Abstract
Conditioned medium from adipose derived stem cells (ADSC-CM) stimulates both collagen synthesis and migration of fibroblasts, and accelerates wound healing in vivo. Recently, the production and secretion of growth factors has been identified as an essential function of adipose-derived stem cells (ADSCs). However, the main soluble factor of ADSC-CM which mediates paracrine effects and its underlying mechanism has not been elucidated yet. In this study, we considered transforming growth factor-beta1 (TGF-β1) as a strong candidate for paracrine effect of ADSC-CM and investigated collagen synthesis and hyaluronic acid synthase (HAS) expression. After ADSC-CM addition, collagen type I, type III, HAS and hyaluronic acid (HA) expressions on human dermal fibroblasts (HDFs) were evaluated. Furthermore, to clarify effects of TGF-β1 as a paracrine mediator, TGF-β1 antibody and external supplementary TGF-β1 were treated to HDFs. Collagens type I, type III, HAS-1 and HAS-2 mRNA expressions of HDFs were greatly increased by ADSC-CM treatment, however there was no change in TGF-β1 antibody treated HDFs compared with non-treated control. These results strongly demonstrate that TGF-β1 plays an important role as a paracrine mediator of ECM synthesis. The fact that TGF-β1 contained in ADSC-CM not only accelerates collagen deposition but also increase hyaluronic acid synthesis of HDFs through HAS-1 and HAS-2 expression was also elucidated in this study. Therefore, ADSC-CM shows promise for the treatment of cutaneous wounds and accelerates granulation formation during healing process.
Keywords: Adipose derived stem cells (ADSCs), Paracrine effect, TGF-β1, Hyaluronic acid synthase (HAS), Human dermal fibroblasts (HDFs)
Introduction
Since the existence of multipotent stem cells within adipose tissue was reported in 1964, it has been documented that adipose tissue-derived stem cells (ADSCs) act in a similar way with the bone marrow-derived mesenchymal stem cells, including their morphology, colony frequency, immune phenotype and surface markers (Kern et al. 2006). However, due to the abundance, practicality and accessibility in the human body, interest has rapidly grown in the plasticity and therapeutic potential of ADSCs (Zhu et al. 2008). Moreover, the production and secretion of various extracellular matrix (ECM) components and cytokines such as vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), platelet derived growth factor (PDGF), transforming growth factor-beta (TGF-β) and fibroblast growth factor (FGF) from ADSCs have been received extensive attention as autologous inducing factors for tissue regeneration (Kinnaird et al. 2004; Kratchmarova et al. 2002; Maeda et al. 1996, 1997; Rehman et al. 2004).
ADSCs and their secretomes mediate diverse skin regeneration processes, such as wound healing, antioxidant protection, anti-wrinkle and hair-growth effects (Kim et al. 2007; Park et al. 2008). Wound healing effects of ADSCs were also reported with a conditioned-medium of ADSCs (ADSC-CM) (Park et al. 2010). In response to injury, ADSCs not only directly migrate to the wound area, differentiate and repopulate the wound with healthy skin appendages, but also show paracrine activation of human dermal fibroblasts (HDFs) and keratinocytes, resulting in accelerating proliferation and synthesis of ECM components on the wound site (Kim et al. 2009). In a highly coordinated biological process of dermal wound healing, by these syntheses and cell-to-cell, cell-to-cytokine interdependencies, skin fibroblasts repair wounds and maintain the integrity of skin as well (David-Raoudi et al. 2008).
In spite of various approaches to demonstrate availability of ADSC-CM, the underlying biology related to the ECM production especially hyaluronic acid (HA) has not been well elucidated. HA, a large-molecular-weight glycosaminoglycan (GAG) which is widely expressed in tissues remodeling, is known to affect collagen synthesis and maturation via the HA receptor-mediated signaling pathway (Allemann et al. 2001). In addition, HA plays a critical role in various biological events such as differentiation of the HDFs, wound closure, anti-inflammatory response, cell proliferation, differentiation, and GAG synthesis (Akmal et al. 2005; Meran et al. 2007; Turino and Canor 2003; Williams et al. 2003).
