Abstract
Summary: The airway epithelium acts as a frontline defense against respiratory viruses, not only as a physical barrier and through the mucociliary apparatus but also through its immunological functions. It initiates multiple innate and adaptive immune mechanisms which are crucial for efficient antiviral responses. The interaction between respiratory viruses and airway epithelial cells results in production of antiviral substances, including type I and III interferons, lactoferrin, β-defensins, and nitric oxide, and also in production of cytokines and chemokines, which recruit inflammatory cells and influence adaptive immunity. These defense mechanisms usually result in rapid virus clearance. However, respiratory viruses elaborate strategies to evade antiviral mechanisms and immune responses. They may disrupt epithelial integrity through cytotoxic effects, increasing paracellular permeability and damaging epithelial repair mechanisms. In addition, they can interfere with immune responses by blocking interferon pathways and by subverting protective inflammatory responses toward detrimental ones. Finally, by inducing overt mucus secretion and mucostasis and by paving the way for bacterial infections, they favor lung damage and further impair host antiviral mechanisms.
INTRODUCTION
Besides its role in maintaining the conduit for air to and from the alveoli, the airway epithelium is central to the defense of the lung against pathogens, through the combined function of ciliated epithelial and secretory cells maintaining efficient mucociliary clearance and through a variety of other host defense mechanisms (45, 65, 144). Taking over the first line of defense, the airway epithelium can be considered a soldier in the fight against airborne pathogens.
Airway epithelial cells regulate both innate and adaptive immunity through production of functional molecules and physical interactions with cells of the immune system. Activation of epithelial cells results in immediate host defense responses that include production of antiviral substances as well as proinflammatory cytokines which recruit and activate other mucosal innate immune cells and initiate mechanisms of adaptive immunity (108).
Viral respiratory tract infections (vRTIs) are the most common illnesses worldwide, resulting in a wide range of severities, from the common cold to severe life-threatening respiratory tract infections (87, 229, 269, 284, 291). People of all ages experience several vRTIs each year, with young children having the largest number of illnesses. Molecular diagnostic techniques have consistently identified rhinoviruses as the most frequent agents of vRTIs (86, 168). Other common respiratory viruses include influenza virus (72, 122, 150, 279), parainfluenza virus (313), respiratory syncytial virus (RSV) (199), adenovirus (102, 164), human metapneumovirus (134), human coronavirus (74, 314), and enteroviruses (mostly echovirus) (121). Human bocavirus (133, 256) and polyomaviruses (KI and WU) (312) have also been identified in vRTIs, but their pathogenic role remains to be defined. vRTIs are associated with both short- and long-term morbidity. They are important triggers of acute exacerbations of chronic airway diseases such as asthma, cystic fibrosis (CF), and chronic obstructive pulmonary disease (COPD) (31, 44, 170, 202, 216, 276, 311). During infancy, they are associated with an increased risk of development of recurrent wheeze and asthma later in life (79, 322).
While airway defense mechanisms efficiently fight respiratory viruses most of the time, resulting in rapid clearance of the virus with minimal clinical consequences, viruses have found ways of avoiding immune responses in the airways, with the potential consequence of severe respiratory disease. This review focuses on the role of the airway epithelium in the fight against viral infections, delineating intact defense mechanisms (the epithelium as a healthy soldier) and situations where these are insufficient for efficient virus control (the epithelium as a wounded soldier).
THE HEALTHY SOLDIER
The Airway Epithelium as a Barrier
Physical barrier.
The airway epithelium is at the interface of the human body with the inhaled environment and forms a complex physicochemical barrier complemented by the mucociliary escalator to provide the first line of defense against inhaled pathogens (Fig. 1).
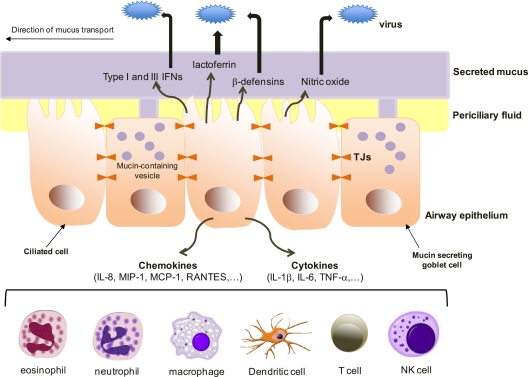
FIG. 1.
Defenses of the healthy epithelium (the healthy soldier). Epithelial cells act as a barrier against respiratory viruses. The mucociliary apparatus (ciliary movement of mucus) and tight junctions (TJs) add mechanical, biological, and chemical protection. The airway epithelium also regulates both innate and adaptive immune responses, through production of antiviral substances such as IFNs, lactoferrin, β-defensins, and nitric oxide (NO) in the mucus layer and production of cytokines and chemokines which recruit and activate immune cells in the submucosa.
Epithelial cells, which cover the whole mucosal surface in contact with the air, are the central component of this physical barrier. They are attached to their neighbors by cell-cell junctions, including tight junctions (TJs), adherens junctions (AJs), gap junctions, and desmosomes (234). These structures form an impermeable and effective mechanical barrier and permit maintenance of an ionic gradient for directional secretion of many substances (40). Among cell-cell junctions, TJs are the most important for maintaining epithelial integrity. They consist of a series of interacting proteins and receptors which ensure impermeability of the barrier and also enable communication between adjacent cells and regulate intercellular transport (234). Located below TJs, AJs provide important adhesive contacts between neighboring epithelial cells. Gap junctions are unique cell-to-cell channels that allow diffusion of small metabolites, second messengers, ions, and other molecules (<1 kDa) between neighboring cells (177). Desmosomes are intercellular junctions that provide strong adhesion between cells and form adhesive bonds in a network which gives mechanical strength to tissues (85). Cell-cell junctions act as a barrier to virus entry and dissemination into the airway submucosa. They hinder viral access to receptors within the epithelial basolateral membrane, which is an important interaction and entry site for several viruses (18, 19). Interestingly, recent work has demonstrated a high concentration of retinoic acid-inducible gene I (RIG-I), a key regulator of type I interferon (IFN) antiviral signaling at TJs, suggesting that these could also interact with antiviral innate responses (see “The Airway Epithelium and Innate Immune Recognition”) (189).
Thus, merely through their physical presence and the network they form at the surface of the airway mucosa, epithelial cells represent an efficient and crucial first-line defense barrier against viral invasion.
Mucociliary escalator.
Mucus overlying the airway epithelium provides further protection of the mucosa by creating a semipermeable barrier that enables the exchange of nutrients, water, and gases while being impermeable to most pathogens. It also allows effective lung clearance through the mucociliary escalator. Approximately 90% of inhaled particles, including respiratory viruses, are transported with mucus from the bronchioles to the trachea by the beats of cilia present on the surfaces of airway epithelial cells (299).
The airway mucus is a viscoelastic gel with a complex composition in which almost 200 different proteins have been identified, such as antimicrobial substances (lysozyme and defensins), cytokines, and antioxidant proteins (195). It is secreted continuously by intraepithelial goblet cells and by mucous cells of submucosal glands toward the surface of the epithelium. The main components of respiratory mucus are mucins, which are large highly charged glycoproteins that cross-link to form the structural framework of the mucous barrier (239, 281). They contribute to the innate immune defense through antiviral and anti-inflammatory properties and through interaction with other mucus components, such as IgA, collectins, and defensins. To date, at least 11 mucins (MUC1, -2, -3, -4, -5AC, -5B, -6, -7, -8, -13, and -19) have been detected in human airways, but MUC5AC and MUC5B are the predominant mucins in human sputum (238, 298). MUC genes are regulated mainly by the transcription factors nuclear factor κB (NF-κB) and/or Sp1 (238).
