Abstract
The feasibility of using genipin cross-linked type II collagen scaffold with rabbit bone marrow mesenchymal stem cells (RBMSCs) to repair cartilage defect was herein studied. Induction of RBMSCs into chondrocytic phenotype on type II collagen scaffold in vitro was conducted using TGF-β 3 containing medium. After 3-weeks of induction, chondrocytic behavior, including marker genes expression and specific extracellular matrix (ECM) secretion, was observed. In the in vivo evaluation experiment, the scaffolds containing RBMSCs without prior induction were autologous implanted into the articular cartilage defects made by subchondral drilling. The repairing ability was evaluated. After 2 months, chondrocyte-like cells with lacuna structure and corresponding ECM were found in the repaired sites without apparent inflammation. After 24 weeks, we could easily find cartilage structure the same with normal cartilage in the repair site. In conclusion, it was shown that the scaffolds in combination of in vivo conditions can induce RBMSCs into chondrocytes in repaired area and would be a possible method for articular cartilage repair in clinic and cartilage tissue engineering.
Keywords: Cartilage defect, Type II collagen scaffold, Rabbit bone marrow mesenchymal stem cells, Chondrocyte-like cells, In vivo
Introduction
Articular cartilage damages can be caused by different types of diseases and injuries, being osteoarthritis one of the most common causes (Mandelbaum et al. 1998; Yan and Yu 2007; Fritz et al. 2008). In some cases, this condition might cause severe walking dissability that can be very limiting for a patient. Due to the limited ability for healing of the articular cartilage, the treatments for defects in the articular cartilage are complicated to manage. Traditional approaches of therapy include physical therapy, joint debridement, and autologus chondrocyte or cartilage transplantation (Fritz et al. 2008; Peterson et al. 2002). Nevertheless, a common drawback of the repaired cartilage is the appearance of fibrocartilage (Steinwachs et al. 2008), instead of hyaline-cartilage (Rodrigo et al. 1994; Steadman et al. 2003). Tissue engineering approach using appropriate scaffolds might be able to induce a correct type of cartilage from different cell sources such us bone marrow-derived stem cells (Caplan and Goldberg 1999; Caplan 2000; Shao et al. 2006; Lin et al. 2006; Haasper et al. 2008; Raghunath et al. 2005). A three dimensional scaffolds can be used to expand the number of chondrocytes without dedifferentiation. An excellent scaffold for cartilage tissue engineering should be biocompatible, biodegradable, with a proper degradation and adsorption rate for tissue replacement. So far, a number of scaffolds made of biological or synthetic materials were used in combination with chondrocytes or mesenchymal/progenitor cells in osteochondral defect repair experiments (Filová et al. 2008; Lind et al. 2008; Løken et al. 2008; Swieszkowski et al. 2007). Type II collagen is the major extracellular matrix (ECM) component of normal cartilage, its use as scaffold should have some advantages. First, the scaffold would mimic the natural cartilage micro-environment, and the RGD amino sequences in collagen might facilitate cell-attachment, proliferation and maintain cellular activity (Blewitt and Willits 2007). It was noted that type II collagen scaffold as compared to alginate or type I collagen scaffold exhibited the best chondrocytic gene expression (Blewitt and Willits 2007; Bosnakovski et al. 2006). This study used type II collagen scaffold previously developed for our cartilage repairing experiments (Ko et al. 2009). Another main concern in tissue engineered cartilage was the cell source (Yan and Yu 2007; Shao et al. 2006). Many reports indicated that using autologous chondrocyte transplantation (ACT) or osteochondral transplantaion (OCT) to repair injured cartilage might have some problems like donor site morbidity or fibrocartilage-like tissue just because the isolated chondrocytes were not able to proliferate in vitro (Peterson et al. 2002; Haasper et al. 2008). Therefore, cell source for cartilage tissue engineering is a very important factor. Chondrocytes are easy to harvest, but it had been found that the cells needed a long time of culturing to increase to a sufficient number before implantation (Raghunath et al. 2005). On the other hand, stem cells could be expanded and be able to differentiate into many types of tissues or organs (Yao et al. 2004; Scapinelli et al. 2002). Adult stem cells had been applied to therapy in hemoglobinopathies and other blood-related disorders, cancers and immunodeficiencies in recent years (Bloom 1999). Wakitani found that mesenchymal stem cells (MSCs) differentiated to chondrocytes 2 weeks after tranplantation, and 6 months after tranplantation the subchondral bone was completely repaired (Wakitani et al. 1994). Therefore, it would be possible that the use of stem cells might overcome the problem of scarcity of suitable chondrocytes. And when used in combination of proper scaffolds and proper administration of growth factors, stem cells could have a great impact in cartilage repair. Current medical practice prefered minimal manipulation of MSCs and in vitro induction of MSCs toward chondrocytes had to be avoided if possible. If there were more manipulations of stem cells, the cells would be regarded as a certain kind of drug and would not be conducive to clinical surgery. Therefore, in this study, adult stem cells obtained from bone marrow without any lengthy in vitro treatment were used on type II collagen scaffold for direct implantation. In the animal experiment the osteochondral defect repair potential was evaluated in rabbits with autologous rabbit bone marrow stem cells.