In an attempt to explore the contribution of ADSC-CM to the cutaneous wound healing, we investigated the underlying biology of HA and collagen production mediated by ADSC-CM treatment onto HDFs. Moreover, based on the fact that TGF-β stimulates HA synthesis in HDFs, the paracrine mediator released from ADSCs which determines mRNA and protein expression profile was investigated.
Materials and methods
Cell cultures
Human Dermal Fibroblasts (HDFs) were purchased from Modern Cell & Tissue Technologies (MCTT, Seoul, Korea) and American Type Culture Collection (ATCC, VA, USA) respectively. HDFs were cultured in Dulbecco’s modified Eagle medium (DMEM, WelGENE, Daegu, Korea) supplemented with 10% FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml). The cells were maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% air and the growth medium was changed every 3–4 days. The cells were cultured with serum-free medium for 24 h before experiment.
Adipose-derived stem cells (ADSCs) were purchased from Invitrogen (StemPro® Human Adipose-Derived Stem Cell Kit, Cat. No. R7788-110, CA, USA). As per the user’s manual, ADSCs were isolated from human lipoaspirate tissue and expanded for one passage before cryopreservation. The ADSCs express the following flow cytometry cell-surface protein profile: positive CD29, CD44, CD73, CD90, CD105, and CD166; negative CD14, CD31, CD45, and Lin1. ADSCs were cultured in serum-free DMEM/F12 (WelGENE, Daegu, Korea) medium and no cellular death was observed during the culture period.
Preparation and treatment of ADSC-CM
ADSCs (4 × 105 cells) of passage 3–5 were seeded on a 100 mm dish and cultured with a serum free medium for 72 h and the medium was then collected. The collected ADSC-CM was sterilized with 0.22 μm syringe filter (Millex®-GS Filter Unit, MA, USA) after centrifugation at 300 × g for 5 min.
Confluent HDFs were treated with serum-free medium containing 5 ng/ml of TGF-β1 or 0 (control), 10, 50 and 100% concentration of ADSC-CM. TGF-β1 in 10 mM citric acid (pH 3.0) was reconstituted with 1 mg/ml of bovine serum albumin to activate TGF-β1 from its latent form prior to use. After 4 h of treatment, HDFs were processed for further total RNA extraction to confirm the dose-effects of ADSC-CM treatment. After 3, 12, 36, 48 h of treatment, the culture medium supernatant was utilized for enzyme-linked immunosorbent (ELISA) assay and HDFs were processed for further total RNA extraction.
TGF-β1 blocking assay
To clarify the effects of the TGF-β1, the action of TGF-β1 composed in ADSC-CM was neutralized with a chicken anti-human TGF-β1 antibody (Abcam, MA, USA). HDFs cultured in 6-well plates were cultured with serum-free medium containing 0.1% BSA for starvation for 24 h prior to use.
5 ng/ml of TGF-β1 and 50% ADSC-CM were pre-incubated with a chicken anti-human TGF-β1 antibody (100 ng/ml) for 1 h and added to the HDFs. After 24 h, the cells were washed with cold PBS and prepared for RT–PCR analysis.
Total RNA isolation and RT–PCR analysis
The total RNA from each sample was extracted using easy-BLUE™ Total RNA Extraction Kit (iNtRON Biotech, Gyeonggi-Do, Korea). According to the manufacturer’s instruction, 1 mg of total RNA was used for reverse transcription reaction with the first-strand cDNA synthesis mix containing 20 mM Tris–HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 10 mM dithiothreitol, 0.25 mM of each dNTP, and 100 U of Moloney murine leukemia virus reverse transcriptase. The sequences of the sense (+) and antisense (−) primer pairs of HAS-1, HAS-2, HAS-3, collagen type I, collagen type III and control were as follows: HAS-1 (+) 5′-ggtgcttctgtcgctctacg-3′ and (−) 5′-gctactgggtggccatgttgac-3′ (product size 306 bp); HAS-2 (+) 5′-tggggcggcaagcgcgaggtcat gtacacagc-3′ and (−) 5′-caccagagcgcgttgtacagccactcacggaag-3′ (product size 250 bp); HAS-3 (+) 5′-tggcctactttggctgtgtgcag-3′ and (−) 5′-agatcatctctgcattgccc-3′ (product size 300 bp); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (+) 5′-agccgcatcttcttttgcgtc-3′ and (−) 5′-tcatatttggcaggtttttct-3′ (product size 580 bp). Amplification of cDNA fragments was performed on PCR through 30 cycles with 1 μl of each RT product as a template DNA in a 60 mM Tris–HCl (pH 9.1) buffer containing 18 mM (NH4)2SO4, 16 mM MgCl2, 0.25 mM of each dNTP, 0.1 nmol of each primer, and iMax-Taq DNA polymerase. Each sample (5 μl) of the final PCR product was separated using a 1% agarose gel and visualized using UV fluorescence after staining with ethidium bromide. Template control, GAPDH, was included in each run. The intensity of the bands was measured using the Quantity One Software (Bio-Rad, CA, USA).