Airway mucin production is induced by many respiratory viruses, including rhinovirus and influenza virus (278, 336). The increased production of mucus allows for better trapping and clearance of viruses. However, mucin overproduction and excessive mucus formation can lead to airway obstruction and exacerbation of preexisting airway disease, turning a powerful innate cleaning defense system into a detrimental mechanism (see The Wounded Soldier) (239, 298).
The Airway Epithelium and Innate Immune Recognition
In addition to their physical protective role, airway epithelial cells possess important immune functions in the fight against respiratory viruses. Innate immune responses of the epithelium allow for control of viral invasion and replication, and adaptive responses allow for effective viral clearance through cells of the adaptive immune system. Airway epithelial cells rapidly recognize pathogens through pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) and intracellular viral sensors (3) (Fig. 2).
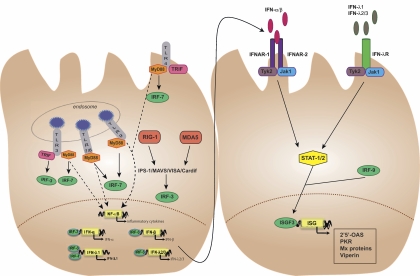
FIG. 2.
Key airway signaling pathways induced by respiratory viruses. TLR3 is expressed in intracellular endosomes and recognizes viral ssRNAs, such as those of rhinovirus, RSV, and influenza virus. TLR3 activates IRF-3 via the Toll/IL-1 receptor domain-containing adaptor (TRIF), resulting in IFN-β and IFN-λ1 production. TLR3 also activates IRF-7 and NF-κB through MyD88 activation. Activation of NF-κB and IRF-7 leads to the production of proinflammatory cytokines and the production of IFN-α, -λ1, and -λ2/3, respectively. Other endosomal TLRs (TLR7/8 and TLR9) detect ssRNAs and dsRNAs, such as those of influenza virus and adenovirus. Both TLR7/8 and TLR9 signal through a MyD88-dependent pathway, leading to the activation of NF-κB and IRF-7. TLR4 is expressed on the cell surface and responds to the RSV fusion protein. TLR4 signals through MyD88 to activate NF-κB and through TRIF to activate IRF-7. The two RNA helicases RIG-I and MDA5 detect viral replication products in the cytosol to activate IRF-3 via the adaptor protein IPS-1/MAVS/VISA/Cardif.
Virus recognition through TLRs.
TLRs are type I integral membrane glycoproteins which recognize a variety of pathogen-associated molecular patterns (PAMPs) (151, 286). In mammals, 13 members of the TLR family have been identified so far. The distribution and function of TLRs depend on the type of cells and their localization (62, 176, 331). Primary human airway epithelial cells and cell lines derived from them have been shown to express 10 TLRs (TLR1 to -10) (188, 260), and TLR expression has been documented for human biopsy tissue (101). TLRs play a crucial role in the initiation of immune responses in the respiratory epithelium (45, 175). TLRs trigger activation predominantly of mitogen-activated protein (MAP) kinases and of several key transcription factors, including NF-κB, interferon regulatory factor 3 (IRF-3), and IRF-7, resulting in the induction of numerous proinflammatory cytokines and type I (α and β) and type III (λ) IFNs (120).
TLR3, TLR7, TLR8, and TLR9 represent a TLR subfamily found in cytoplasmic endosomes of airway epithelial cells. They recognize viral nucleic acids and lead to the induction of type I IFNs. TLR3 responds to the presence of many respiratory viruses, including rhinoviruses (107, 137), RSV (89), and influenza viruses (90). TLR7, TLR8, and TLR9 recognize double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), and CpG DNA, respectively (141). TLR7 and TLR9 use MyD88 as an essential adaptor for the activation of NF-κB and IRF-7, leading to the production of proinflammatory cytokines (140) and type I IFNs (88). Only a few studies have investigated the interaction between respiratory viruses and TLR7, TLR8, or TLR9. Interaction of influenza virus with TLR7 and of adenoviral vectors with TLR9 has been shown to be important in immune responses of dendritic cells (DCs) (167, 323), but it is unknown whether such interaction is also relevant in airway epithelial cells.
Although intracellular TLRs, and TLR3 in particular, play a major role in viral recognition, the extracellular receptors TLR2, -4, and -6, known to be activated by bacterial products, might also be involved in the recognition of respiratory viruses by the airway epithelium. It has been shown that the RSV fusion protein interacts with TLR4, which then signals through MyD88 to induce immune responses in airway epithelial cells (28, 92, 103, 152), and a recent study demonstrated RSV interaction with TLR2 and TLR6, promoting a proinflammatory response (190).
Virus recognition through intracellular viral sensors.
It has become apparent that viral RNA is also detected by intracellular viral sensors such as RIG-I and melanoma differentiation-associated gene 5 (MDA5), both of which are members of the RIG-I-like RNA helicase family (231). In airway epithelial cells, induction of an antiviral response via RIG-I/MDA5 has been shown for several respiratory viruses. For example, RSV induces replication-mediated activation of the IFN-β promoter via RIG-I signaling (253). The RNA helicases RIG-I and MDA5 are present in the cytosol and detect intracellular dsRNA and ssRNA. MDA5 has been shown to detect small RNA viruses such as rhinoviruses and is critical in regulating IFN-β, IFN-λ, and proinflammatory cytokine transcription (138). In contrast, RIG-I has been shown to detect negative-sense ssRNA viruses that bear a 5′-triphospho-RNA genome, such as influenza virus and RSV, and appears to play a crucial role in the antiviral response to infection with these viruses (138). Both RIG-I and MDA5 proteins contain an RNA helicase domain which binds viral RNA and caspase recruitment domains (CARDs) that are involved in signaling (198). Recently, a new member of the RIG-I-like RNA helicase family, namely, LGP2, has been suggested to play an important role in picornavirus recognition by facilitating viral RNA recognition by RIG-I and MDA5 through its ATPase domain (255).
Binding of RNA species to RIG-I and MDA5 leads to interaction of CARDs within an adaptor protein called IPS-1, MAVS, VISA, or Cardif (222). This outer mitochondrial membrane protein mediates the recruitment and activation of protein kinases that phosphorylate the transcription factor IRF-3, leading to the synthesis of type I and type III IFNs (155). Although RIG-I and MDA5 share similar signaling features, the two helicases are able to discriminate among different ligands and therefore trigger innate immune responses to a broad variety of respiratory viruses (138).
Recent studies have described further intracellular sensors involved in the recognition and initiation of immune responses to respiratory viruses. Among them, some NOD-like receptors (NLRs) have been shown to play a role in the defense against respiratory viral infections. While NOD1 and NOD2, the first NLRs identified, are involved mainly in the recognition of bacterial peptidoglycans, NLRP3 seems to be important for the immune response to respiratory viruses (5, 118, 192, 280). Sensing or signaling through NLRP3 results in activation of the inflammasome, a signaling complex composed of an NLR protein, the adaptor apoptotic speck-containing protein with a CARD (ASC), and procaspase-1, which processes the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 into their respective mature secreted forms (282). NLRP3 expression and production of IL-1β are induced in primary human airway epithelial cells in response to influenza A virus infection, but it has yet to be determined whether NLRP3 acts directly as a PRR for viral RNA or if it is activated only indirectly in response to PRR signaling (5).
