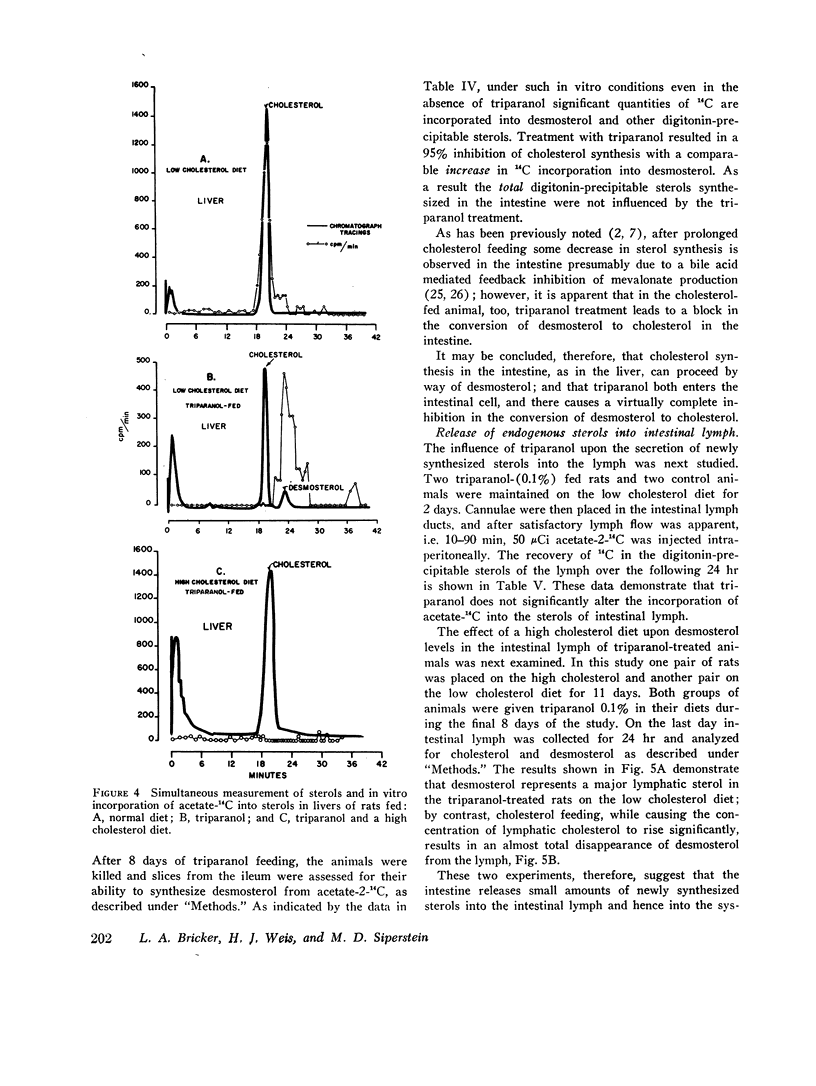
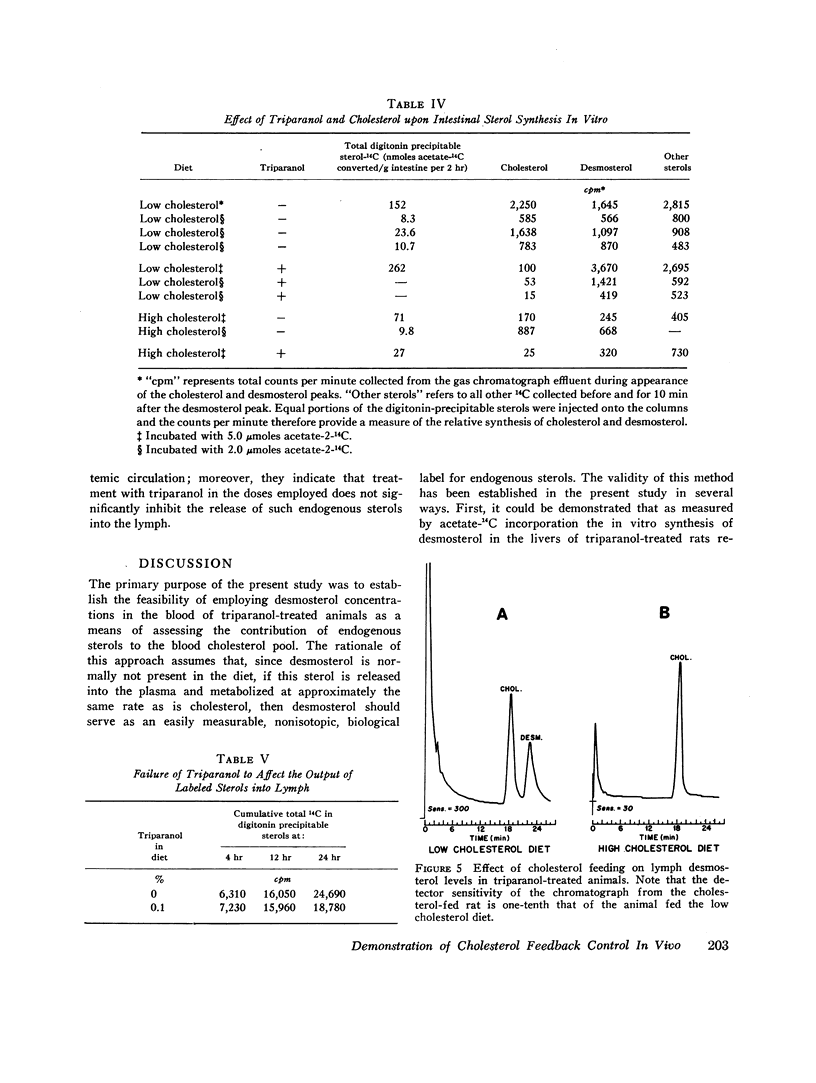
Abstract
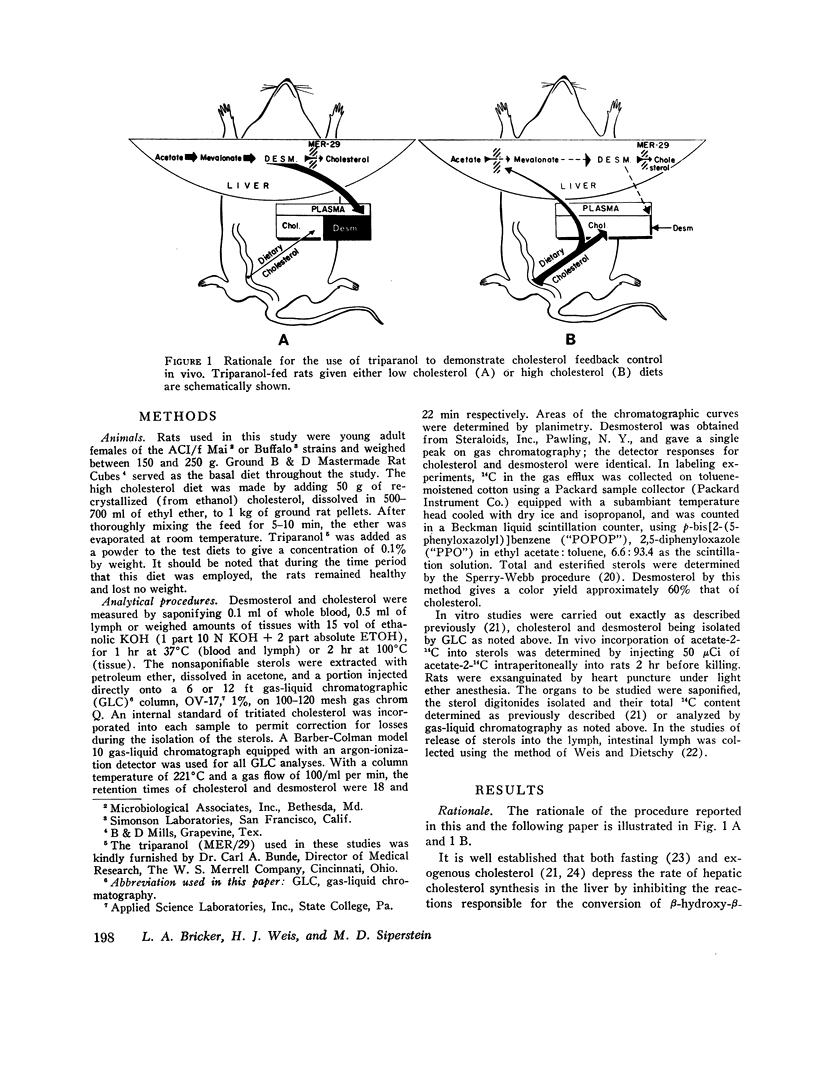
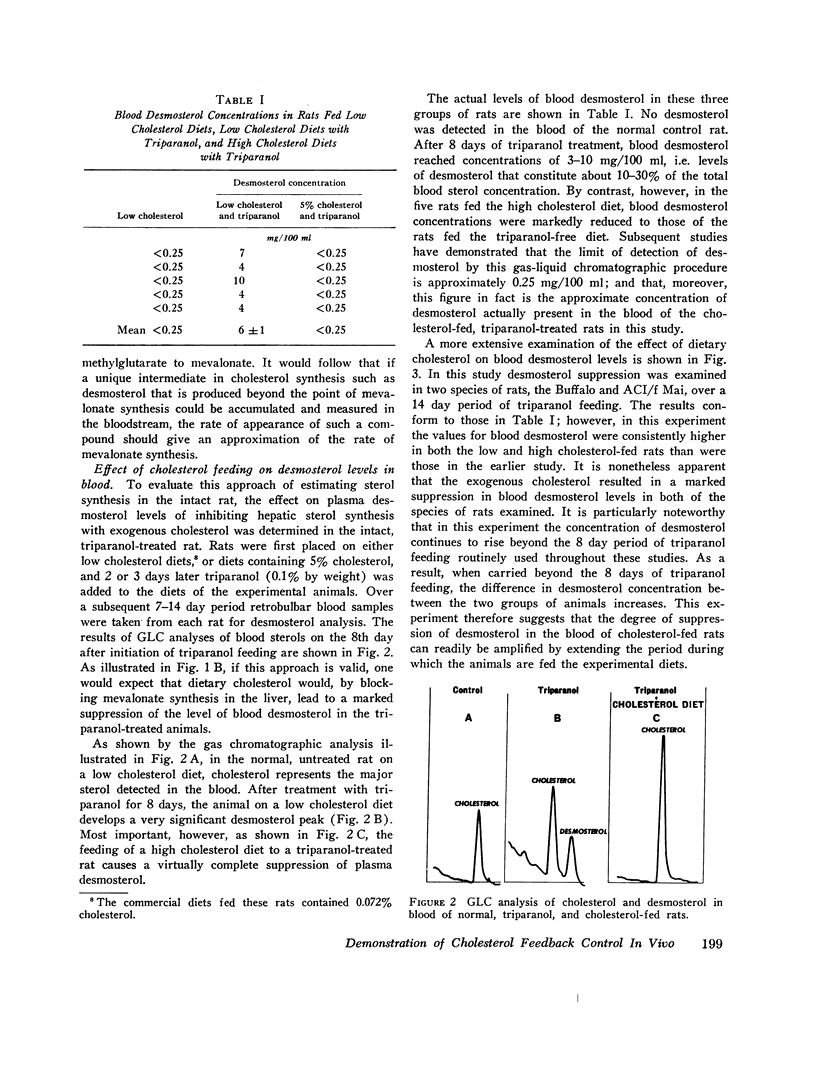
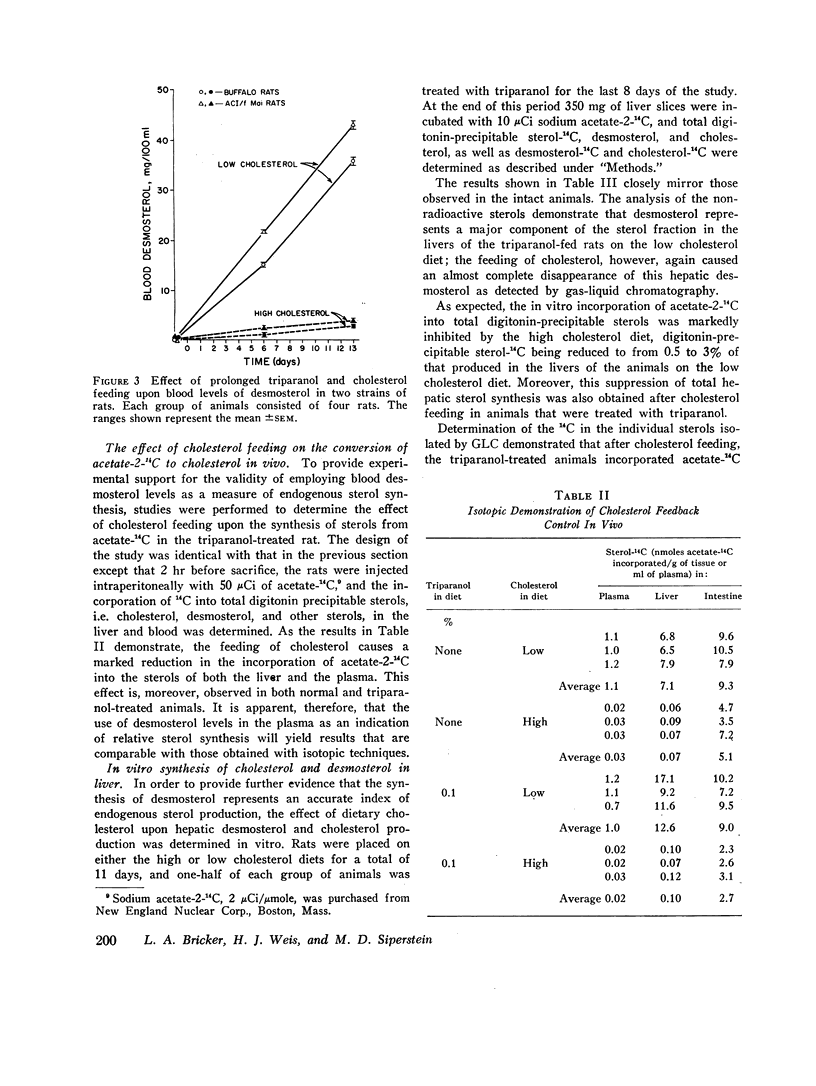
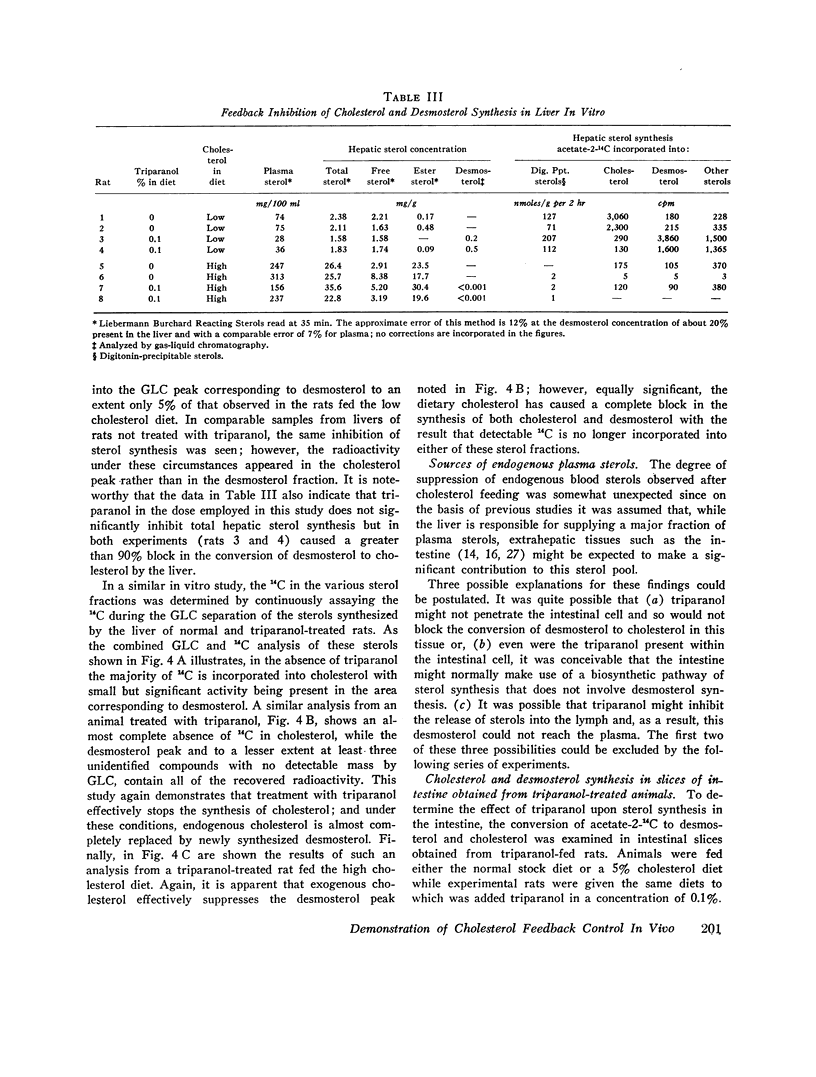
This report describes a “desmosterol suppression” technique with which it has been possible to demostrate the operation of the cholesterol negative feedback system in the intact animal. 0.1% triparanol in the diet causes a virtually complete block in the conversion of desmosterol to cholesterol by liver and intestine. Since desmosterol is not consumed in the diet, the level of plasma desmosterol can be employed as an index of endogenous sterol production and release into the bloodstream. With this technique it was shown that the feeding of cholesterol for 8 days to rats decreases blood desmosterol levels to less than 5% of control values. Very similar results were obtained when cholesterol synthesis was assayed in vivo with acetate-14C as a cholesterol precursor. These