescence measurements in single HEK293 cells were described previously (Luo et al. 2001). In brief, the coverslips with attached cells were loaded with acetoxymethyl ester of fura2 (fura2/AM, Molecular Probes) 1 μM at 37°C in the dark for 25 min, and then washed three times and incubated for 30 min in HEPES-buffered physiological saline solution (HBSS: NaCl, 120 mM; KCl, 5.4 mM; Mg2SO4, 0.8 mM; HEPES, 20 mM; CaCl2, 1.8 mM; and glucose, 10 mM; with pH 7.4 adjusted by NaOH).
Fluorescence was monitored by placing the Teflon chamber with fura2-loaded cells onto the stage of a Nikon Diaphot microscope (403 Neofluor objective). The cells were excited alternatively by 340 and 380 nm wavelength, and the emission fluorescence intensity at 510 nm was recorded by a photomultiplier tube (Omega Optical). All experiments were conducted at room temperature (23 ± 2°C), except indicated, and carried out within 2 h of loading for each coverslip. The data are expressed as the ratio of fura2 fluorescence due to excitation at 340 nm to that due to excitation at 380 nm (F340/F380).
Assessment of fura2 loading
The fura2 loading inside cells was assessed by the background subtracted fluorescence values (counts per seconds) obtained at 380 nm excitation light before any stimulation. Background fluorescence was the value of F380 after complete quench of fura2 by MnCl2.
Statistics
Data were analyzed and presented as means ± (S.E.) of n separate measurements. When appropriate, statistical comparisons between groups were carried out with 2-way unpaired Student’s t test or χ2 test. The accepted level of significance was p < 0.05.
Reagents
All reagents and drugs were purchased from Sigma–Aldrich, except for those indicated.
Results and discussion
Intracellular Ca2+ responses to different concentrations of carbachol in single HEK293 cells
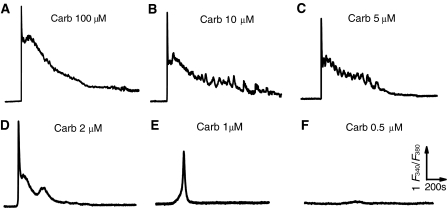
To obtain endogenous Ca2+ oscillation signal, concentration-dependent [Ca2+]i responses to a physiological relevant stimulator, carbachol (Carb), were conducted in the wild type HEK293 cells loaded with 1 μM fura2/AM, a concentration commonly used for fura2/AM loading. Treatments of the cells incubated in HBSS containing 1.8 mM Ca2+ with different concentrations of Carb usually induced a significant [Ca2+]i increase due to the activation of membrane muscarinic receptor, a member of Gq protein coupled receptor superfamily (Fig. 1). Obviously, the patterns of the [Ca2+]i rises were altered with the stimulation by different concentrations of the agonist. Carb at concentration of 100 μM elicited maximal overshot followed by a sustained but gradually declined [Ca2+]i signal (Fig. 1a). As the concentrations of Carb were lowered to 10, 5 and 2 μM, the amplitude and the lasting duration for the Ca2+ increase were reduced accordingly (Fig. 1b–d) until finally a single Ca2+ transient occurred in approximately 90% (29/32) or 25% (6/28) tested cells stimulated with 1 or 0.5 μM Carb, respectively (Fig. 1e–f). Although some of the cells gave a sharp transient followed by a somewhat high frequency fluctuation or sinusoidal response at a raised plateau level of [Ca2+]i, the typical repetitive [Ca2+]i transients or oscillations displayed in the transfected HEK293 cells with the muscarinic receptor (Shuttleworth and Thompson 1998) or the extracellular Ca2+-sensing receptor (Gerbino et al. 2005; Rey et al. 2005) could not be produced in these wild type cells by any concentration of Carb in this study.
Fig. 1.