Materials and methods
Type II collagen extraction
Native type II collagen was extracted from bovine trachea and scaffold fabrication was made as previously described (Ko et al. 2009). Briefly, small pieces cut from bovine trachea incubated with 50 mM Tris–HCl (Sigma, St. Louis, MO), containing 25 mM EDTA-Na2 (Sigma), and 2 mM N-ethylmaleimide (Sigma). The collagen was digested by incubation in 0.5 M acetic acid (HAc) (Sigma) containing 8 × 106 units pepsin/L (Sigma) for 16 h. Crude type II collagen was harvested from the digested collagen by salting out with sodium chloride (Sigma). The atelocollagen pellet was re-dissolved in 0.5 M HAc three times. The atelocollagen was removed by dialysis against 0.02 M phosphate buffer (pH = 7.4). After centrifugation (20,000g, 20 min), the collagen was washed in distilled water twice, and centrifuged again (20,000g, 20 min). Then, collagen was frozen at −20 °C and lyophilized.
Type II collagen scaffold preparation
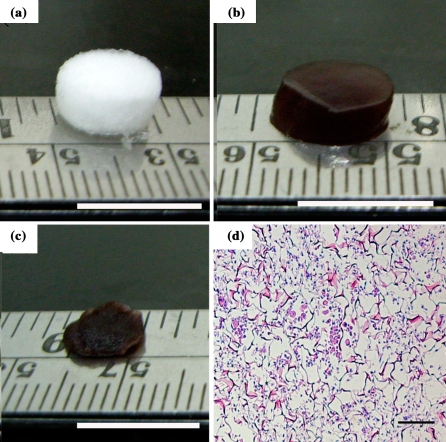
For experiments, collagen was dissolved in 0.5 M CH3COOH (Sigma) to make a 1% (w/v) solution. The solution was dissolved and then poured into 8 mm diameter plastic mold at a depth of approximately 5 mm. The solution was placed in a −20 °C freezer for 24 h, and then lyophilized at −60 °C for 24 h, to result in porous scaffolds, as shown in Fig. 1a. Each fabricated scaffold (about 3 mg) was immersed for 48 h in 4.4 mM genipin (Wako Pure Chemical Industries, Osaka, Japan), for cross-linking getting a deep blue type II collagen scaffold, as shown in Fig. 1b.
Fig. 1.
Collagen type II scaffold mophorology white color before crosslinking (a); deep blue color after crosslinking with genipin (b); the excluded water scaffold showed a much smaller volume than water-containing one (c); RBMSCs cultured on collagen type II scaffold with fresh medium (d). a, b and cscale bar: 1 cm. dscale bar: 100 μm
Rabbit bone marrow stem cells (RBMSCs) preparation
Six two-month-old New Zealand White rabbits weighing three kilograms on average were used in this study. The RBMSCs were obtained as described previously (Wakitani et al. 1994). Briefly, the rabbits were anesthetized by intramuscular injection of Zoletil 50 10–15 mg/kg (Virbac Laboratoires, Carros, France), desinfected and shaved for surgery. Two ml of marrow was aspirated using an 18-gauge needle, mixed with 0.1 ml of heparin (3000 units per ml) (Sigma) in a 15-ml centrifugation tube and then washed with PBS twice. The pellet was suspended with fresh medium containing α-MEM (Invitrogen, Carlsbad, CA), 20% fetal bovine serum (FBS) (Invitrogen), 4 ng/ml FGF-2 (R&D Systems, Minneapolis), 1% antibiotics (Invitrogen), cultured in a T75 flask. The medium was changed every 5 days, and cells were harvested after 14 days. The harvested cells were used as autologous RBMSCs for transplanting back to the original rabbits after the cartilage defect was created. The detailed transplantation protocol is described in the “In vivo Evaluation” section.