observations indicate that the cholesterol feedback system operates very effectively in the intact animal in suppressing the endogenous contribution to the circulating cholesterol pool. Since intestinal cholesterol synthesis is only slightly inhibited by exogenous cholesterol, these results also indicate that the intestine does not represent a significant source of plasma sterols in the rat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAN J., STEINBERG D., THOMPSON M. J., MOSETTIG E. Mechanism of action of MER-29, an inhibitor of cholesterol biosynthesis. Biochem Biophys Res Commun. 1960 Jan;2:63–65. doi: 10.1016/0006-291x(60)90266-7. [DOI] [PubMed] [Google Scholar]
- AVIGAN J., STEINBERG D., VROMAN H. E., THOMPSON M. J., MOSETTIG E. Studies of cholesterol biosynthesis. I. The identification of desmosterol in serum and tissues of animals and man treated with MER-29. J Biol Chem. 1960 Nov;235:3123–3126. [PubMed] [Google Scholar]
- BHATTATHIRY E. P., SHIPERSTEIN M. D. FEEDBACK CONTROL OF CHOLESTEROL SYNTHESIS IN MAN. J Clin Invest. 1963 Oct;42:1613–1618. doi: 10.1172/JCI104846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHER N. L., OVERATH P., LYNEN F. beta-Hydroxy-beta-methyl-glutaryl coenzyme A reductase, cleavage and condensing enzymes in relation to cholesterol formation in rat liver. Biochim Biophys Acta. 1960 Jun 3;40:491–501. doi: 10.1016/0006-3002(60)91390-1. [DOI] [PubMed] [Google Scholar]
- Bricker L. A., Morris H. P., Siperstein M. D. Loss of the cholesterol feedback system in the intact hepatoma-bearing rat. J Clin Invest. 1972 Feb;51(2):206–215. doi: 10.1172/JCI106805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Cholesterol synthesis by the gastrointestinal tract: localization and mechanisms of control. J Clin Invest. 1965 Aug;44(8):1311–1327. doi: 10.1172/JCI105237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Siperstein M. D. Effect of cholesterol feeding and fasting on sterol synthesis in seventeen tissues of the rat. J Lipid Res. 1967 Mar;8(2):97–104. [PubMed] [Google Scholar]