The Airway Epithelium as a Source of Antiviral Substances
Upon viral recognition, airway epithelial cells produce a variety of antiviral substances that are important in immediate innate immune responses. Searches for such agents in the mucous layer covering the airway epithelium have identified a wide range of peptides, proteins, and organic molecules. Among them, IFNs, lactoferrin (LF), β-defensins (BDs), and nitric oxide (NO) are considered the most important epithelium-derived antiviral host defense molecules (Fig. 1).
Type I and III IFNs and their downstream signaling pathways.
IFN-α and IFN-β were among the first antiviral agents to be characterized and are still seen as central to the early antiviral response of virus-infected cells. They represent the classical antiviral IFNs and are expressed by many epithelial cell types. They bind to the type I IFN receptor complex (IFNAR1 and -2) and induce a range of different genes encoding proteins that selectively interfere with virus replication, protein synthesis, or protein trafficking (16, 139, 245). Recently, a novel class of antiviral cytokines was discovered and classified as type III IFNs: IFN-λ1/IL-29, IFN-λ2/IL-28A, and IFN-λ3/IL-28B. Type III IFNs possess antiviral properties similar to those of type I IFNs but appear to be expressed especially by epithelial cells and consequently exert host protection primarily at epithelial surfaces (8, 9, 335). These IFNs use a unique receptor complex composed of the IL-10 receptor beta (IL-10Rβ) chain and the IFN-λR1 chain (50, 149, 261).
The regulation of IFN synthesis requires the participation of several transcription factor complexes, such as NF-κB, ATF2, and particularly IRFs (13), which are activated in response to virus-specific signals, including dsRNA, ssRNA, and viruses such as rhinovirus, RSV, and influenza virus. Type I IFN induction is regulated mainly by IRF-3 and IRF-7 (254, 327). It has been shown that IFN-β is initially produced from infected cells via IRF-3 and that IFN-β receptor binding induces IRF-7, leading to late-phase IFN-β and IFN-α production (254). IFN-λ1 gene induction is regulated by both IRF-3 and IRF-7, whereas IFN-λ2/3 genes appear to be induced only by IRF-7 (201).
After binding to their specific receptors, type I and type III IFNs send a signal to the nucleus through the Jak-STAT pathway. Activated STAT1/2 forms a complex with IRF-9 (also called ISGF3) which translocates to the nucleus and induces IFN-stimulated genes (ISGs), leading to the production of the proapoptotic p53 protein (277, 326) and of several antiviral proteins (9, 35, 159, 245). These include dsRNA-activated protein kinase R (PKR), 2′,5′-oligoadenylate synthetase (2′,5′-OAS), myxovirus resistance proteins (Mx proteins), and viperin, which ultimately mediate the antiviral actions of IFNs by inhibiting viral replication (158). Activated PKR phosphorylates cellular proteins, including the translation initiation factor eIF-2, resulting in arrest of translation of both cellular and viral mRNAs (75). Viral activation of PKR also causes apoptosis through a Fas-dependent pathway (82) and leads to the production of nitric oxide (287) (see “Nitric oxide”). 2′,5′-OAS leads to cleavage of ssRNA and thus to degradation of viral RNA (214, 274). The Mx proteins impair intracellular trafficking of viral proteins and thus reduce virus replication (94). Viperin is induced upon rhinovirus (219) and influenza virus (306) infections. The precise mechanisms by which viperin blocks viral replication are unclear, but they seem to involve inhibition of virus release from the plasma membranes of infected cells (306).
Most respiratory viruses induce IFNs in airway epithelial cells. The importance of IFN responses to respiratory viruses by the airway epithelium is underscored by studies of asthmatics which suggest that these patients have a deficient IFN-β and IFN-λ response to rhinovirus infection (35, 310). This defective IFN response may promote susceptibility to infection by impairing the ability of the infected host cell to undergo early apoptosis and by allowing increased virus replication and, ultimately, cytolysis of infected cells. Based on these findings, IFN-based treatments for asthmatic patients have been proposed (57). In a clinical study, IFN-α was used intranasally to treat 10 corticosteroid-resistant asthmatics (265). Treatment resulted in improved lung function and allowed a reduction in oral corticosteroid use. However, due to side effects (particularly nasal bleeding and blockage), the clinical use of IFNs in asthmatic patients is limited (237). Nevertheless, IFN treatment may be of use to combat severe diseases such as pandemic influenza virus infections. The pandemic 2009 (H1N1) influenza virus is extremely sensitive to the antiviral actions of type I and type III IFNs (200).
LF.
LF is a member of the transferrin gene family. Epithelial cells produce LF, which is present at high concentrations in the bronchial mucus (307). LF displays antiviral activity against both DNA and RNA viruses, including RSV and rhinovirus. It prevents entry of virus into the host cell, either by blocking cellular receptors or by direct binding to virus particles (290). For example, LF interacts with the RSV fusion protein, leading to a decrease of the viral concentration in epithelial cells (252).
hBDs.
Human BDs (hBDs) are the most abundant antimicrobial peptides expressed at epithelial surfaces, including the airway epithelium (143, 171). Besides their antibacterial properties, these cationic peptides have been shown to act as antiviral agents (325). Four hBDs (hBD-1 to -4) for airway mucosal defense have been identified in respiratory epithelial cells, but only hBD-2 and hBD-3 act against respiratory viruses (52, 73, 148, 267, 324). Accordingly, expression of hBD-2 and hBD-3, but not that of hBD-1, is induced in bronchial epithelial cells after rhinovirus infection (55, 218). hBDs can suppress viral replication by interfering with T cells, monocytes, and immature DCs, by inducing cytokine production by epithelial cells, and by directly binding to certain viruses (257).
Nitric oxide.
Respiratory epithelial cells produce high levels of NO, as evidenced by detection of NO in exhaled breath and of NO reaction products (e.g., NO2− and NO3−) in bronchoalveolar lavage fluid from human lungs (77). Three NO synthases (NOS) present in airway epithelial cells contribute to NO production: the constitutive NOS-1 and NOS-3 proteins and the inducible, calcium-dependent NOS-2 protein. NOS-2 is expressed constitutively in normal human airway epithelium under basal conditions, and its synthesis is increased in response to proinflammatory mediators (59).
NOS-2 is induced and NO production increased after infection with respiratory viruses (136, 191, 246, 287). Intracellular dsRNA formed during viral replication activates PKR, which is implicated in NOS-2 gene expression (287). Several studies have demonstrated an inhibition of viral replication in human respiratory epithelial cells by addition of chemical donors of NO or by overexpression or induction of NOS-2 (233, 247). NO inhibits rhinovirus replication and virus-induced cytokine expression through inhibition of IRF-1 and NF-κB pathways in airway epithelial cells (146, 247), but other transcription factors may be involved (248). NO also possesses proapoptotic properties through induction of caspase activity (25).
The importance of nitric oxide production in antiviral defense of the airway epithelium is demonstrated by studies showing that increased virus (parainfluenza virus 3) replication in airway epithelial cells from CF patients is due at least partly to a lack of NOS-2 induction in response to virus and that NO donor or NOS-2 overexpression provides protection from virus infection (333, 334). In vivo, NO deficiency is implicated in the development of airway hyperresponsiveness after viral respiratory infection in guinea pigs (67). Data for humans show that increased exhaled nitric oxide after rhinovirus infection is correlated with reduced airway hyperresponsiveness in asthmatic patients, suggesting a beneficial effect of NO in virus-induced asthma exacerbations (42).