In vitro evaluation
The cells were harvested by trypsin (Invitrogen) for the experiments and resuspended in fresh medium. Cell suspension (2 × 106 cells/100 μl) was injected inside the collagen type II scaffold. Scaffolds were incubated at 37 °C in an atmosphere of 5% CO2 and 95% humidity. Cells were completely attached to the scaffold after 1 h of incubation, and then 0.5 ml fresh medium was added. After 1 h, when the cells were completely attached to the scaffold, 0.5 ml fresh medium was added. After 3-days of culture, medium was changed to the serum-free induction medium for chondrocyte differentiation. The serum free-medium based on DMEM was supplemented with ITS+1 Premix (BD, Franklin Lakes, NJ), and bovine serum albumin (1.25 mg/ml)). Pyruvate (1 mM) (Sigma), ascorbate 2-phosphate (37.5 mg/ml) (Sigma), dexamethasone (10−7 M) (Sigma) and TGF-β3 (10 ng/ml, recombinant, human) (R&D Systems) were also added. Medium changes were made at 2- to 3-day intervals and scaffolds were examined at time points up to 21 days. Cell morphology and marker genes expression were examined.
In vivo evaluation
Experimental rabbits were maintained and used in accordance with the animal regulations of the ethics committee of Nation Tsing Hua University, Taiwan. The research protocol of this experiment was reviewed and approved by the ethics committee of the university and the rabbits were cared following the international rules.
Twelve two-month-old New Zealand White rabbits weighing three kilograms on average were used in the study. The data collected in this study were based on sixplicate tests (six rabbits, n=6). RBMSCs harvest protocol was the same as in the previous section. Cartilage defects of rabbits were made by the following steps. Briefly, the patella was dislocated laterally, and the patellar groove was exposed. The articular cartilage defect (diameter about 4.5 and 3 mm depth) was created through the articular cartilage and into the subchondral bone of patellar groove of the knee with a 4.5 mm diameter dermal punch. Before transplanting to the defect, the scaffold with cells was washed twice with PBS for avoiding FBS effects. After removing the PBS from scaffolds, the sacffolds were used for transplantation (Fig. 1c). In the left knee, the defect was filled with the scaffold seeded with the RBMSCs (2 × 106 cells each scaffold) (Cell-Scaffold, CS). The cell distribution was evaluated by H&E stain before transplanted into defects (Fig. 1d). In the other (right) knee, the defect was filled with an empty scaffold without cells using the same protocols (Empty Cell-Scaffold, ECS). After surgery, all rabbits were allowed unrestricted cage activity without immobilization until sacrificed 8 and 24 weeks later.
Real-Time PCR for gene expression measurement
The cultured cells in the scaffold with chondrogenic medium were analyzed by real-time PCR to investigate temporal mRNA expression changes of collagen type II, and aggrecan. Total RNA was extracted from the cultured cells inside the scaffolds. The scaffolds were minced by surgical blade and incubated with TRIZOL reagent (Life Technologies). Subsequent steps were followed as of manufacturer’s instruction. Total RNA of each sample was measured by a spectrophotometer (Beckman, Fullerton, CA) and then was reverse transcribed with oligo (dT) primer using MMLV reverse transcriptase by First strand synthesis kit (Fermentas, Glen Burnie, Maryland) for cDNA synthesis. The resultant solution (2.0 μL) was amplified in triplicate by real-time PCR (Applied Biosystems, Foster, CA) with SyBr Green Master Mix reagent (Applied Biosystems). Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as internal control. The primers for the rabbit specific genes were designed according to the published sequences available in GenBank using Primer Express (Applied Biosystems) as shown in Table 1. Genes related to ECM components were selected, namely, collagen type II and aggrecan. The comparative Ct method was used for gene expression quantification.
Table 1.
Sequences of primers used in real-time PCR
Histology and immuno-histology staining
For in vitro experiments, RBMSCs-laden scaffold samples were washed in PBS, fixed in 10% neutral buffered formalin, and embedded in paraffin. For in vivo experiments, after gross examination, the harvested samples were fixed in 10% formalin (Sigma), decalcified in 30% formic acid (Sigma), and then embedded in paraffin (Sigma). Then sections of 5 μm were cut and collected on slides. Slides were stained with hematoxylin and eosin (H&E) (Sigma) for histological evaluation according to manufacturing protocol (Ko et al. 2009). Alcian blue staining (Sigma) was used for glycosaminoglycans (GAGs) according to manufacturing protocol (Ko et al. 2009). For immunohistochemistry staining, the slide firstly was blocked with normal goat serum for 45 min. Mouse anti-human monoclonal primary antibodies of collagen II diluted 1:100 (Chemicon, CA) and aggrecan diluted 1:100 (Chemicon), were applied at 4 °C for 24 h, followed by incubation with HRP conjugated anti-mouse secondary antibody diluted 1:250. Diaminobenzidine (DAB) (Sigma) was used as the substrate, which presents the brown color of the immuno-positive cells. Counterstaining was done with hematoxylin. The slides were dehydrated before being coverslipped.