ELISA assay for protein expression
The concentrations of collagen and hyaluronic acid in the culture medium aliquots were measured using Hyaluronan Enzyme-Linked Immunosorbent Assay Kit (HA-ELISA, Echelon Bioscience Incorporated, UT, USA) and Human Collagen1 ELISA (COSMO BIO CO., LTD, Tokyo, Japan) according to the manufacturer’s instruction.
In brief, standards and samples were transferred into the HA ELISA plate and treated with Working Enzyme. Working substrates were used for further color development. The amount of collagen secreted into culture medium was assessed by pepsin digestion. Biotinylated anti-collagen antibody and standard solutions or assay samples were mixed well then transferred into 96 well plates for following avidin-HRP conjugate solution treatment. Routinely, the plates were incubated with the substrate at 37 °C for 1–2 h before reading the optical density at 405 and 450 nm. Optical densities were determined by using a microtiter plate spectrophotometer. Two independent experiments were performed and the blank reading was subtracted from the values for both standards and samples. A standard curve was created by plotting the logarithm of the mean absorbance of each standard versus the logarithm of the HA and collagen concentration.
Statistical analysis
Data are expressed as mean ± standard error mean (S.E.M.). Statistical significance was assessed using one-way analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test or Student t-test. Statistical analysis was performed with the GraphPad Prism package (GraphPad Software, CA, USA). P values less than 0.05 were considered statistically significant.
Results
HAS-1, HAS-2, collagen type I and collagen type III mRNA expression level of HDFs
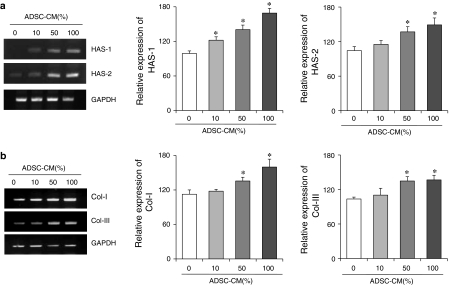
HDFs were treated with various concentrations of ADSC-CM (0, 10, 50 and 100%) to investigate dose effects of ADSC-CM on HAS-1, HAS-2, collagen type I and collagen type III mRNA expression. HDFs treated with 0, 10, 50, 100% of ADSC-CM for 4 h showed dose-dependent increase in HAS-1 and HAS-2 mRNA expression as shown in the representative picture of agarose gel of Fig. 1a. Relative mRNA expression of HAS-1 and HAS-2 to GAPDH was determinated and is also shown in Fig. 1a. HDFs cultured with 100% ADSC-CM showed the highest value in both HAS-1 and HAS-2 mRNA expression. A dose-dependent increase in the HAS-1 and HAS-2 expression by ADSC-CM treatment was confirmed although the amount of mRNA expression was generally higher in HAS-1.
Fig. 1.