The Airway Epithelium as a Source of Cytokines and Chemokines
Airway epithelial cells participate actively in immune responses to viral infection by releasing chemokines and cytokines into the submucosa (Fig. 1). These proteins have a wide range of effects, acting on both innate and adaptive processes of the immune system. During respiratory viral infections, large amounts of cytokines and chemokines are found in the airways, with some of them produced by airway epithelial cells (80, 173, 174).
Signaling pathways inducing cytokines and chemokines.
Infection of human airway epithelial cells with respiratory viruses induces rapid cytokine and chemokine gene expression through activation of several proinflammatory transcription factors. These include NF-κB, AP-1, and STAT1/2 (123, 181). Activation of NF-κB is common to many respiratory viruses, as demonstrated for rhinovirus (129, 203), RSV (24), and influenza virus (145). NF-κB is also the most widely studied pathway, responsible for the transcription of over 100 different genes, and has long been the focus of academic scientists and the pharmaceutical industry in search of new anti-inflammatory agents for respiratory diseases such as asthma and COPD.
Virus-induced cytokines and chemokines in innate immune responses.
The recruitment of immune cells is essential to promote an efficient antiviral response. Cytokines and chemokines secreted by respiratory virus-infected epithelia promote recruitment of inflammatory cells, including neutrophils, eosinophils, NK cells, and macrophages, from blood into infected tissues, as well as their activation (178).
Neutrophil recruitment is induced mainly by epithelium-derived IL-8/CXCL8, Gro-α/CXCL1, and ENA-78/CXCL5 (142, 178, 223). Through the release of antiviral proteins such as lactoferrin and defensins, neutrophils actively participate in the fight against respiratory viruses. Interestingly, however, inhibition of neutrophil recruitment to the lung had no effect on virus clearance in a mouse model of influenza virus infection, suggesting only a minor role of neutrophils in this model of respiratory viral infection (309).
Under the influence of IL-5, granulocyte-macrophage colony-stimulating factor (GM-CSF), eotaxin-1/CCL11, eotaxin-2/CCL24, and RANTES/CCL5, eosinophils are recruited to the lung and activated (83). Antiviral activity of eosinophils is promoted by eosinophil products such as eosinophil cationic protein (ECP) and eosinophil-derived neurotoxin (49). The protective effect of eosinophils against respiratory viruses was demonstrated in one study, among others, showing eosinophil-mediated decreased virus loads in the lungs of guinea pigs infected by parainfluenza virus (1).
NK cells also play an important role in the innate immune response against respiratory viruses, with their function being the elimination of a variety of target cells, including virus-infected cells, and the modulation of adaptive immunity toward an efficient antiviral response (22). Cytokines and chemokines shown to enhance NK cell recruitment and activity include IFN-α/β and macrophage inflammatory protein-1α (MIP-1α)/CCL3 (22, 285). NK cells are rapid and efficient producers of cytokines, such as IFN-γ, that are important in early antiviral responses and in the antigen-independent activation of antigen-presenting cells. A study demonstrating that influenza virus infection was lethal in mice lacking the NK cell-activating receptor NCR1 suggests that NK cells are indeed crucial for protection against respiratory virus infections (78).
Monocyte/macrophage recruitment is induced by IL-1β, MIP-1α/CCL3, monocyte chemoattractant protein 1 (MCP-1)/CCL2, and tumor necrosis factor alpha (TNF-α), all of which can be produced by airway epithelial cells upon viral infection (178, 258). For instance, an active role of airway epithelial cells in monocyte recruitment, involving both chemokines and adhesion molecules, has been demonstrated during influenza virus infection (106). Macrophages internalize and process viruses and present antigens to adaptive immune cells, thus playing a central role in antiviral immunity. They also release cytokines such as IL-12 and IL-10, which lead to activation of NK cells and to regulation of Th1 responses and enhanced B-cell survival, proliferation, and antibody production, respectively (see next paragraph).
Virus-induced cytokines and chemokines in adaptive immune responses.
Airway epithelial cells not only mediate innate immune responses but also regulate adaptive immune responses through interactions with DCs and T and B lymphocytes.
DCs, which induce the proliferation and activation of T cells, are crucial to the initiation of adaptive immune responses to viral infections in the lung (84). Two major types of pulmonary DCs have been identified: conventional DCs (cDCs; formerly called myeloid DCs [mDCs]) and plasmacytoid DCs (pDCs) (293, 320). cDCs or their precursors originate in the bone marrow and are recruited continuously from the blood into the lungs, where they lay in close proximity to the airway epithelium, even in the absence of inflammation (111). They express specific chemokine receptors such as CCR6, which is the ligand for the chemokine MIP-3α/CCL20 (217). Airway epithelial cells have been shown to enhance cDC migration into the epithelium via MIP-3α/CCL20 production (232), and MIP-3α/CCL20 induction has been demonstrated in several viral infections, such as influenza virus infection (178, 308). cDCs form a recognition network beneath the epithelial layer, where they sense and take up foreign antigens. Upon antigen recognition, they undergo a process called maturation, during which they downregulate chemokine receptors involved in homing to the lung and express the chemokine receptor CCR7, which drives migration to lymph nodes (243). Once in the lymph nodes, cDCs further mature into antigen-presenting cells, expressing molecules such as CD80, CD86, and major histocompatibility complex (MHC) class I and II molecules, allowing cognate stimulation of CD8+ and CD4+ T cells (166). Airway epithelial cells are increasingly recognized as important players in the processes driving local cDC differentiation and maturation via production of various cytokines, including IL-15, thymic stromal lymphopoietin (TSLP), and type I IFNs (227, 230, 272, 289). In contrast to cDCs, pDCs are not efficient antigen-presenting cells. Rather, their antiviral action is mediated by production of large amounts of IFN-α, which delivers a powerful innate response to limit viral replication. pDCs migrate into draining lymph nodes via high endothelial venules, where they modulate adaptive immune responses and promote a protective Th1 response through secretion of type I IFNs and IL-12 (2, 196, 263, 270). Upon viral infection, pDCs are recruited rapidly from the bone marrow, and within several days, they form the majority of DCs in the lung (300). Note that cDCs and pDCs express different TLRs on their surfaces and thus are activated preferentially by different stimuli: human cDCs express primarily TLR2 and TLR4, responding to viral proteins such as the RSV fusion protein, whereas pDCs typically express TLR7 and TLR9, recognizing single-stranded RNA and viral DNA, e.g., influenza viruses (63, 132).
T-cell and B-cell recruitment is central to adaptive immune responses to viral infections (147). Bronchial biopsy specimens from subjects undergoing experimental rhinovirus infection show T-cell infiltration of the airway epithelium (69). The appearance of antigen-specific effector T cells in the airways is observed around 6 to 7 days after infection with influenza and parainfluenza viruses (275). Airway epithelial cells induce migration of Th1 cells into the mucosa via production of RANTES/CCL5 and IP-10/CXCL10 and that of Th2 cells via production of IL-1β (178). Both Th1 and Th2 cells are important in antiviral adaptive responses: Th1 cells produce IFN-γ, IL-2, IL-12, and TNF-α, which all contribute to efficient cellular virus clearance, whereas Th2 cells produce IL-4, IL-5, and IL-13 and play an important role in humoral immunity against viruses. The Th1/Th2 balance plays a central role in the control of respiratory viruses and the outcome of disease, and the normal T-cell response to virus infection is thought to be of the Th1 type (225). For instance, studies of mice presensitized by recombinant vaccinia viruses showed that after RSV infection, animals exhibiting mainly Th2 cytokine responses developed enhanced disease with severe symptoms, whereas those with Th1 responses had reduced immunopathology and enhanced viral clearance (6).