Statistical analysis
The data of gene expression results are expressed as means ± standard deviation (S.D.) by over three experiments for each test. The control and experimental group of in vitro study were compared with each other by t test. The control and experimental group of in vivo evaluation were compared with each other and unoperated tissue.
Results
In vitro study
In order to check the cell distribution, we used the H&E staining to identify the location of the cells within the scaffolds before transplantation. Our results showed that RBMSC were distributed homogenously in the scaffolds before transplantation and kept their fibroblast-like shape without extracellular matrix secretion (Fig. 1d).
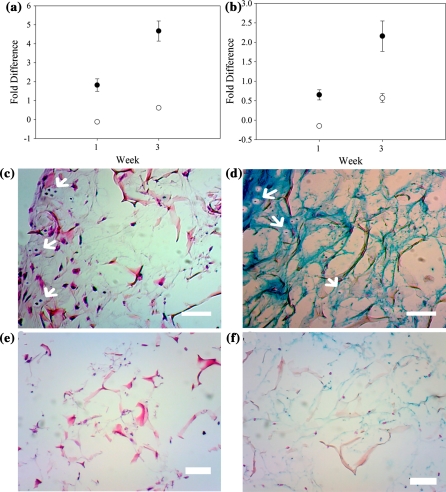
In order to evaluate the differentiation potential of RBMSCs, the histology staining was conducted and the gene expression was evaluated by Real-Time PCR. According to previously study, MSCs could be induced into chondrocytes by pellet culture using growth factors of the TGF-β family (Im et al. 2006; Johnstone et al. 1998). To induce RBMSCs to undergo chondrogenesis, TGF-β3 was used for 3 weeks in this study. The gene expression data of type II collagen and aggrecan are presented in Fig. 2a and b. The results showed that the chondrogenesis gene expression of type II collagen and aggrecan of cells with TGF-β3 were significantly higher than cells without TGF-β3 at 1-week induction. After 3-week induction, our results showed that RBMSC culture with and without TGF-β3 could be induced to chondrocyte differentiation paralleled by up-regulation of aggrecan and type II collagen gene expressions (Fig. 2a and b), although these genes were expressed obviously in the group with TGF-β3. The H&E and alcian blue staining results also confirmed the point that RBMSCs had differentiated into the round-shaped chondrocyte-like cells with lacuna structures (as arrows indicate), and secretion of GAGs in their ECM (Fig. 2c and d). The groups of the RBMSCs cultured in the scaffold without TGF-β3 showed lower cellular proliferation and less amount of GAG secretion than the groups with TGF-β3.
Fig. 2.
Quantitative analysis of type II collagen, aggrecan at 1 week and 3 weeks; type II collagen (a) and aggrecan (b) of RBMSCs induction with or without TGF-β3 (filled and open circles represent RBMSCs after induction by medium with or without TGF-β3, respectively); H&E staining (c) and alcian blue stain (d) of RBMSCs induction with TGF-β3; H&E staining (e) and alcian blue stain (f) of RBMSCs induction without TGF-β3. The arrows point out the chondrocyte cells with lacuna. Scale bar: 100 μm
In vivo study
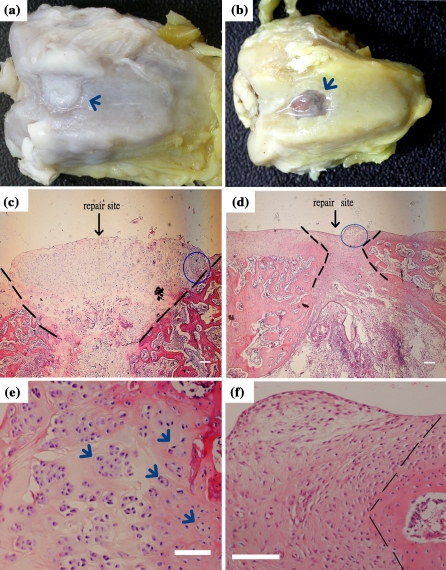
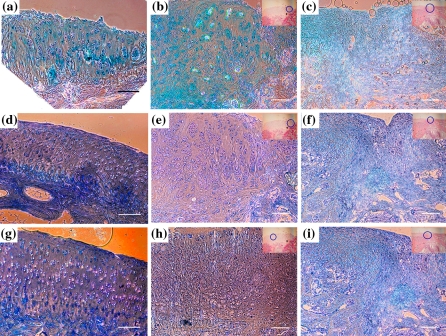
Cartilage defect repairing potential of our method was studied in a rabbit model. The scaffolds with RBMSCs (CS) or without RBMSCs (ECS) were used for comparison. We had chosen to use the minimally manipulated RBMSCs as our cell source. If the RBMSCs after culture on our scaffolds could differentiate into functional chondrocytes and repair the cartilage defect in the in vivo microenvironment without prior induction, it would be more convenient for the clinical application of this technique. Based on this reason, RBMSCs prepared from rabbits without any processing (including prior induction or labelling with the fluorescent dye) were cultured on collagen type II scaffold directly for autologous transplantation. Eight weeks after transplantation, the rabbits were sacrificed and the cartilage was examined. Grossly to say, the defects were completely filled by the repair tissue 8 weeks after