Abstract
Glucose-induced insulin release is thought to result from the following sequence of events in the beta cell: glucose metabolism leading to the production of a metabolic signal, net calcium uptake by the beta cell in response to the signal, and interaction between calcium and a microtubular-microfilamentous system, leading to emiocytosis of the secretory granules. Dibutyryl-cyclic AMP (db-cAMP) and theophylline are known to potentiate glucose-induced insulin release, their insulinotropic action being most marked at high glucose concentrations. Based on the above mentioned concepts, it was considered in the present experiments that the primary site of action of cAMP in the beta cell could correspond to either a facilitation of glucose metabolism, a modification of calcium distribution, or an interaction with the microtubular-microfilamentous system.
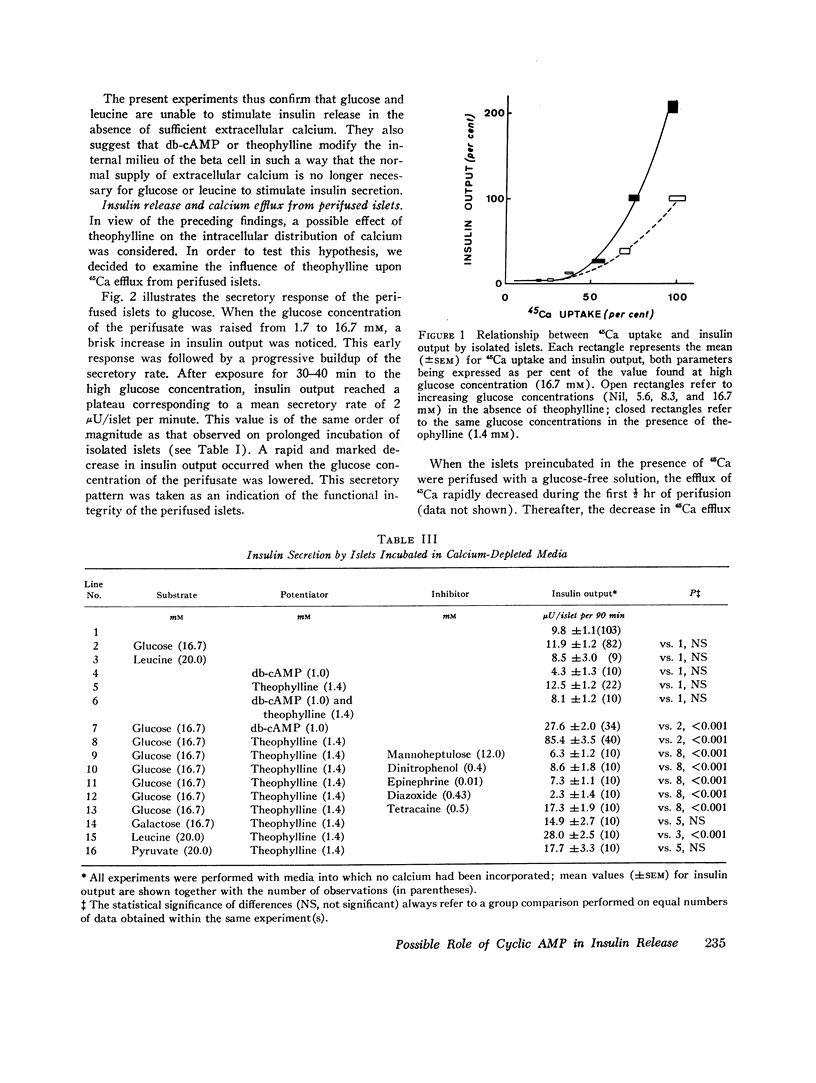
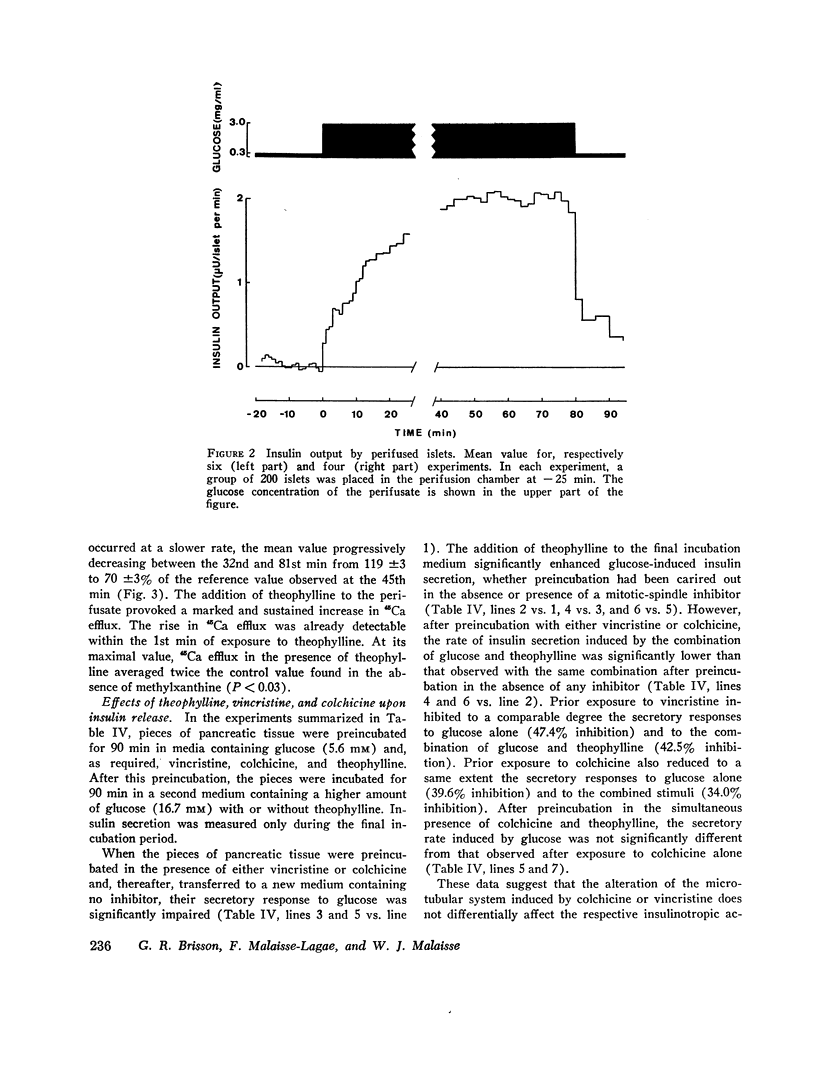
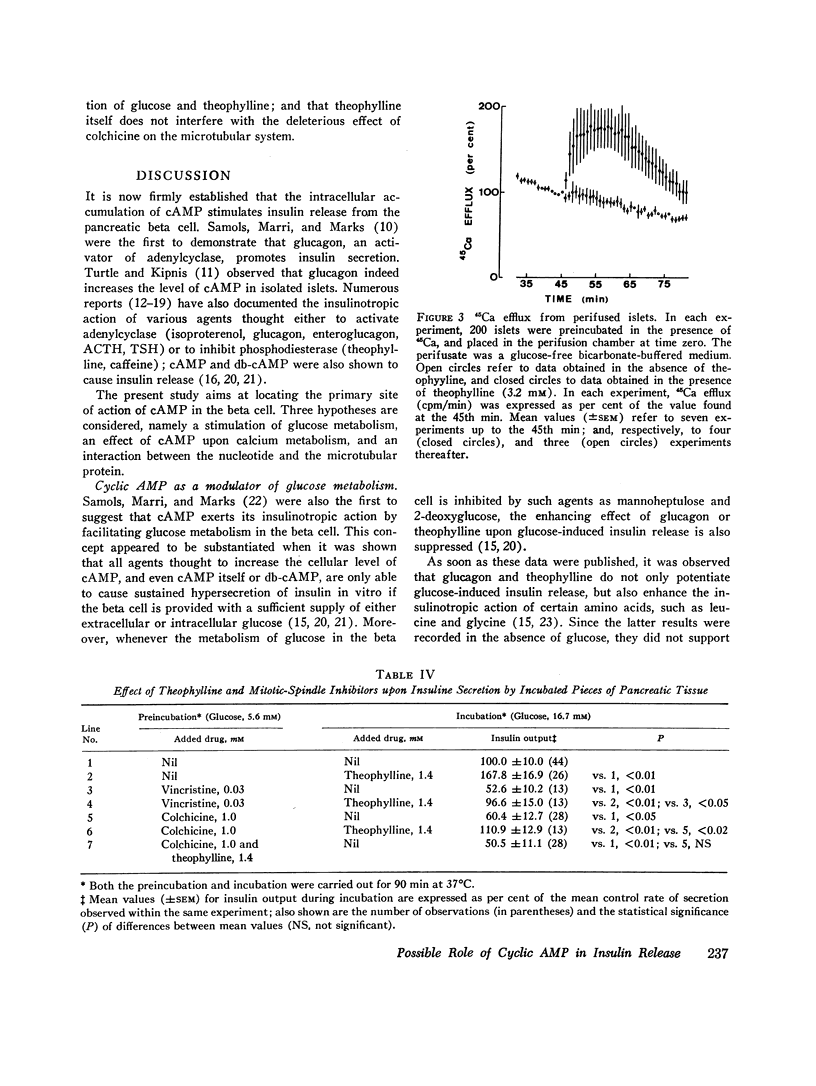
The first of these hypotheses appeared unlikely because db-cAMP and theophylline, in sharp contrast with other agents known to affect glucose metabolism in the beta cell, did not modify glucose-induced calcium uptake by isolated islets incubated at high glucose concentrations. The last hypothesis also appeared unlikely since theophylline did not interfere with the deleterious effect of colchicine on the microtubular system, and since vincristine or colchicine did not differentially affect the respective insulinotropic action of glucose and theophylline. An effect of cAMP upon calcium distribution in the beta cell was suggested by the following findings. Whereas glucose and leucine were unable to promote insulin release in the absence of extracellular calcium, the addition of db-cAMP or theophylline to the calcium-depleted media partially restored theinsulinotropic action of glucose and leucine. Moreover, theophylline caused a dramatic increase in 45Ca efflux from perifused islets, even in the absence of glucose. It is concluded that the insulinotropic action of cAMP could be due to a glucose-independent translocation of calcium within the beta cell, from an organelle-bound pool to a cytoplasmic pool of ionized calcium readily available for transport across the cell membrane.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Randle P. J. [Metabolism and insulin secretion in isolated islets]. Acta Diabetol Lat. 1969 Sep;6 (Suppl 1):538–553. [PubMed] [Google Scholar]