- Dietschy J. M. The role of bile salts in controlling the rate of intestinal cholesterogenesis. J Clin Invest. 1968 Feb;47(2):286–300. doi: 10.1172/JCI105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy J. M., Wilson J. D. Cholesterol synthesis in the squirrel monkey: relative rates of synthesis in various tissues and mechanisms of control. J Clin Invest. 1968 Jan;47(1):166–174. doi: 10.1172/JCI105706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECKLES N. E., TAYLOR C. B., CAMPBELL D. J., GOULD R. G. The origin of plasma cholesterol and the rates of equilibration of liver, plasma, and erythrocyte cholesterol. J Lab Clin Med. 1955 Sep;46(3):359–371. [PubMed] [Google Scholar]
- GOULD R. G. Lipid metabolism and atherosclerosis. Am J Med. 1951 Aug;11(2):209–227. doi: 10.1016/0002-9343(51)90107-6. [DOI] [PubMed] [Google Scholar]
- GOULD R. G., TAYLOR C. B., HAGERMAN J. S., WARNER I., CAMPBELL D. J. Cholesterol metabolism. I. Effect of dietary cholesterol on the synthesis of cholesterol in dog tissue in vitro. J Biol Chem. 1953 Apr;201(2):519–528. [PubMed] [Google Scholar]