Different patterns of intracellular Ca2+ signal in response to different concentrations of Carb in single HEK293 cells. HEK293 cells loaded with 1 μM fura2/AM were respectively stimulated with 100 (a), 10 (b), 5 (c), 2 (d), 1 (e) or 0.1 μM (f) fura2 in HBSS containing 1.8 mM extracellular Ca2+ as indicated. Note that repetitive Ca2+ spikes are not seen in any group of Carb-treated cells. Similar results were obtained from 24–38 cells in at least 6 independent experiments for all panels
The inconsistent responses between the wild type and the transfected HEK293 cells may suggest that somewhat insufficiency of key elements involved in oscillatory signaling system might undermine the genesis of this Ca2+ signal in the wild type cells, so that an artificial enhancement of the relevant receptor expression could give an typical and measurable oscillatory response (Shuttleworth and Thompson 1998; Rey et al. 2005). Nevertheless, lines of evidence indicate that the endogenous complex temporal Ca2+ signals including repetitive oscillations do commonly occur to physiological stimulation or lower concentrations of agonist in vivo and even in vitro circumstances (Thomas et al. 1996; Shuttleworth 1999; Berridge et al. 2000; Zhu et al. 2008). Thus, because no any oscillating response could be found in the wild type cells (Fig. 1), we speculated that the failure of [Ca2+]i oscillating measurement may be due to some extrinsic factors like the experimental conditions that we adopted rather than the cellular intrinsic regulators such as the status of endoplasmic reticulum load (Bird and Putney 2005), the degree of Ca2+ influx across cell membrane (Luo et al. 2001; Sneyd et al. 2004) or the mitochondrial buffering ability (Vay et al. 2007) as reported.
Intracellular Ca2+ responses in single HEK293 cells loaded with different concentrations of fura2/AM
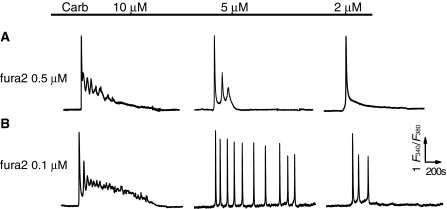
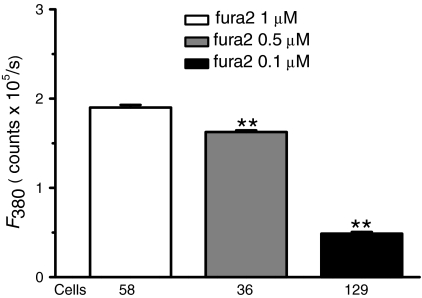
To verify whether the experimental treatment(s) adopted in this study affected the measurement of Ca2+ spiking in the wild type HEK293 cells, we first assessed the factor of the concentration for fluorescence Ca2+ indicator, fura2/AM. The protocol of loading cells with fura2/AM at concentrations of 1, 0.5 and 0.1 μM, respectively, and then stimulating cells with lower concentrations of Carb (≤10 μM) were performed under exactly identical experimental conditions. As showed in Fig. 2, the patterns of Ca2+ signals in response to Carb at lower concentrations ranging from 2 to 10 μM were changed significantly with the different loading dose of the Ca2+ indicator. For example, the fluctuating Ca2+ signals in response to Carb were gradually showing up in cells loaded with 0.5 and 0.1 μM fura2/AM, compared with the responses in 1 μM fura2/AM loaded cells (Fig. 1). Importantly, while sinusoidal Ca2+ signals and transient oscillations were seen in most of 0.5 μM fura2/AM loaded cells (panels 10 and 5 μM Carb in Fig. 2a), the typical Ca2+ oscillations showed up in partial cells loaded with fura2/AM at much lower concentration (0.1 μM) and activated with 5 μM Carb as indicated in Fig. 2b. These cells loaded with 0.1 μM fura2/AM demonstrated less than 30% fluorescence loading intensity proportional to the value of cells loaded with 1 μM fura2/AM (Fig. 3), but 1/3 of them gave the long lasting repetitive Ca2+ spikes upon 5 μM Carb stimulation (27/87 cells from independent experiments) during 40 min period of recording, a response rarely seen in 0.5 μM (Fig. 2a) and never observed in 1 μM fura2/AM loaded cells (Fig. 1). The others exhibited sinusoidal signal or a single transient followed by a slight plateau of Ca2+ rise (Table 1). Additionally, unlike the results obtained in the transfected HEK293 cells with the muscarinic receptors (Shuttleworth and Thompson 1998), the wild type HEK293 cells scarcely gave rise to repetitive Ca2+ transients upon stimulation with Carb at the concentration range between 1 to 3 μM, except to a higher concentration of Carb (5 μM). This is likely due to the difference in receptor expression density and receptor sensitivity to stimulations between transfected and wild type cells.