Effect of ADSC-CM treatment on mRNA expression of HAS and collagen in HDFs. (a) The agarose gels indicate HAS-1 and HAS-2 mRNA expression (left) and the quantified data of HAS-1 and HAS-2 mRNA expression relative to GAPDH expression show dosedependent increase in mRNA expression (right). (b) The agarose gels indicate collagen type I and type III mRNA expression (left) and the quantified data of collagen type I and type III mRNA expression relative to GAPDH expression show dosedependency (right). Each bar indicates average value ± S.E.M.; *p < 0.05 versus 0% ADSC-CM control. Three independent experiments were carried out in duplicate
As shown in the agarose gel picture in Fig. 1b, treatment with ADSC-CM for 4 h increased expression of both collagen type I and collagen type III mRNA levels in a dose-dependent manner. Relative mRNA expression of collagen type I and collagen type III to GAPDH showed the highest value in 100% ADSC-CM treated HDFs. Compared to the control (ADSC-CM 0%), a 1.5-fold of increase in collagen type I mRNA were observed with 100% ADSC-CM treated HDFs.
Vertical (y) axes of the graph in Fig. 1 were assessed as % of control (ADSC-CM 0%) with relative mRNA expression values to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression.
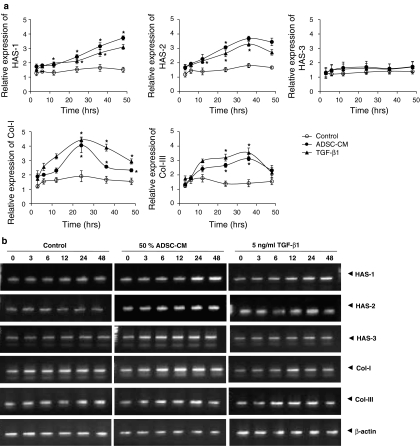
Time-course effects of TGF-β1 and ADSC-CM on collagen type I and type III synthesis and HAS expression in HDFs
In order to confirm a dominant paracrine mediator which determines HAS and collagen mRNA expression profile, TGF-β1, one of the paracrine factors present in ADSC-CM, was used to treat HDFs. Then the time course effect of TGF-β1 was compared with that of 50% ADSC-CM on HDFs.
The expression of HAS-1 mRNA was gradually increased in the ADSC-CM and the TGF-β1 treated groups. This increased expression was a dose dependant and was sustained for about 50 h. However, there was no change in HAS-1 mRNA expression level of control group (ADSC-CM 0%) (Fig. 2). Increased mRNA expression of HAS-2 was also observed and the up-regulated peaks were detected after 36 h of 50% ADSC-CM and 5 ng/ml TGF-β1 treatment. On the other hand, after 48 h of treatment mRNA expression of HAS-2 showed slight decrease both in ADSC-CM and TGF-β1 treated HDFs. There was no change in HAS-2 mRNA expression level of ADSC-CM 0% control (Fig. 2). In case of HAS-3, there was no observable change in mRNA expression in neither experimental groups nor control. The increased expression of HAS-3 mRNA by ADSC-CM and TGF-β1 treatment was not identified. Collagen type I and collagen type III mRNA expression also started to increase gradually after treatment and the highest expression was observed after 24 h in collagen type I and after 36 h in collagen type III mRNA expression. After this up-regulation, mRNA expression of collagen type I and collagen type III were again down-regulated to basal level. Although there was no change in mRNA expression of control, expression profiles of collagen type I and collagen type III observed to be similar in control groups.
Fig. 2.
Time-course effects of 50% ADSC-CM and 5 ng/ml TGF- β1 on HAS-1, HAS-2, HAS- 3 and collagen type I, type III mRNA expression in HDFs. (a) mRNA expression was estimated relative to β-actin expression after 3, 12, 36, 48 h of treatment. (b) the representative pictures of mRNA expression on agarose gel. The mRNA expression of HAS-1, HAS-2, HAS-3, collagen type I, collagen type III and β-actin were assessed with RT–PCR. The experiments shown in (a) and (b) were repeated three times in duplicate. Each bar indicates average value ± S.E.M.; *p < 0.05 versus 0% ADSCs-CM control
Although it is hard to confirm the difference in mRNA expression level with naked eye, the pictures of agarose gel obtained from RT–PCR are shown in Fig. 2b. In TGF-β1 treated group, the pattern of increasing and decreasing mRNA expression appeared to be similar to the group treated with 50% ADSC-CM (Fig. 2b).