- Cerasi E., Luft R. [Is diabetes mellitus a disorder of the cellular information transmission?]. Acta Diabetol Lat. 1970 Sep;7 (Suppl 1):278–299. [PubMed] [Google Scholar]
- Efendić S., Alm B., Löw H. Effects of Ca ++ on lipolysis in human omental adipose tissue in vitro. Horm Metab Res. 1970 Sep;2(5):287–291. [PubMed] [Google Scholar]
- Entman M. L., Levey G. S., Epstein S. E. Demonstration of adenyl cyclase activity in canine cardiac sarcoplasmic reticulum. Biochem Biophys Res Commun. 1969 Jun 6;35(5):728–733. doi: 10.1016/0006-291x(69)90466-5. [DOI] [PubMed] [Google Scholar]
- Friedmann N., Park C. R. Early effects of 3',5'-adenosine monophosphate on the fluxes of calcium end potassium in the perfused liver of normal and adrenalectomized rats. Proc Natl Acad Sci U S A. 1968 Oct;61(2):504–508. doi: 10.1073/pnas.61.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D. B., Rasmussen H., DiBella F., Guthrow C. E., Jr Cyclic adenosine 3':5'-monophosphate-stimulated phosphorylation of isolated neurotubule subunits. Proc Natl Acad Sci U S A. 1970 Oct;67(2):652–659. doi: 10.1073/pnas.67.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerström C., Gunnarsson R. [Bioenergetics of islet function: oxygen utilization and oxidative metabolism in the beta-cells]. Acta Diabetol Lat. 1970 Sep;7 (Suppl 1):127–158. [PubMed] [Google Scholar]
- Hellman B., Idahl L. A. [Control of ATP levels in stimulated pancreatic B-cells]. Acta Diabetol Lat. 1969 Sep;6 (Suppl 1):597–611. [PubMed] [Google Scholar]
- KNOPF R. F., FAJANS S. S., FLOYD J. C., Jr, CONN J. W. Comparison of experimentally induced and naturally occurring sensitivity to leucine hypoglycemia. J Clin Endocrinol Metab. 1963 Jun;23:579–587. doi: 10.1210/jcem-23-6-579. [DOI] [PubMed] [Google Scholar]
- Kipnis D. M. [Studies of insulin secretion: radioimmunoassay of cyclic nucleotides and the role of cyclic amp]. Acta Diabetol Lat. 1970 Sep;7 (Suppl 1):314–337. [PubMed] [Google Scholar]