Fig. 2.
Different patterns of intracellular Ca2+ signal in responses to Carb in single HEK293 cells loaded with different concentrations of fura2/AM. Typical patterns of [Ca2+]i responses to 10, 5, or 2 μM Carb in single HEK293 cells loaded with 0.5 (a) or 0.1 μM (b) fura2 in HBSS containing 1.8 mM of extracellular Ca2+ as indicated. Note that the typical Ca2+ oscillations are observed in the cells loaded with 0.1 μM fura2. Similar results were obtained from 28–87 cells in at least 10 independent experiments for all panels
Fig. 3.
Comparison of fluorescent loading with different concentrations of fura2 dye in HEK293 cells. The intracellular fluorescent loading states in single HEK293 cells were evaluated in cells loaded with 1, 0.5, or 0.1 μM fura2/AM, respectively, in Dulbecco’s Eagle’s medium for 25 min at temperature of 37°C and the F380 values were measured (see Methods). Each bar stands for mean ± SE of 58–129 cells from at least 10 independent experiments. **p < 0.01 versus group of 1 μM fura2 loading
Table 1.
Ca2+ response patterns to 5 μM Carb in single HEK293 cells loaded with 0.1 μM fura2 in HBSS containing different concentrations of extracellular Ca2+
| [Ca2+]o (mM) | Occurrence of different Ca2+ response patterns to 5 μM Carb (%) | ||||
|---|---|---|---|---|---|
| Single rise | Transient spikes | Spiking < 20 min | Spiking > 30 min | N | |
| 1.2 | 19 (32.8) | 32 (55.2) | 5 (8.6) | 2 (3.4) | 58 |
| 1.5 | 16 (7.4) | 48 (22.2) | 50 (23.1) | 102 (47.2)** | 216 |
| 1.8 | 41 (47.1) | 10 (11.5) | 9 (10.3) | 27 (31.1) | 87 |
[Ca2+]o: concentration of extracellular Ca2+. N: cells from independent experiments. ** p < 0.01 compared with the group of 1.2 mM or 1.8 mM [Ca2+], respectively
Although not all the cells tested oscillated in response to Carb, these results did demonstrate that under condition of loading cells with a proper concentration of fura2/AM the native Ca2+ oscillations can be produced upon a stimulation of physiologically-relevant agonist concentration in wild type HEK293 cells. This requirement for an indicator concentration indicates that the loading state of fura2/AM, or exactly the quantity of fura2 inside the cells (Fig. 3), greatly affects the observation or actually the formation of repetitive Ca2+ transient signaling and finally determination of Ca2+ signal types. Therefore, caution should be taken in using Ca2+ indicators, especially for a high-affinity Ca2+ indicator to detect small [Ca2+]i signals like sparks, waves or repetitive transients. In fact, ion indicators function as chelators for a specific ion. They determine a specific ion concentration via changes in their fluorescence properties like absorption, intensity, or spectra, meanwhile, they also cause experimental problem, in particular the high-affinity Ca2+ indicators such as fura2 and calcium green-1 may buffer small amounts of intracellular free Ca2+ when they enter into the cells at higher concentrations (Hofer and Machen 1994; Takahashi et al. 1999). In the current study, the repetitive Ca2+ spikes could not be detected by fura2 at commonly used concentration (1 μM), but was slightly exhibited at concentration of 0.5 μM and eventually detected by loading cells with 0.1 μM fura 2 (Figs. 1 and 2). This phenomenon was also noticed in other studies in different cell lines and a notion is reached that low-affinity Ca2+ indicator such as fluo 3 or fura-5F are preferred for such measurements of small [Ca2+]i signals as spikes, waves and oscillations (Hofer and Machen 1994; Takahashi et al. 1999; Bird and Putney 2005). However, if fura2 is optional, lower concentration of the dye is ought to be taken for measurement of small Ca2+ signals, at which the measurement of large [Ca2+]i response to 100 μM Carb stimulation was not influenced either (data not shown).