General expression level of mRNA appeared to be different between the ADSC-CM and TGF-β1 treated groups, however, the time points of the increase and decrease, and expression profile were similar. This similarity between 5 ng/ml TGF-β1 and 50% ADSC-CM treated group in time-course effects implies TGF-β1 to be a potent mediator among ADSC-CM components which determines mRNA and protein expression profiles of ECM.
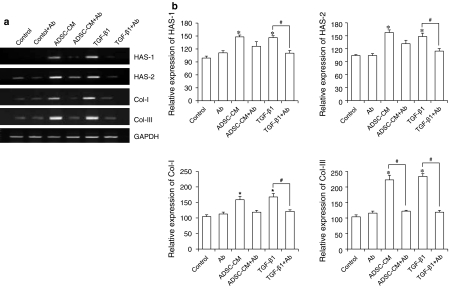
TGF-β1 blocking assay
Figure 3 shows similar increased expression patterns in collagen and HAS expression between TGF-β1 and ADSC-CM treated groups, and decreased expression patterns in TGF-β1 and ADSC-CM treated groups resulted from anti-human TGF-β1 antibody addition. For this TGF-β1 blocking assay, TGF-β1 solution and ADSC-CM were neutralized with TGF-β1 antibody and used for the treatment of HDFs for 24 h, and we observed increased expression in HAS-1, HAS-2, collagen type I and collagen type III mRNA at the time point of 24 h Fig. 2.
Fig. 3.
HAS-1, HAS-2, collagen type I, and type III mRNA expression profile in HDFs after treatment with ADSCCM or TGF-β1 with or without neutralization with anti-TGF-β1 antibody. (a) Representative pictures of agarose gels showing mRNA expression obtained using RTPCR. (b) Quantified mRNA expression relative to GAPDH mRNA expression. HDFs were treated with 50% ADSC-CM or 5 ng/ml TGF-β1 which were neutralized with 100 ng/ml TGF-β1 antibody for 1 h. The results are reported as mean ± S.E.M. (n = 6) and with an analysis of variance (ANOVA), which was followed by the Bonferroni’s adjustment. The value marked with an asterisk is significantly (p < 0.05) different from the control. The value marked with a # is significantly (p < 0.05) different from the other groups
The concentration of the TGF-β1 antibody which shows complete inhibition of TGF-β1 (0.25 ng/ml) was indicated to be 5–10 ng/ml in the instructor’s manual. The concentration of TGF-β1 antibody used in this study (100 ng/ml) seems to be sufficient to block the activity of TGF-β1 in both TGF-β1 solution and ADSC-CM. The antibody treatment suppressed increase of mRNA expression in HAS and collagen by TGF-β1 and ADSC-CM treatment on HDFs. The representative pictures of agarose gel obtained from RT–PCR (Fig. 3a) show visible suppression in HAS and collagen mRNA expression after TGF-β1 neutralization.
As shown in Fig. 3b, when HDFs were cultured under TGF-β1 neutralized condition, the mRNA expressions of HAS-1, HAS-2, collagen type I and collagen type III showed no significant difference to non-treated control even though cells were treated with ADSC-CM or TGF-β1. Furthermore, the suppressing activity of TGF-β1 antibody treatment was most significant in collagen type III mRNA expression. In general, the suppressive effects of TGF-β1 antibody neutralization appeared to be greater in collagen than HAS. As a result, the increased collagen type I and type III mRNA expression by 50% ADSC-CM and TGF-β1 treatment were reversed to nontreated control level.
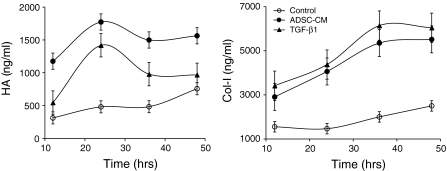
Time-course effects of TGF-β1 and ADSC-CM on HA and collagen type I, type III synthesis of HDFs
The actual HA and collagen type I protein expression facilitated by mRNA up-regulation as a result of ADSC-CM or TGF-β1 treatment were confirmed using ELISA.