transplantation (Fig. 3). The repair sites of the ECS showed blue color (Fig. 3a), indicating the ginipin-crooslinked scaffolds were still present, while those of the CS showed only the white color of cartilage-like tissue (Fig. 3b). A closer examination of histology of CS or ECS is shown in Fig. 3. The most important findings were that the repair site of CS showed a considerable number of chondrocyte-like cells with lacuna structures (Fig. 3c and e). However, the repair site of the ECS showed only cells without chondrocytes morphology (Fig. 3d and f). Besides, cell clusters of typical cartilage characteristics were observed in Fig. 3e, as indicated by the arrows. These observations indicated that the RBMSCs cultured on the scaffolds helped to form chondrocyte-like cells and regenerated cartilage-like tissue in the repair sites, while ECS by themselves failed to form cartilage tissue in the repair sites. Further comparisons of the repair sites of both scaffolds with natural cartilage are shown in Fig. 4. Put into evidence by alcian blue staining, as shown in Fig. 4a, b and c, both types of scaffolds resulted in GAGs secretion in the repair sites, though the CS seemed to induce more GAGs formation surrounding the cells. In immunostainings for type II collagen and aggrecan, positive results were shown for the case of CS (Fig. 4e, h). This result is comparable with the features found in normal cartilage, as in Fig. 4d, g. The repair sites of ECS did not show much type II collagen, as in Fig. 4f, and only a weak staining for aggrecans, as in Fig. 4i. In contrast to the scaffold without RBMSCs (Fig. 4f and i), large amounts of aggrecan and type II collagen were secreted by the induced chondrocytes spread all over the repair sizes. Our results also indicated that the RBMSCs in the scaffold helped to form cartilage tissue in vivo, without prior induction to chondrocyte differentiation before implantation. This finding indicated that the RBMSCs could be induced into functional chondrocytes within the scaffolds under physiological environment at the defect site.
Fig. 3.
Gross morphology and histology examination of H&E stain of defects after 8 weeks. CS showed only the white color of cartilage-like tissue (a) and in the contrast, those of the ECS showed blue color (b). H&E stain: the stain shows the full vision of repair site CS (c) and ECS (d), the figs (e) and (f) were zoomed in from the area by gating of circle in figs (c) and (d), repectively (the arrows indicated the cell location). Scale bar: 100 μm
Fig. 4.
Alcian blue stain, type II collagen and aggrecan secretion in the cartilage defect after 8 weeks of scaffold transplantation. Alcian blue stain: normal cartilage (a), CS (b) and ECS (c). Type II collagen: normal cartilage (d), CS (e) and ECS (f). Aggrecan: normal cartilage (g), CS (h) and ECS (i). Figs (b), (c), (e), (f), (h) and (i) were zoomed in from the area by gating of circle in the upper right side of figures. Scale bar: 100 μm
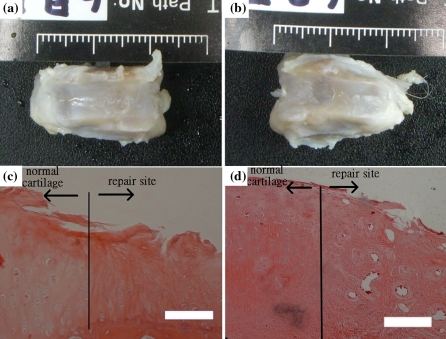
Twenty-four weeks after transplantation, the repair sites of the ECS showed white color of tissue, like the CS, indicating ginipin-crooslinked scaffolds had disappeared completely (Fig. 5a and b). After 24 weeks of transplantation, the histology of CS group showed that repaired cells with typical cartilage characteristics and high ECM secretion (dense red color) compared to normal group (Fig. 5c), for which the ECS group showed less cell growth. These observations also indicated the potential usefulness of direct stem cell implantation within type II collagen scaffold as treatment for cartilage repair.
Fig. 5.