Intracellular Ca2+ responses in single HEK293 cells incubated in medium containing different extracellular Ca2+ concentration
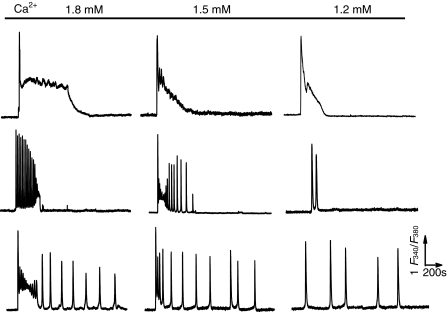
As most of the cells tested in 0.1 μM fura2 loaded cells gave less than 5 repetitive transients to Carb stimulation (Table 1. 51/87 cells from independent experiments), we further determined whether other factors, in addition to fura2, also affected the repetitive spiking occurrence in the wild type HEK293 cells. It has been reported that extracellular Ca2+ concentration can be an important factor determining the frequency of Ca2+ oscillations (Clapham 1995; Shuttleworth and Thompson 1998; Luo et al. 2001; Bird and Putney 2005; Gerbino et al. 2005). Thus, we next testified the influence of Ca2+ concentrations in the medium on the formation of Ca2+ oscillatory response in these cells. Three groups of cells loaded with 0.1 μM fura2 were respectively incubated with HBSS containing 1.8, 1.5 or 1.2 mM CaCl2 throughout the experiment, and exposed to 5 μM Carb for at least 40 min. As shown in Fig. 4 and Table 1, Carb elicited various types of Ca2+ signaling response in 0.1 μM fura2-loaded cells. However, the main patterns of the response could be summarized into three patterns; sustained single Ca2+ rise (the upper panel), short lasting (middle panel) and the typical Ca2+ transients (the bottom panel) that were all present in the three groups of cells but with different proportions (Table 1). Cells incubated with 1.5 mM extracellular Ca2+ exhibited the most Ca2+ spikes with the longest duration, whereas cells exposure to 1.2 mM extracellular Ca2+ showed the least transients and the shortest duration among the three groups.
Fig. 4.
Influence of intracellular Ca2+ oscillations by extracellular Ca2+ concentration in single HEK293 cells. Typical traces represent the three main patterns of Ca2+ signal responses, sustained single Ca2+ rise (the upper panel), short lasting (the middle panel) and the typical Ca2+ transients (the bottom panel), to 5 μM Carb in 0.1 μM fura2 loaded cells incubated in the HBSS containing 1.8, 1.5 or 1.2 mM extracellular Ca2+ (3 groups) as indicated. These patterns of Ca2+ signals were found in the 3 groups of cells but with different occurrence proportion, which are further summarized in Table 1. Similar results were obtained from 58–216 cells in at least 16 independent experiments for all panels
Previous studies have implicated that Ca2+ oscillations can not be induced in null extracellular Ca2+ (Clapham 1995; Shuttleworth and Thompson 1998; Luo et al. 2001; Bird and Putney 2005); neither in 2.5 mM extracellular Ca2+ (Paltauf-Doburzynska et al. 2000). In agreement with the reports, these data also demonstrates that extracellular Ca2+ can be an important factor affecting the oscillatory responses, and the concentration of 1.2–1.5 mM Ca2+ outside the cells is relatively appropriate for the generation of Ca2+ oscillation in the wild type HEK293 cells.