HA protein was increased and up-regulated peak was observed after 24 h of ADSC-CM and TGF-β1 treatment (Fig. 4). HA expression was induced in the initial stage of ADSC-CM or TGF-β1 treatment (Fig. 4). In addition, HA expression was facilitated without HAS-3 mRNA up-regulation (Fig. 2a). HA and collagen type I were gradually increased without ADSC-CM or TGF-β1 treatment in non-treated control owing to the nature of HDFs which synthesize various kind of ECM. Collagen type I expression was gradually increased for 48 h after ADSC-CM and TGF-β1 treatment (Fig. 4). Although collagen type I mRNA was most abundant after 24 h of treatment then decreased (Fig. 2a), the collagen type I protein expression prolonged for 48 h after treatment (Fig. 4). Whereas the concentration of TGF-β1 in ADSC-CM was confirmed to be 103 pg/ml and we only used 50% of ADSC-CM in this experiment, the amount of collagen type 1 was similar to that of HDFs treated with 5 ng/ml TGF-β1. The existence of other factors which support the activity of TGF-β1 can be implied with this result.
Fig. 4.
Time-course effects of 50% ADSC-CM and 5 ng/ml TGF-β1 on HA and collagen expression. Protein expression of HA and collagen type I by HDFs was assessed with ELISA after 3, 12, 36, 48 h of ADSC-CM or TGF-β1 treatment. The experiments were repeated two times (n = 2). Each bar indicates average value ± STDEV
Discussion
In the present study, paracrine effects of ADSC-CM on HDFs were demonstrated with dose-dependent increased mRNA expression levels of HAS-1, HAS-2, collagen type I and collagen type III and further HA and collagen type I expression. In addition, we showed that mRNA and protein expression profile of HDFs which was induced by ADSC-CM treatment was similar to that induced by external TGF-β1 treatment. HA, one of the major ECM components is responsible for skin moisture and collagen type I and type III play a pivotal role in maintaining skin elasticity. These findings are closely related to wound healing mediated by ADSC-CM as previously reported (Turino and Canor 2003).
HA is synthesized on the inner surface of the plasma membrane by three different isoenzymes, HAS-1, HAS-2 and HAS-3. Each isoform is capable of synthesizing HA molecules of a given size and exhibits different kinetic properties plus different cell-type characteristics (Itano et al. 1999). It has been reported that many cytokines are able to modulate HAS transcription in cell cultures. Data obtained for the treatment of fibroblasts with transforming nuclear factor-alpha (TNF-α) and TGF-β1 showed a significant increase in mRNA expression and protein synthesis of HAS-1 and HAS-2, key enzymes for high-molecular-weight HA. Recently, the possibility of high-molecular-weight HA to protect stressed cells has been implied and it is also considered to be related to in vivo antioxidant effects of ADSCs (Campo et al. 2006). ADSC-CM which was used in this study increased mRNA expression of HAS-1 and HAS-2 that are closely related to high-molecular-weight HA production.
Although the role of HA in wound healing has been clarified with various research (David-Raoudi et al. 2008; Guo et al. 2010; Larson et al. 2010), the specialized role of HAS-1, HAS-2 and HAS-3 has not yet been elucidated. HA accumulation during wound healing process is known to be induced by TGF-β1 which facilitates up-regulation of HAS-2 (Guo et al. 2010). It has been known for a long time that HA is abundant in the early granulation tissue of dermal wounds, and associates with scar-less healing (Larson et al. 2010). Recently it was reported that the wounding-induced up-regulation of HA synthesis is not limited to mesenchymal cells, but is also very prominent in the epithelium. The discovery that epidermal injury up-regulates the expression of HAS-2 and HAS-3 in keratinocytes and causes a sixfold increase of epidermal HA was reported by Tammi et al. (2005) . HAS-2 and HAS-3 are considered to be especially related to the initial state of wound healing, and therefore mediate cell migration. However, it is hard to determine which one is more relevant to wound healing.