There is a highly regulated interplay between airway epithelial cells and all other immune cells in the lung (153). Under normal conditions, the coexistence of these cells is well controlled and is elementary in maintaining the tolerogenic immune environment of the healthy lung. For example, healthy epithelial cells inhibit lymphocyte function and associated cytokine secretion, and alveolar macrophages inhibit DC function through the production of soluble mediators such as NO and IL-10 (110, 112, 301). Epithelial cells provide both membrane-bound and soluble inhibitory factors with direct inhibitory effects on T cells, but they also stimulate the secretion of transforming growth factor beta (TGF-β), which leads to the activation of regulatory T cells (297, 301). However, upon extrinsic and intrinsic stimuli such as viral infections, this complex regulatory mechanism is lost or shifts and proinflammatory immune responses are initiated (113, 301).
Inflammatory response according to virus type.
While it is almost impossible to compare the inflammatory responses of different viruses in vitro or in animal models, epidemiological evidence and clinical observations of natural infections in humans suggest that different viruses may be associated with different magnitudes of airway inflammation. For instance, pandemic influenza viruses, including H5N1 avian influenza virus and the H1N1 2009 California influenza virus strain, have been associated with more severe inflammation than seasonal influenza virus strains, and it is believed that the magnitude of inflammation is a major contributing factor to disease severity (17, 200, 209, 283, 319). Similarly, rhinovirus group C strains are thought to be associated with increased inflammation and morbidity compared with rhinovirus group A or B strains (10, 125). RSV strains have also been documented to induce different patterns and severities of inflammatory responses (173, 174, 271). Whether or not these differences can be attributed to the viruses themselves or to hosts that are susceptible to severe infection or prone to produce high levels of inflammation with a given virus is unknown. The role of airway epithelial cell-induced inflammation in this context is therefore rather speculative. However, considering that the airway epithelium is the main site of viral infection, it is likely to contribute significantly to the type and magnitude of the inflammatory response.
Besides the virus type, the environment to which airway epithelial cells are exposed influences the inflammatory response to respiratory viruses. For instance, it has been shown that under the influence of an atopic environment, the epithelial inflammatory response to rhinoviruses is downregulated and associated with increased viral proliferation and augmented cell damage, suggesting a potential mechanism to explain the propensity of atopic asthmatic individuals to develop lower airway symptoms after respiratory infections (321).
Similarities and differences in the upper and lower airways.
The general consensus is that the physiochemical characteristics of the upper (nasal) and lower (bronchial) epithelia are very similar, and the idea that the two airways are linked in function is reinforced by the observation that upper airway disorders such as occupational rhinitis, chronic rhinosinusitis, and nasal polyposis are linked to lower airway disorders such as asthma (reviewed in reference 61). An emerging theme is how the host defense differs between the epithelia of the upper and lower airways, although much remains to be explored. Using in vitro culture systems, some differences between susceptibilities to rhinovirus infection have been noted, with bronchial epithelium generating higher viral loads and more expression of virus-inducible mediators than those in bronchial epithelial cells cultured in the same manner (163).
THE WOUNDED SOLDIER
In the next sections, we focus on different strategies employed by respiratory viruses to escape immune defenses (Fig. 3 and 4; Table 1). We further discuss how vRTIs can lead to perturbed inflammatory responses and to increased mucus production, which will be detrimental rather than protective to the host. Finally, we review how viruses might facilitate bacterial colonization, with further negative consequences for the affected individual (Table 2).
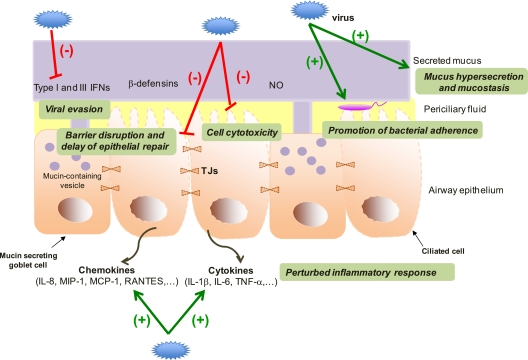
FIG. 3.
The airway epithelium under attack (the wounded soldier). Most respiratory viruses have elaborated strategies to evade antiviral mechanisms, for instance, by interfering with IFN signaling. During viral infection, the epithelium can be injured (through cytotoxic effects, disruption of tight junctions, or interference with epithelial repair), with the consequence of a loss of integrity and protection. Furthermore, respiratory viruses can lead to perturbed (skewed or exaggerated) inflammatory responses. Respiratory viruses might also facilitate bacterial colonization, with further negative consequences for the affected individual.
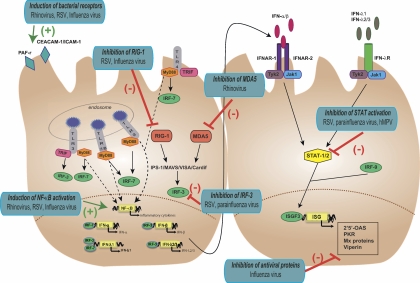
FIG. 4.
Examples of immune response disruption by respiratory viruses. Respiratory viruses are able to modulate the immune response by interfering with antiviral signaling pathways. They can inhibit IFN synthesis, for instance, by blocking IRF-3 activation (RSV and parainfluenza virus) or by interfering with RIG-1/MDA5 signaling (rhinovirus, RSV, and influenza virus). They can interfere with the production of antiviral molecules by blocking IFN signaling, for instance, through inhibition of STAT-1/2 activation (RSV, parainfluenza virus, and human metapneumovirus [hMPV]), or can directly inhibit antiviral proteins (influenza virus). They can interfere with NF-κB signaling (rhinovirus, RSV, and influenza virus), resulting in an excessive production of proinflammatory cytokines and in mucus hypersecretion. They are also able to induce bacterial adherence through an upregulation of host surface receptors such as PAF-r, CEACAM-1, and ICAM-1.
TABLE 1.
Key features of the wounded soldier model of the airway epithelium
| Feature |
|---|
| Loss of epithelial integrity |
| Virus-induced cytotoxicity (direct viral effects on the epithelium, exaggerated apoptosis) |
| Epithelial barrier disruption (cell-cell contacts) |
| Delayed and abnormal epithelial repair |
| Disruption of immune responses |
| Virus subversion of IFN signaling (synthesis and downstream pathways) |
| Virus-induced shifting of Th1-dominated responses toward a Th2-dominated pattern |
| Exaggerated and deleterious virus-induced immune response |
| Increased mucus production and mucostasis (ciliary impairment) |
| Pathways promoting bacterial infection |
| Lethal synergy between respiratory viruses and bacteria (short-term and long-term effects) |
| Decreased clearance and facilitated bacterial penetration (mucus trapping, increased paracellular permeability) |
| Virus-induced bacterial adherence (damaged epithelium, increased epithelium-bacterium interactions, exposition of bacterial receptors) |
TABLE 2.