In addition to the elements tested above, we also examined whether the experimental temperature might share influence on the formation of Ca2+ oscillations. As this type of Ca2+ signal usually takes place under physiological condition, we set the temperature at 35 ± 1°C and found that cells stimulated with 5 μM Carb at such temperature produced more repetitive Ca2+ spikes with long duration than those activated at room temperature 22 ± 2°C (60.7% in 56 cells vs. 47.2% in 216 tested cells), suggesting that this type of Ca2+ signals were generated more easily at physiological temperatures.
Taken in all, the current study demonstrates that versatile temporal Ca2+ signals in response to different potent stimulations can be produced in single wild type HEK293 cell, a cell line widely used in investigations of intracellular signaling transduction pathways and their modulations and functions (Shuttleworth and Thompson 1998; Luo et al. 2001; Sneyd et al. 2004; Rey et al. 2005; Gerbino et al. 2005). However, unlike the capture of general Ca2+ signal responses, the repetitive oscillations in [Ca2+]i in response to low concentrations of agonist using fluorescent ion indicator are dramatically affected by several experimental factors. The most important factor is the option of Ca2+ indicator and its concentration used for loading cells because inappropriate adoption of dye and its concentration will cause failure in obtaining repetitive Ca2+ transients (Figs. 1 and 2). Additionally, the extracellular Ca2+ concentration in the cell medium should also be modified carefully because this factor can affect the transient frequency as well as occurrence rate (Fig. 3 and Table 1). Also, the physiological temperature ought to be taken since more cells oscillate at this condition. Therefore, this study provides a useful approach for the measurement of the physiological [Ca2+]i oscillatory response in a wild type cell line.
Intracellular Ca2+ oscillations to physiological relevant stimulations open a wide door to Ca2+ signaling system. They may protect cells from the toxic effects of sustained increases in [Ca2+]i and allow information to be encoded in the frequency, amplitude and duration of the Ca2+ spikes. Currently, many studies demonstrate that multiple cellular events are tuned to respond optimally to specific frequencies and patterns of Ca2+ spiking ((Berridge et al. 2000; Frey et al. 2000; Watt et al. 2000; Reither et al. 2006; Zhu et al. 2008), but the mechanisms underlying their formation and dynamic characteristics are diverse and remained to be explained (Frey et al. 2000; Carafoli 2002; Berridge et al. 2003). In this regard, investigation in cell lines in vitro, particularly in HEK293 cells, has appeared to be one of the important approaches for obtaining evidence of and information on this type of Ca2+ signal response. This study demonstrates optimized experimental conditions for the measurement of Ca2+ oscillatory responses in wild type HEK293 cells, which are definitely more resembling to the cells in physiological context, thus providing a useful approach for the observation of native Ca2+ spiking response to relevant physiological stimulations.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation (30772574 and 30973537), the Beijing Natural Science Foundation (7082018) and the Scientific Research Common Program of Beijing Municipal Commission of Education (D.L.).