HA accumulation has been shown to enhance the TGF-β1 driven differentiation of HDFs to myofibroblasts and the effects of TGF-β1 on proliferation (Meran et al. 2007, 2008). In precise, TGF-β1 dependant differentiation of fibroblasts is antagonized by inhibition of HAS, confirming that the TGF-β1 driven differentiation of fibroblasts is a major source of myofibroblasts, and that HA is a major modulation of this process (Webber et al. 2009). Myofibroblasts are specialized cells which exist in granulation tissue and mediate the closure of wounds and formation of collagen (Kim et al. 2008). In this study, ADSC-CM treatment to HDFs resulted in similar mRNA and protein expression profiles with those of the HDFs which exhibit the TGF-β1 dependant myofibroblastic responses. Increase in collagen synthesis which might result from myofibroblastic responses of HDFs was also confirmed by ADSC-CM treatment. This finding implies TGF-β1 be a potent mediator of HA synthesis which determines mRNA and protein expression profiles among ADSC-CM constituents. Without presuming the involvement of myofibroblastic differentiation, TGF-β1 is known as a potent factor accelerates wound healing by stimulating collagen deposition and HA accumulation (Puolakkainen et al. 1995) and seems to played an important role in ECM synthesis as a major component of ADSC-CM.
Whereas the amount of TGF-β1 (approx. 103 pg/ml) in ADSC-CM was miniscule compared with the concentration of independently added TGF-β1 (5 ng/ml), there was no significant difference in HAS-1, HAS-2, collagen type I and collagen type III mRNA expression. Moreover, the amount of HA synthesized after ADSC-CM treatment was larger than after TGF-β1 treatment. With these results, it can be implied that growth factors or cytokines in ADSC-CM amplified ECM synthesis is induced by TGF-β1. ADSC-CM treatment can be an efficient way of treatment to augment ECM in the tissue.
Recently, several studies have reported the various pharmacological effects of ADSC-CM in wound healing and photoaging via activation of dermal fibroblasts (Kim et al. 2007; Planat-Benard et al. 2004). However, underlying mechanism has not been fully characterized and few studies focus on underlying biology of soluble factors composed in ADSC-CM. Especially involvement of HAS isoforms to activation of ADSC-CM treated fibroblasts has not yet been reported. We confirmed paracrine effects of ADSC resulted in up-regulation of HA through increased expression of HAS-1 and HAS-2 mRNA. The main mediator of these paracrine effects present in ADSC-CM was elucidated by introducing TGF-β1 and TGF-β1 antibody. TGF-β1 is assumed to be a potent mediator that modulates paracrine effects of ADSC-CM. However, to clarify the mechanisms of synergistic effects and the direct role of TGF-β1 within ADSC-CM, signal transduction of TGF-β1 and other cytokines present in ADSC-CM which are involved in ECM synthesis should be studied in further wound healing research. Moreover, myofibroblastic differentiation and phenotypic change in fibroblasts which is induced by ADSC-CM treatment is currently under investigation in our research group.
In conclusion, we confirmed the paracrine effects of ADSC-CM on HDFs which activate collagen synthesis and HA accumulation. The production of collagen and HA is known to be mediated by several cytokines; however TGF-β1 was implied to play a pivotal role in increasing mRNA expression and to impact further HA and collagen expression profile. Moreover, our study suggests the usefulness of ADSC-CM as a wound healing agent.