Studies on bacterial adherence to human respiratory epithelial cells during viral infection
| Study (yr [reference]) | Virus or virusesa | Bacterium or bacteria | Cell type |
|---|---|---|---|
| 2010 (213) | Influenza virus | S. pneumoniae | Murine tracheal explants |
| 2009 (302) | RV | S. aureus, S. pneumoniae, H. influenzae | Primary human nasal cells |
| 2009 (70) | RSV | Nontypeable H. influenzae, S. pneumoniae | A549 |
| 2008 (172) | Influenza virus | S. pneumoniae | A549 |
| 2007 (12) | RSV | Nontypeable H. influenzae, S. pneumoniae | A549 |
| 2007 (292) | RSV | P. aeruginosa | A549, IB3-1 |
| 2006 (11) | RSV, influenza virus, HPIV-3 | Nontypeable H. influenzae, S. pneumoniae | A549, BEAS-2B, primary human normal small airway cells, primary human bronchial cells |
| 2005 (95) | RSV | S. pneumoniae | A549 |
| 2003 (119) | RV | S. pneumoniae | Primary human tracheal cells |
| 1994 (93) | Adenovirus | S. pneumoniae | A549 |
| 1992 (204) | RSV | Nontypeable H. influenzae | Rat airway epithelial cells |
| 1987 (250) | Influenza virus | S. aureus, S. pneumoniae | Ferret respiratory tracts |
| 1986 (215) | Influenza virus | S. pneumoniae | Mouse tracheal epithelial cells |
| 1982 (131) | Influenza virus | S. pneumoniae | Mouse tracheal tissue |
| 1980 (60) | Influenza virus | S. aureus, S. pneumoniae, H. influenzae | Primary human tracheal epithelial cells |
RV, rhinovirus; HPIV-3, human metapneumovirus type 3.
Loss of Epithelial Integrity
The airway epithelium plays an important role as a protective physical and functional barrier between the external environment and underlying tissues and as a central element in the initiation and regulation of innate and adaptive immune responses in the lung. During viral infection, the epithelium can be injured, with the consequence of loss of integrity and protection (Fig. 3).
Virus-induced cytotoxicity.
Airway epithelial cells are the principal hosts for respiratory viruses. These can cause widespread damage to the epithelium during replication (66). Epithelial damage not only is characterized by modifications in morphology, such as loss of ciliated cells (29), but also is associated with perturbations in airway homeostasis, such as decreased production of smooth muscle relaxing factors (e.g., nitric oxide) leading to airway hyperresponsiveness (67). Influenza virus and RSV have been shown to have important cytotoxic effects on the bronchial epithelium (66). However, the extent of cell damage following infection with other respiratory viruses, such as rhinovirus, is less clear. Whereas rhinovirus-induced cytotoxicity was shown in bronchial epithelial cells after rhinovirus infection (27), no cellular damage was observed in rhinovirus-infected nasal epithelial cells (318). Cytotoxicity upon infection with certain viruses may depend on the virus strain, infection conditions, and infected cell type and density (27).
Respiratory viruses can also induce cytotoxicity of airway epithelial cells by increased cell apoptosis. Apoptosis acts as a protective mechanism to limit replication and spread of the virus to other cells, but exaggerated apoptosis can lead to a loss of integrity and protection of the epithelium, with detrimental consequences. Respiratory viruses may lead directly to exaggerated cell apoptosis (116, 186) or trigger overt production of proapoptotic mediators such as NO (210).
Epithelial barrier disruption.
Respiratory viruses also induce perturbations in the continuity of the epithelial barrier. They can interfere with tight junctions, leading to an increase of paracellular permeability. For example, rhinoviruses are able to disrupt tight junctions by interfering with the ZO-1 protein (244). Infection of human epithelial cells with RSV results in significant epithelial membrane barrier disruption, characterized by a decrease in transepithelial electrical resistance and changes in paracellular permeability mediated by a cellular cytoskeletal rearrangement (266).
In addition, cytokines produced by the epithelium or by migrating inflammatory cells upon viral infection may affect cell-to-cell contact through interaction with TJs and desmosomes and thus disrupt the continuity of the epithelial barrier (135). In support of this notion, studies of asthmatic patients have shown a correlation between the number of inflammatory cells in the airways and the degree of epithelial barrier disruption (7).
Delayed and abnormal epithelial repair.
Epithelial repair initiates quickly after injury. Migrating basal airway epithelial cells repopulate damaged areas, proliferate, and differentiate until epithelial integrity has been restored (242). Numerous factors released by epithelial cells are involved in epithelial repair, including matrix metalloproteinases (MMPs), cytokines, and growth factors. MMPs play a crucial role in the remodeling of the matrix onto which cells migrate, and many pathways are involved in this process (294). For instance, TGF-β increases airway wound repair through MMP-2 upregulation (154).
Abnormal repair processes of the airway epithelium leading to abnormal organization and integrity of the respiratory epithelium, with subsequent perturbations in innate immune functions, have been proposed as key mechanisms in the pathogenesis of chronic airway diseases such as asthma (38, 48) and COPD (221).
Rhinoviruses have been shown to be major regulators of such airway remodeling processes, involving not only the airway epithelium but also other components of the airway mucosa. They are capable of delaying wound healing in bronchial epithelial cells (27). In addition, they may lead to subepithelial fibrosis by the induction of TGF-β, MMP-9, and MMP-10 production, resulting in enhanced degradation and abnormal repair of the extracellular matrix (26, 303). Furthermore, they induce the production of proangiogenic molecules, including amphiregulin (a member of the epidermal growth factor [EGF] family) and vascular endothelial growth factor (VEGF), and therefore may lead to increased angiogenesis (156, 220). Treatments aimed at inhibiting the production of profibrotic and/or angiogenic factors induced after rhinovirus infection have therefore been suggested as therapeutic options, for instance, in the context of virus-induced asthma (268, 296).
RSV has also been described as interfering with normal repair processes by increasing MMP-2 and MMP-9 activity (160) and by leading to excessive production of fibroblast growth factor basic (FGF-β) and EGF (51).
Disruption of Immune Responses
Virus subversion of IFN signaling.
Respiratory viruses have elaborated many strategies to evade the host IFN system (Fig. 4). Most viruses have efficient ways to inhibit IFN synthesis, to bind and inactivate secreted IFN molecules, to block IFN-activated signaling, or to disturb the action of IFN-induced antiviral proteins. In this section, we briefly explore two major mechanisms by which viruses evade antiviral defense: (i) inhibition of IFN synthesis and (ii) inhibition of IFN-induced antiviral proteins.
(i) Inhibition of IFN synthesis.
Respiratory viruses have devised various ways to inhibit IFN induction. These mechanisms range from the inhibition of specific viral sensors to the targeting of critical end points of several signaling pathways, such as transcription factors. For instance, influenza virus (91) and RSV (161) bind RIG-I and rhinovirus binds MDA5 to inhibit their activity (304), and IRF-3 activation is inhibited by RSV (273) and parainfluenza viruses (165). RSV targets the cytoplasmic RIG-I sensor through the NS2 protein (161). It is currently believed that NS2 prevents RIG-I signaling by interfering with the RIG-I-MAVS interaction, therefore severely limiting signaling mediated by the critical interaction between the sensor RIG-I and its specific adaptor, MAVS (161). In contrast, parainfluenza viruses target the phosphorylation of IRF3 specifically, by encoding V accessory proteins which act as substrates for the kinases TBK1 and IKK-ι/ɛ. V proteins may be expressed at high levels during the initial stages of infection and therefore limit IRF3 activation by binding IKK-ɛ and preventing IRF-3 activation, and hence IFN induction (165). By interfering with IFN production, respiratory viruses can block multiple antiviral pathways simultaneously, including IFN synthesis, ISG induction, and recruitment of inflammatory cells (71, 226).
(ii) Inhibition of IFN downstream signaling.