- Lacy P. E. Beta cell secretion--from the standpoint of a pathobiologist. Diabetes. 1970 Dec;19(12):895–905. doi: 10.2337/diab.19.12.895. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lambert A. E., Jeanrenaud B., Renold A. E. Enhancement by caffeine of glucagon-induced and tolbutamide-induced insulin release from isolated foetal pancreatic tissue. Lancet. 1967 Apr 15;1(7494):819–820. doi: 10.1016/s0140-6736(67)92782-1. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Brisson G. R., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. VI. Analogy between the insulinotropic mechanisms of sugars and amino acids. Horm Metab Res. 1971 Nov;3(6):374–378. doi: 10.1055/s-0028-1094124. [DOI] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Brisson G., Malaisse-Lagae F. The stimulus-secretion coupling of glucose-induced insulin release. I. Interaction of epinephrine and alkaline earth cations. J Lab Clin Med. 1970 Dec;76(6):895–902. [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Mayhew D. A possible role for the adenylcyclase system in insulin secretion. J Clin Invest. 1967 Nov;46(11):1724–1734. doi: 10.1172/JCI105663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. Stimulation of insulin secretion by noncarbohydrate metabolites. J Lab Clin Med. 1968 Sep;72(3):438–448. [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Walker M. O., Lacy P. E. The stimulus-secretion coupling of glucose-induced insulin release. V. The participation of a microtubular-microfilamentous system. Diabetes. 1971 May;20(5):257–265. doi: 10.2337/diab.20.5.257. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., King S. Effects of neutral red and imidazole upon insulin secretion. Diabetologia. 1968 Dec;4(6):370–374. doi: 10.1007/BF01211774. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F., Wright P. H. A new method for the measurement in vitro of pancreatic insulin secretion. Endocrinology. 1967 Jan;80(1):99–108. doi: 10.1210/endo-80-1-99. [DOI] [PubMed] [Google Scholar]
- Malaisse W., Malaisse-Lagae F. [A possible role for calcium in the stimulus-secretion coupling for glucose-induced insulin secretion]. Acta Diabetol Lat. 1970 Sep;7 (Suppl 1):264–277. [PubMed] [Google Scholar]
- Montague W., Cook J. R. The role of adenosine 3':5'-cyclic monophosphate in the regulation of insulin release by isolated rat islets of Langerhans. Biochem J. 1971 Mar;122(1):115–120. doi: 10.1042/bj1220115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda A., Aguilar-Parada E., Eisentraut A. M., Unger R. H. Control of pancreatic glucagon secretion by glucose. Diabetes. 1969 Jan;18(1):1–10. doi: 10.2337/diab.18.1.1. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr Beta adrenergic stimulation of insulin release in man. Diabetes. 1967 Mar;16(3):150–155. doi: 10.2337/diab.16.3.150. [DOI] [PubMed] [Google Scholar]