Gross morphology and histology examination of H&E stain of defects after 24 weeks. The CS showed the white color of cartilage-like tissue (a). The ECS showed white color (b). H&E stain: CS (c) and ECS (d). Scale bar: 100 μm
Discussions
In vitro study
Several studies have been performed on scaffold-guided chondrogenesis with growth factors using stem cell–derived chondrogenic cells. Caterson et al. characterized the chondrogenic potential of human marrow stroma-derived cells in both a PLA/alginate amalgam and pure PLA scaffold with or without transforming growth factor beta (TGF-β) treatment (Caterson et al. 2001). The results showed that cells could differentiate to chondrocyte-like cells in the presence of TGF-β in the medium. Williams et al. studied MSCs from goats that were encapsulated in a photopolymerizing poly-ethylene glycol based hydrogel and cultured with or without TGF-β (Williams et al. 2003). The results showed that the encapsulated MSCs were able to form cartilage-like tissue in vitro with TGF-β. Shen et al. addressed synergistic effects of bone morphogenetic protein-2 (BMP-2) and TGF-β3 in the induction of chondrogenesis of bone marrow MSCs in vitro (Shen et al. 2009). Janjian et al. presented positive results of mold-shaped, nanofiber scaffold combined with TGF-β1 or insulin-like growth factor-I (IGF-1) for chondrogenesis of human MSCs (Janjanin et al. 2008). They showed that their constructs exhibited hyaline cartilage histology with desired thickness and shape as well as favorable tissue integrity and shape retention. Recently, Fan et al. fabricated a novel PLGA-gelatin/chondroitin/hyaluronate (PLGA-GCH) hybrid scaffold with TGF-β1-impregnated microspheres (MS-TGF) for chondrogenesis of MSCs in vivo or in vitro (Fan et al. 2006). Their results indicated that PLGA-GCH/MS-TGF and in vivo microenvironment helped to keep chondral phenotype and enhanced repair. Our results further showed that RBMSCs could be differentiated into chondrocyte-like cells using type II collagen with TGF-β3.
In vivo study
For in vivo cartilage defect repair, RBMSCs without prior treatment of TGF-β3 showed correct differentiation to chondrocyte-like cells, and good repair of the site. These results can be attributed to two factors in the in vivo environment: cell to cell interactions of the implanted cells with the surrounding host cells, and the resultant growth factors secreted from these interactions. Host tissue cells (including synovial fluids and bleeding by drilling to subchondral bone) might penetrate the pores of the scaffold and attach to the collagen fiber. Theoretically these host tissue cells could make contact with the seeded bone marrow stem cells. The cell to cell interactions might promote differentiation of the seeded cells to chondrocyte-like cells. Several studies indicated that cell to cell interactions promoted differention to chondrocyte-like cells. Richardson et al. cultured nucleus pulposus (NP) cells with MSCs using a monolayer coculture model which allows direct cell to cell contact (Richardson et al. 2006). Their findings showed a significant increase of sox9, type II collagen and aggrecan mRNA in MSCs after only 7 days. Li et al. cocultured fat-derived MSCs with nucleus pulposus in vitro (Li et al. 2005). Under the culture system the expression of SRY-related high mobility group-box gene 9, aggrecan and collagen type II increased significantly in both MSCs and nucleus pulposus cells. Furthermore, Chen et al. showed that sheep MSCs cocultured with synovial fluid cells 2 weeks, on poly L-lactic-co-glycolic acid porous scaffold in both co-culture systems (in vitro and in vivo), promoted chondrogenesis (Chen et al. 2005). The synovial fluid is an important agent for chondrogenesis of MSCs. Their findings suggested that growth factor-mediated signaling between the two cell types induced chondrogenic stem cell differentiation. Although we have not identified if any growth factors were involved in the in vivo situation in the defect repair study, the successful differentiation of MSCs to chondrocyte-like cells demonstrated the possibility of appropriate growth factors production in the microenvironment of the repair site.
Genipin was derived from geniposide, which was extracted from the Gardenia jasminoides. It was used in Chinese medicine as an antiphlogistic against inflammation. Its anti-inflammatory characteristics could be useful for tissue engineering. Studies had been reported to be significantly less cytotoxic than glutaraldehyde in vitro or in vivo (Chang et al. 2001; Sung et al. 1999). Recently, Dare et al. fabricated a genipin cross-linked human fibrin hydrogel system as a scaffold for articular cartilage tissue engineering. Their results showed that genipin appeared to inhibit the inflammatory reaction observed 3 weeks after subcutaneous implantation of the fibrin hydrogel into rats (Dare et al. 2009). Our results were in agreement with their reports showing that the repair site did not result in the inflammation during experimental period.