Another efficient way to escape epithelial IFN responses is to inhibit IFN signaling implicated in the production of specific antiviral proteins. A frequent point of interference by viruses is the Jak-STAT signaling pathway, used by IFNs to induce transcription of antiviral genes. Human metapneumovirus and the V protein of parainfluenza virus 5, for instance, block IFN signaling by inhibiting or degrading STAT-1 (47, 329). The NS1 and NS2 proteins of RSV inhibit STAT-2 activation, thus blocking IFN-α/β function (161, 162). Respiratory viruses can also directly block antiviral proteins. The influenza virus NS1 protein, for example, inhibits PKR activation, resulting in increased viral replication in the lung (20).
Virus-induced shifting of Th1-dominated responses toward a Th2-dominated pattern.
As reviewed above, an efficient antiviral adaptive response is thought to be of the Th1 type (225). Respiratory viruses can modulate the Th1/Th2 balance in favor of a Th2 response, resulting in the development or exacerbation of chronic airway diseases such as asthma (36, 179). In allergic asthmatic subjects, Th1 responses to rhinovirus are downregulated, as indicated by lower levels of IL-12 and IFN-γ, whereas Th2 responses are increased, with higher levels of IL-4, IL-5, and IL-13 (179). Whether this is the result of a preexisting allergic phenotype of the host or whether rhinovirus infection can exaggerate Th2 responses is a key question, and it is worth noting that murine models of combined rhinovirus infection and exposure to airway allergens give enhanced Th2 responses compared to those to virus or allergen challenge alone (14, 193).
A role for airway epithelial cells in contributing to the Th2 phenotype is possible, as they have receptors for and respond to IL-4, IL-13, and IL-25 (68, 115). Furthermore, RSV and rhinovirus can lead to induction of the pro-Th2 cytokine TSLP, suggesting a direct link between viral infection of the epithelium and an altered immune response toward a Th2 pattern (4, 157, 264).
Deleterious aspects of virus-induced immune responses.
Whereas any dysfunction of the innate immune response is obviously detrimental to the host, an exaggerated response may also contribute to disease severity. The regulation of cytokine and chemokine production by the airway epithelium upon viral exposure is complex, and there is only a fine line between induction of a protective antiviral response and an exaggerated, deleterious inflammatory response.
Many clinical studies have identified positive associations between levels of airway epithelial cell-derived inflammatory mediators and disease severity. For instance, dose-related associations were found between several cytokines and chemokines (including IL-8/CXLC8, MIP-1α/CCL3, MCP-1/CCL2, and the IL-6/TNF-α ratio) in respiratory secretions and the severity of RSV bronchiolitis (76, 114, 271), suggesting a relationship between overly aggressive innate immune responses and disease following infection. Epithelium-derived cytokines and chemokines, by increasing cellular recruitment into infected airways, are believed to be responsible for damaging both infected and uninfected areas of the lung (117, 305). Similar associations have been demonstrated in the context of influenza virus (21, 43, 206, 283), coronavirus (328), and parainfluenza virus (58) infections. It is unclear, however, whether the high levels of inflammatory mediators found in severe disease reflect an exaggerated and possibly dysregulated inflammatory response or are simply a reflection of increased virus replication and increased pathology (205, 315).
Increased Mucus Production and Mucostasis
Increased mucus production by the airway epithelium upon viral infection is detrimental to respiratory function, as it leads to airway blockade. Increased expression of MUC5AC mRNA in bronchial epithelial cells has been reported for mice after RSV infection (97), and rhinovirus infection induces MUC5AC production in the human airway epithelium (104). Mucus hypersecretion plays a central role in the pathogenesis of severe airway obstruction in virus-induced exacerbations of asthma, cystic fibrosis, and COPD (15, 99, 235, 236).
Respiratory viruses are also able to induce ciliary impairment, including disruption of ciliary beating and loss of ciliated cells, leading to mucostasis (318, 332). Mechanisms involved in ciliostasis are poorly understood but could be mediated by ion alterations during viral attachment and replication, by direct damage to the ciliary system, or by damage through the induction of NO, cyclic AMP, calmodulin, and inositol 1,4,5-triphosphate (InP3) production, which alters baseline ciliary beat frequency (33) or modulates hfh4 and mdnh5 gene expression, resulting in absent or dysfunctional ciliary function (169).
Pathways Promoting Bacterial Infection
Lethal synergy between respiratory viruses and bacteria.
Fortunately, most respiratory viral infections are self-limited and associated with minor morbidity. However, the course of the disease can be severe and even lead to death. In many of these cases, morbidity is not due to the viral infection itself but is the result of associated complications, including secondary bacterial infections.
Clinical studies have shown that respiratory viruses increase the incidence and severity of severe secondary bacterial complications (130, 207, 330). During influenza pandemics, for instance, bacterial coinfections caused by Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus have been important contributors to mortality. The majority of deaths during the 1918 to 1919 flu pandemic were due to bacterial pneumonia (185). Concerning the 2009 influenza A (H1N1) pandemic, the role of bacterial coinfection in influenza-related illnesses and deaths appears less clear (31a, 31b, 31c).
In addition to these acute effects of secondary bacterial coinfections, epidemiological evidence indicates that viral infections predispose patients to bacterial respiratory colonization in chronic airway diseases such as CF or COPD (34, 109, 126, 211).
In this section, we focus on pathogenic mechanisms implicated in the coinfection of viruses and bacteria.
Decreased clearance and facilitated bacterial penetration.
Mucus contains many components that bind bacteria. When viruses impair mucociliary clearance mechanisms, bacteria can attach to mucins and colonize the airway epithelium. Furthermore, penetration of immune cells and antibacterial substances into the thickened mucus is decreased (317). This is illustrated by studies showing that influenza virus infection results in decreased tracheal mucociliary velocity and clearance of S. pneumoniae (172, 213).
In addition, perturbation of the epithelial barrier upon virus infection may increase paracellular permeability and facilitate translocation of bacteria and their soluble products into the submucosa. For instance, rhinoviruses disrupt airway epithelial barrier functions and promote the paracellular migration of bacteria such as H. influenzae (244).
Virus-induced bacterial adherence.
Early studies demonstrated increased susceptibility of mammalian cells to bacterial adherence as a result of viral infection in vitro (60, 251) and in vivo (131, 204, 213, 215, 250). Adherence of S. aureus and Bordetella pertussis to Hep-2 (human epidermoid cancer) epithelial cells was found in greater quantities for cells infected with RSV than for noninfected cells (240, 241), and similar observations were made with airway epithelial cells. RSV was shown to induce Pseudomonas aeruginosa, S. pneumoniae, and H. influenzae adherence to airway epithelial cells (12, 70, 95, 292), and increased pneumococcal adherence was found after prior adenovirus or rhinovirus infection of airway epithelial cells (93, 302). Interestingly, rhinovirus preinfection enhanced staphylococcal and streptococcal adherence, whereas measles virus and adenovirus infection decreased adherence of both bacterial species, suggesting a virus-specific induced change on the host cell membrane to which all bacteria adhered in the same way (96, 259, 302). Increased bacterial adherence facilitates biofilm formation and could represent a critical step in permanent airway colonization by P. aeruginosa in cystic fibrosis patients (98). In addition, respiratory viruses such as rhinovirus, RSV, and influenza virus have been shown to increase pneumococcal biofilm formation (187).