References
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nature Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling, dynamics, homeostasis and remodeling. Nat Rev Mol Cell Biol. 2003;14:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bird GS, Putney JWJr. Ca2+ entry by whatever mechanism(s) keeping the store filled and oscillating. J Physiol. 2005;562:697–706. doi: 10.1113/jphysiol.2004.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/S0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci USA. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat Med. 2000;6:1221–1227. doi: 10.1038/81321. [DOI] [PubMed] [Google Scholar]
- Gerbino A, Ruder WC, Curci S, Pozzan T, Zaccolo M, Hofer AM. Termination of cAMP signals by Ca2+ and Gαi via extracellular Ca2+ sensors: a link to intracellular Ca2+ oscillations. J Cell Biol. 2005;171:303–312. doi: 10.1083/jcb.200507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- Hofer AM, Machen TE. Direct measurement of free Ca in organelles of gastric epithelial cells. Am J Physiol (Gastrointest Liver Physiol) 1994;267:G442–G451. doi: 10.1152/ajpgi.1994.267.3.G442. [DOI] [PubMed] [Google Scholar]
- Luo D, Broad LM, Bird GS, Putney JWJr (2001) Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J Biol Chem 276:5613–5621 [DOI] [PubMed]
- Meyer T, Stryer L. Calcium spiking. Ann Rev Biophys Biophys Chem. 1991;20:153–174. doi: 10.1146/annurev.bb.20.060191.001101. [DOI] [PubMed] [Google Scholar]
- Paltauf-Doburzynska J, Frieden M, Spitaler M, Graier WF. Histamine induced Ca2+ oscillations in a human endothelial cell line depend on transmembrane ion flux, ryanodine receptors and endoplasmic reticulum Ca2+-ATPase. J Physiol. 2000;524:701–713. doi: 10.1111/j.1469-7793.2000.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reither G, Schaefer M, Lipp P. PKCα: a versatile key for decoding the cellular calcium toolkit. J Cell Biol. 2006;174:521–533. doi: 10.1083/jcb.200604033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey O, Young SH, Yuan J, Yuan JZ, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1, 4, 5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J Biol Chem. 2005;280:22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ. What drives calcium entry during [Ca2+]i oscillations? -challenging the cappacitative model. Cell Calcium. 1999;25:237–246. doi: 10.1054/ceca.1999.0022. [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. Muscarinic receptor activation of arachidonate-mediated Ca2+ entry in HEK293 cells is independent of phospholipase C. J Biol Chem. 1998;273:32636–32643. doi: 10.1074/jbc.273.49.32636. [DOI] [PubMed] [Google Scholar]
- Sneyd J, Tsaneva-Atanasova K, Yule DI, Thompson JL, Shuttleworth TJ. Control of calcium oscillations by membrane fluxes. Proc Natl Acad Sci USA. 2004;101:1392–1396. doi: 10.1073/pnas.0303472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Camacho P, Lechleiter JD, Herman B. Measurement of intracellular calcium. Physiol Rev. 1999;79:1089–1125. doi: 10.1152/physrev.1999.79.4.1089. [DOI] [PubMed] [Google Scholar]
- Thomas AP, Bird GS, Hajnóczky G, Robb-Gaspers LD, Putney JWJr (1996) Spatial and temporal aspects of cellular calcium signaling. FASEB J 10:1505–1517 [PubMed]
- Vay L, Hernández-SanMiguel E, Santo-Domingo J, Lobaton CD, Moreno A, Montero M, Alvarez J. Modulation of Ca2+ release and Ca2+ oscillations in HeLa cells and fibroblasts by mitochondrial Ca2+ uniporter stimulation. J Physiol. 2007;580:39–49. doi: 10.1113/jphysiol.2006.126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt SD, Gu X, Smith RD, Spitzer NC. Specific frequencies of spontaneous Ca2+ transients upregulate GAD 67 transcripts in embryonic spinal neurons. Mol Cell Neurosci. 2000;16:376–387. doi: 10.1006/mcne.2000.0871. [DOI] [PubMed] [Google Scholar]
- Willoughby D, Cooper DMF. Ca2+ stimulation of adenylyl cyclase generates dynamic oscillations in cyclic AMP. J Cell Sci. 2006;119:828–836. doi: 10.1242/jcs.02812. [DOI] [PubMed] [Google Scholar]
- Zhu L, Luo Y, Chen T, Chen F, Wang T, Hu Q. Ca2+ oscillation frequency regulates agonist-stimulated gene expression in vascular endothelial cells. J Cell Sci. 2008;121:2511–2518. doi: 10.1242/jcs.031997. [DOI] [PubMed] [Google Scholar]