Conclusions
Repairing osteochondral defect using tissue engineering technique is a promising alternative to traditional treatment of cartilage defects. In this study we have demonstrated the feasibility of using RBMSCs without prior induction on type II collagen scaffold to repair the defect in vivo. Eight weeks results showed the in vivo microenvironment plus the scaffold can successfully induce the stem cells toward chondrogenesis and produce good response to cartilage defect repair. Twenty-four weeks histology results ascertained the repair cartilage with good cartilage characteristic during the longer observation period. Our results showed that the scaffolds in combination with in vivo conditions could induce RBMSCs differentiation to chondrocytes in repaired areas and would be a possible method for articular cartilage repair in the clinic and cartilage tissue engineering.
Acknowledgments
This research is supported by the Ministry of Economic Affairs, Taiwan, under the Technology Development Program for Academia grant 91-EC-17-A-17-S1-0009.
Contributor Information
Yu-Hong Wei, Email: yhwei@saturn.yzu.edu.tw.
I-Ming Chu, Phone: +886-3-5713704, FAX: +886-3-5715408, Email: imchu@che.nthu.edu.tw.
References
- Blewitt MJ, Willits RK. The effect of soluble peptide sequences on neurite extension on D collagen substrates and within 3D collagen gels. Ann Biomed Eng. 2007;35:2159–2167. doi: 10.1007/s10439-007-9389-4. [DOI] [PubMed] [Google Scholar]
- Bloom FE (1999) Breakthroughs 1999. Science 286:2267 [DOI] [PubMed]
- Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Tissue engineering designs for the future: new logics, old molecules. Tissue Eng. 2000;6:1–8. doi: 10.1089/107632700320838. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Goldberg VM. Principles of tissue engineered regeneration of skeletal tissues. Clin Orthop Relat Res. 1999;367(Suppl):S12–S16. doi: 10.1097/00003086-199910001-00003. [DOI] [PubMed] [Google Scholar]
- Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, Vaccaro AR, Tuan RS. Three-dimensional cartilage formation by bone marrow-derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;257:394–403. doi: 10.1002/1097-4636(20011205)57:3<394::AID-JBM1182>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Chang Y, Tsai CC, Liang HC, Sung HW. Reconstruction of the right ventricular outflow tract with a bovine jugular vein graft fixed with a naturally occurring crosslinking agent (genipin) in a canine model. J Thorac Cardiovasc Surg. 2001;122:1208–1218. doi: 10.1067/mtc.2001.117624. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang C, Lü S, Wu J, Guo X, Duan C, Dong L, Song Y, Zhang J, Jing D, Wu L, Ding J, Li D. In vivo chondrogenesis of adult bone-marrow-derived autologous mesenchymal stem cells. Cell Tissue Res. 2005;319:429–438. doi: 10.1007/s00441-004-1025-0. [DOI] [PubMed] [Google Scholar]
- Dare EV, Griffith M, Poitras P, Kaupp JA, Waldman SD, Carlsson DJ, Dervin G, Mayoux C, Hincke MT. Genipin Cross-Linked Fibrin Hydrogels for in vitro Human Articular Cartilage Tissue-Engineered Regeneration. Cells Tissues Organs. 2009;190:313–325. doi: 10.1159/000209230. [DOI] [PubMed] [Google Scholar]
- Fan H, Hu Y, Zhang C, Li X, Lv R, Qin L, Zhu R. Cartilage regeneration using mesenchymal stem cells and a PLGA-gelatin/chondroitin/hyaluronate hybrid scaffold. Biomaterials. 2006;27:4573–4580. doi: 10.1016/j.biomaterials.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Filová E, Jelínek F, Handl M, Lytvynets A, Rampichová M, Varga F, Cinátl J, Soukup T, Trč T, Amler E. Novel composite hyaluronan/type I collagen/fibrin scaffold enhances repair of osteochondral defect in rabbit knee. J Biomed Mate Res B Appl Biomater. 2008;87:415–424. doi: 10.1002/jbm.b.31119. [DOI] [PubMed] [Google Scholar]
- Fritz J, Janssen P, Gaissmaier C, Schewe B, Weise K. Articular cartilage defects in the knee–basics, therapies and results. Injury. 2008;39(Suppl 1):S50–S57. doi: 10.1016/j.injury.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Haasper C, Zeichen J, Meister R, Krettek C, Jagodzinski M. Tissue engineering of osteochondral constructs in vitro using bioreactors. Injury. 2008;39(Suppl 1):S66–S76. doi: 10.1016/j.injury.2008.01.037. [DOI] [PubMed] [Google Scholar]