- LINDSEY C. A., Jr, WILSON J. D. EVIDENCE FOR A CONTRIBUTION BY THE INTESTINAL WALL TO THE SERUM CHOLESTEROL OF THE RAT. J Lipid Res. 1965 Apr;6:173–181. [PubMed] [Google Scholar]
- MORRIS M. D., CHAIKOFF I. L., FELTS J. M., ABRAHAM S., FANSAH N. O. The origin of serum cholesterol in the rat; diet versus synthesis. J Biol Chem. 1957 Feb;224(2):1039–1045. [PubMed] [Google Scholar]
- SIPERSTEIN M. D., GUEST M. J. Studies on the site of the feedback control of cholesterol synthesis. J Clin Invest. 1960 Apr;39:642–652. doi: 10.1172/JCI104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M. Feedback control of mevalonate synthesis by dietary cholesterol. J Biol Chem. 1966 Feb 10;241(3):602–609. [PubMed] [Google Scholar]
- TAYLOR C. B., PATTON D., YOGI N., COX G. E. Diet as source of serum cholesterol in man. Proc Soc Exp Biol Med. 1960 Apr;103:768–772. doi: 10.3181/00379727-103-25664. [DOI] [PubMed] [Google Scholar]
- TOMKINS G. M., SHEPPARD H., CHAIKOFF I. L. Cholesterol synthesis by liver. III. Its regulation by ingested cholesterol. J Biol Chem. 1953 Mar;201(1):137–141. [PubMed] [Google Scholar]
- Weis H. J., Dietschy J. M. Failure of bile acids to control hepatic cholesterogenesis: evidence for endogenous cholesterol feedback. J Clin Invest. 1969 Dec;48(12):2398–2408. doi: 10.1172/JCI106206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D. Biosynthetic origin of serum cholesterol in the squirrel monkey: evidence for a contribution by the intestinal wall. J Clin Invest. 1968 Jan;47(1):175–187. doi: 10.1172/JCI105707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Lindsey C. A., Jr Studies on the influence of dietary cholesterol on cholesterol metabolism in the isotopic steady state in man. J Clin Invest. 1965 Nov;44(11):1805–1814. doi: 10.1172/JCI105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. D., Reinke R. T. Transfer of locally synthesized cholesterol from intestinal wall to intestinal lymph. J Lipid Res. 1968 Jan;9(1):85–92. [PubMed] [Google Scholar]