- Randle P. J., Ashcroft S. J. [Bioenergetics of islet function: islet glucose metabolism]. Acta Diabetol Lat. 1970 Sep;7 (Suppl 1):159–180. [PubMed] [Google Scholar]
- Rasmussen H., Tenenhouse A. Cyclic adenosine monophosphate, CA++, and membranes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1364–1370. doi: 10.1073/pnas.59.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMOLS E., MARRI G., MARKS V. PROMOTION OF INSULIN SECRETION BY GLUCAGON. Lancet. 1965 Aug 28;2(7409):415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- Samols E., Marri G., Marks V. Interrelationship of glucagon, insulin and glucose. The insulinogenic effect of glucagon. Diabetes. 1966 Dec;15(12):855–866. doi: 10.2337/diab.15.12.855. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Vaughan G. D. Insulin release after ACTH, glucagon and adenosine-3'-5'-phosphate (cyclic AMP) in the perfused isolated rat pancreas. Diabetes. 1967 Jul;16(7):449–454. doi: 10.2337/diab.16.7.449. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic AMP in the control of carbohydrate metabolism. Diabetes. 1969 Dec;18(12):797–819. doi: 10.2337/diab.18.12.797. [DOI] [PubMed] [Google Scholar]
- Turner D. S., McIntyre N. Stimulation by glucagon of insulin release from rabbit pancreas in vitro. Lancet. 1966 Feb 12;1(7433):351–352. doi: 10.1016/s0140-6736(66)91327-4. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Kipnis D. M. An adrenergic receptor mechanism for the control of cyclic 3'5' adenosine monophosphate synthesis in tissues. Biochem Biophys Res Commun. 1967 Sep 7;28(5):797–802. doi: 10.1016/0006-291x(67)90388-9. [DOI] [PubMed] [Google Scholar]
- Turtle J. R., Littleton G. K., Kipnis D. M. Stimulation of insulin secretion by theophylline. Nature. 1967 Feb 18;213(5077):727–728. doi: 10.1038/213727a0. [DOI] [PubMed] [Google Scholar]
- Vecchio D., Luyckx A., Zahnd G. R., Renold A. E. Insulin release induced by glucagon in organ cultures of fetal rat pancreas. Metabolism. 1966 Jul;15(7):577–581. doi: 10.1016/0026-0495(66)90119-3. [DOI] [PubMed] [Google Scholar]
- Wright P. H., Malaisse W. J., Reynolds I. J. Assay of partially neutralized guinea pig anti-insulin serum. Endocrinology. 1967 Aug;81(2):226–234. doi: 10.1210/endo-81-2-226. [DOI] [PubMed] [Google Scholar]