- Im GI, Jung NH, Tae SK. Chondrogenic differentiation of mesenchymal stem cells isolated from patients in late adulthood: the optimal conditions of growth factors. Tissue Eng. 2006;12:527–536. doi: 10.1089/ten.2006.12.527. [DOI] [PubMed] [Google Scholar]
- Janjanin S, Li WJ, Morgan MT, Shanti RM, Tuan RS. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J Surg Res. 2008;149:47–56. doi: 10.1016/j.jss.2007.12.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- Ko CS, Huang JP, Huang CW, Chu IM. Type II collagen-chondroitin sulfate-hyaluronan scaffold cross-linked by genipin for cartilage tissue engineering. J Biosci Bioeng. 2009;107:177–182. doi: 10.1016/j.jbiosc.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Li X, Lee JP, Balian G, Greg AD. Modulation of chondrocytic properties of fat-derived mesenchymal cells in co-cultures with nucleus pulposus. Connect Tissue Res. 2005;46:75–82. doi: 10.1080/03008200590954104. [DOI] [PubMed] [Google Scholar]
- Lin Z, Willers C, Xu J, Zheng MH. The chondrocyte: biology and clinical application. Tissue Eng. 2006;12:1971–1984. doi: 10.1089/ten.2006.12.1971. [DOI] [PubMed] [Google Scholar]
- Lind M, Larsen A, Clausen C, Osther K, Everland H. Cartilage repair with chondrocytes in fibrin hydrogel and MPEG polylactide scaffold: an in vivo study in goats. Knee Surg Sports Traumatol Arthrosc. 2008;16:690–698. doi: 10.1007/s00167-008-0522-1. [DOI] [PubMed] [Google Scholar]
- Løken S, Jakobsen RB, Arøen A, Heir S, Shahdadfar A, Brinchmann JE, Engebretsen L, Reinholt FP. Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2008;16:896–903. doi: 10.1007/s00167-008-0566-2. [DOI] [PubMed] [Google Scholar]
- Mandelbaum BR, Browne JE, Freddie F, et al. Articular cartilage lesions of the knee. Am J Sports Med. 1998;26:853–861. doi: 10.1177/03635465980260062201. [DOI] [PubMed] [Google Scholar]
- Peterson L, Brittberg M, Kiviranta I, et al. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- Raghunath J, Salacinski HJ, Sales KM, Butler PE, Seifalian AM. Advancing cartilage tissue engineering: the application of stem cell technology. Curr Opin Biotechnol. 2005;16:503–509. doi: 10.1016/j.copbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Richardson SM, Walker RV, Parker S, Rhodes NP, Hunt AJ, Freemont AJ, Hoyland AJ. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- Rodrigo JJ, Steadman JR, Silliman JF, et al. Improvement in full thickness chondral defect healing in the human knee after debridement and microfracture using continuous passive motion. Am J Knee Surg. 1994;7:109–116. [Google Scholar]
- Scapinelli R, Aglietti P, Baldovin M, Giron F, Teitge R. Biologic resurfacing of the patella: current status. Clin Sports Med. 2002;21:547–573. doi: 10.1016/S0278-5919(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Shao X, Goh JC, Hutmacher DW, Lee EH, Zigang G. Repair of large articular osteochondral defects using hybrid scaffolds and bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006;12:1539–1551. doi: 10.1089/ten.2006.12.1539. [DOI] [PubMed] [Google Scholar]
- Shen B, Wei A, Tao H, Diwan AD, Ma DD. BMP-2 Enhances TGF-beta3-mediated chondrogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in alginate bead culture. Tissue Eng Part A. 2009;15:1311–1320. doi: 10.1089/ten.tea.2008.0132. [DOI] [PubMed] [Google Scholar]
- Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation and outcomes. Am J Knee Surg. 2003;15:170–176. [PubMed] [Google Scholar]
- Steinwachs MR, Guggi T, Kreuz PC. Marrow stimulation techniques. Injury. 2008;39(Suppl 1):S26–S31. doi: 10.1016/j.injury.2008.01.042. [DOI] [PubMed] [Google Scholar]
- Sung HW, Huang RN, Huang LL, Tsai CC. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J Biomater Sci Polym Ed. 1999;10:63–78. doi: 10.1163/156856299X00289. [DOI] [PubMed] [Google Scholar]
- Swieszkowski W, Tuan BH, Kurzydlowski KJ, Hutmacher DW. Repair and regeneration of osteochondral defects in the articular joints. Biomol Eng. 2007;24:489–495. doi: 10.1016/j.bioeng.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- Williams CG, Kim TK, Taboas A, Malik A, Manson P, Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178–187. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Yao CL, Chu IM, Hsieh TB, Hwang SM. A systematic strategy to optimize ex vivo expansion medium for human hematopoietic stem cells derived from umbilical cord blood mononuclear cells. Exp Hematol. 2004;32:720–727. doi: 10.1016/j.exphem.2004.05.021. [DOI] [PubMed] [Google Scholar]