References
- Akmal M, Singh A, Anand A, Kesani A, Aslam N, Goodship A. The effects of hyaluronic acid on articular chondrocytes. J Bone Joint Surg Br. 2005;87:1143–1149. doi: 10.1302/0301-620X.87B8.15083. [DOI] [PubMed] [Google Scholar]
- Allemann F, Mizuno S, Eid K, Yates KE, Zaleske D, Glowacki J. Effects of hyaluronan on engineered articular cartilage extracellular matrix gene expression in 3-dimensional collagen scaffolds. J Biomed Mater Res. 2001;55:13–19. doi: 10.1002/1097-4636(200104)55:1<13::AID-JBM20>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, Campo S, Angela D, Ferlazzo AM, Calatroni A. TNF-alpha, IFN-gamma, and IL-1beta modulate hyaluronan synthase expression in human skin fibroblasts: synergistic effect by concomital treatment with FeSO4 plus ascorbate. Mol Cell Biochem. 2006;292:169–178. doi: 10.1007/s11010-006-9230-7. [DOI] [PubMed] [Google Scholar]
- David-Raoudi M, Tranchepain F, Deschrevel B, Vincent JC, Bogdanowicz P, Boumediene K, Pujol JP. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen. 2008;16:274–287. doi: 10.1111/j.1524-475X.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Guo N, Li X, Mann MM, Funderburgh LM, Du Y, Funderburgh LJ. Hyaluronan synthesis mediates the fibrotic response of keratocytes to transforming growth factor β. JBC. 2010;285:32012–32019. doi: 10.1074/jbc.M110.127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Kim H, Kawazoe T, Han DW, Matsumara K, Suzuki S, Tsutsumi S, Hyon SH. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen. 2008;16:714–720. doi: 10.1111/j.1524-475X.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- Kim WS, Park BS, Park SH, Kim HK, Sung JH. Antiwrinkle effect of adipose-derived stem cell: activation of dermal fibroblast by secretory factors. J Dermatol Sci. 2009;53:96–102. doi: 10.1016/j.jdermsci.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Kratchmarova I, Kalume DE, Blagoev B, Scherer PE, Podtelejnikov AV, Molina H. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics. 2002;1:213–222. doi: 10.1074/mcp.M200006-MCP200. [DOI] [PubMed] [Google Scholar]
- Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plastic Reconstr Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Mizuno K, Matsuzawa Y, Matsubara K. Analysis of an expression profile of genes in the human adipose tissue. Gene. 1997;190:227–235. doi: 10.1016/S0378-1119(96)00730-5. [DOI] [PubMed] [Google Scholar]
- Meran S, Thoms D, Stephens P, Martin J, Bowen T, Phillips A, Steadman R. Involvement of hyaluronan in regulation of fibroblast phenotype. J Biol Chem. 2007;282:25687–25697. doi: 10.1074/jbc.M700773200. [DOI] [PubMed] [Google Scholar]
- Meran S, Thomas DW, Stephens P, Enoch S, Martin J, Steadman R, Phillips AO. Hyaluronan facilitates transforming growth factor-beta1-mediated fibroblast proliferation. J Biol Chem. 2008;283:6530–6545. doi: 10.1074/jbc.M704819200. [DOI] [PubMed] [Google Scholar]
- Park BS, Jang KA, Sung JH, Park JS, Kwon YH, Kim KJ, Kim WS. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg. 2008;34:1323–1326. doi: 10.1111/j.1524-4725.2008.34283.x. [DOI] [PubMed] [Google Scholar]
- Park BS, Kim WS, Choi JS, Kim HK, Won JH, Ohkubo F, Fukuoka H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: evidence of increased growth factor secretion. Biomed Res. 2010;31:27–34. doi: 10.2220/biomedres.31.27. [DOI] [PubMed] [Google Scholar]
- Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- Puolakkainen PA, Twardzik DR, Ranchalis JE, Pankey SC, Reed MJ, Gombotz WR. The enhancement in wound healing by transforming growth factor-beta 1 (TGF-beta 1) depends on the topical delivery system. J Surg Res. 1995;58:321–329. doi: 10.1006/jsre.1995.1050. [DOI] [PubMed] [Google Scholar]
- Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- Tammi R, Pasonen-Seppänen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 2005;124:898–905. doi: 10.1111/j.0022-202X.2005.23697.x. [DOI] [PubMed] [Google Scholar]
- Turino GM, Canor JO. Hyaluronan in respirator injury and repair. Am J Respir Crit Care Med. 2003;167:1169–1175. doi: 10.1164/rccm.200205-449PP. [DOI] [PubMed] [Google Scholar]
- Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 2009;175:148–160. doi: 10.2353/ajpath.2009.080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Rayan V, Sumner DR, Thonar EJ. The use of intra-articular Na-hyaluronate as a potential chondroprotective device in experimentally-induced acute articular cartilage injury and repair in rabbits. J Orthop Res. 2003;21:305–311. doi: 10.1016/S0736-0266(02)00156-0. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z. Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]