Several mechanisms have been shown to participate in virus-induced bacterial adherence to the airway epithelium (46). First of all, physical damage to epithelial cells leads to increased bacterial adherence to injured cells (288), as shown by increased adherence of P. aeruginosa to injured tracheal cells during systemic influenza virus infection (224). In addition, virus-induced cell death may expose new receptors for bacterial adherence or impair mechanical removal of attached pathogens (30, 41, 96). Furthermore, increased epithelium-bacterium interactions have been demonstrated upon viral infection. Bacteria adhere to various host cell molecules on the airway epithelium, including platelet-activating factor receptor (PAF-r), outer membrane protein P5-homologous fimbriae (P5 fimbriae), carcinoembryonic antigen-related cellular adhesion 1 (CEACAM-1), and intercellular adhesion molecule 1 (ICAM-1) (37, 124, 251). Respiratory viruses modulate the expression of these receptors, resulting in increased bacterial adherence. For example, rhinovirus increases the binding of S. pneumoniae to bronchial epithelial cells by upregulating expression of PAF-r (119). It also increases the expression of ICAM-1, which serves as a receptor both for itself and for H. influenzae (11, 316). Mechanisms responsible for the increased adherence to respiratory epithelial cells differ between viruses and also between cell types. For instance, whereas RSV upregulates cellular receptors such as ICAM-1 and CEACAM-1, leading to an increase of bacterial adherence, influenza virus has no effect on the expression of these receptors (11). Respiratory viruses can also expose bacterial receptors through direct action on the airway epithelial cell surface. The influenza virus neuraminidase protein promotes adherence and invasion of S. pneumoniae through cleavage of sialic acid from the surfaces of host cells, thus exposing receptors for pneumococci (208). Neuraminidase inhibitors such as oseltamivir and zanamivir, which are used against influenza virus, may therefore have effects that extend beyond inhibition of viral replication, but this has not been demonstrated formally.
Mucins also play an important role in bacterial adherence and act as receptors for various bacteria, including P. aeruginosa (228), H. influenzae (39), and S. aureus (262). RSV increases H. influenzae adherence to airway epithelial cells through induction of MUC5AC and MUC1 gene expression (11). In addition, molecules present in the extracellular matrix, which may be exposed through disruption of the epithelial barrier, can also serve to bind bacteria. Fibrinogen, for instance, enhances adherence of group A streptococcus to influenza A virus-infected cells (249). Finally, respiratory viruses interact with TLR pathways, resulting in a prolonged bacterial load in the lung. Impairment of TLR4 and TLR5 has been demonstrated after influenza virus infection, leading to reduced neutrophil recruitment and increased P. aeruginosa and S. pneumoniae adherence in airway epithelial cells (46).
Thus, in addition to their direct pathogenic effects, viruses can promote bacterial infections of the lung.
Therapeutic Applications for the Wounded Soldier
Several processes that are instrumental in producing epithelial damage, delayed or abnormal epithelial repair, altered immune signaling, or mediator production resulting from virus infection and subsequent bacterial adherence and infection (the wounded soldier) may be amenable to therapeutic interventions. For example, steroids are well established as suppressors of viral (56, 268, 296) and bacterial (100) inflammation and mucin production and may modulate epithelial damage, injury, and repair. A combined steroid-β2 agonist combination may enhance this anti-inflammatory effect. β2 agonists have been shown to reduce bacterium- and toxin-induced epithelial cell damage in models of nasal epithelial culture (53, 54). While steroids and β2 agonists may suppress inflammation and bacterium-induced damage in vitro, one area of interest is the net effect of these treatments on the host defense to respiratory pathogens in vivo. Also of note is the recognized disappointing or incomplete protection of steroid-based therapies in asthma exacerbations (64, 197, 295) which are of viral etiology. Whether this is due to steroids suppressing beneficial host responses remains to be explored fully.
Leukotriene receptor antagonists have also been used as treatments for virus-induced inflammation. While a recent study demonstrated that children admitted to hospital with asthma-related symptoms were less likely to have used therapies, including leukotriene receptor antagonists (127), a recent study found no protective effect of the above in a trial of post-RSV bronchiolitis in children (23). Macrolide antibiotics represent another possible therapeutic application for virus-induced inflammation and epithelial damage. A recent study demonstrated effectiveness of the ketomacrolide telithromycin in asthma exacerbations (128). The proposition that macrolides may have antiviral as well as anti-inflammatory and antibacterial effects is also interesting. By use of in vitro culture systems, azithromycin was able to augment rhinovirus-induced IFNs and their inducible genes and to decrease virus replication (81).
CONCLUSIONS
vRTIs are an important cause of morbidity and, in some cases, mortality. They lead to more than 400,000 hospitalizations per year among children in the United States and are therefore a major burden on the health care system (105, 180, 194).
The airway epithelium plays a central role in the first line of defense in the fight against respiratory virus infections. Through its physical barrier and immune functions, it efficiently contributes to viral clearance. However, respiratory viruses have evolved strategies to subvert epithelial defense mechanisms and can lead to complications such as loss of epithelial integrity, perturbed inflammatory responses, increased mucus production, and secondary bacterial infections. A thorough understanding of the mechanisms by which viruses interfere with epithelial innate and adaptive immune responses might contribute to the design of new therapeutic tools to treat or prevent respiratory disorders caused by viral infections.
Acknowledgments
We thank Helen Tinkler for text amendment in English.
Biography
 Marjolaine Vareille obtained a Ph.D. in Immunology in 2008 at the University of Clermont-Ferrand, France. She became a postdoctoral researcher at the Division of Pediatric Respiratory Medicine of the University of Bern, Switzerland. Her research focused on the interactions between rhinoviruses and airway epithelial cells in cystic fibrosis lung disease. Since May 2010, she has been a lecturer at the University of Clermont-Ferrand and works on the interaction between tumor cells and dendritic cells during breast cancer.
Marjolaine Vareille obtained a Ph.D. in Immunology in 2008 at the University of Clermont-Ferrand, France. She became a postdoctoral researcher at the Division of Pediatric Respiratory Medicine of the University of Bern, Switzerland. Her research focused on the interactions between rhinoviruses and airway epithelial cells in cystic fibrosis lung disease. Since May 2010, she has been a lecturer at the University of Clermont-Ferrand and works on the interaction between tumor cells and dendritic cells during breast cancer.
 Elisabeth Kieninger received her M.D. from the University of Graz, Austria, in 2008. Since January 2009, she has been a Ph.D. student in the group of Nicolas Regamey at the Division of Pediatric Respiratory Medicine of the University of Bern, Switzerland. Her research focuses on the inflammatory response upon viral infection in cystic fibrosis lung disease.
Elisabeth Kieninger received her M.D. from the University of Graz, Austria, in 2008. Since January 2009, she has been a Ph.D. student in the group of Nicolas Regamey at the Division of Pediatric Respiratory Medicine of the University of Bern, Switzerland. Her research focuses on the inflammatory response upon viral infection in cystic fibrosis lung disease.
 Michael R. Edwards received his Ph.D. in 2001 from the University of New South Wales, Sydney, Australia, for research regarding IgE antibody expression, and then joined Professor Sebastian Johnston's group at Imperial College London as a postdoctoral research associate. In 2007, Dr. Edwards obtained a fellowship from Asthma UK to investigate potential molecular mechanisms of deficient interferon responses in asthmatic bronchial epithelial cells, and he has attracted research funding from Asthma UK, the British Lung Foundation, GlaxoSmithKline, and the MRC. His research interests also include pattern recognition in viral infections, signaling and transcriptional events leading to virus-induced inflammatory molecules, and interferon gene expression.
Michael R. Edwards received his Ph.D. in 2001 from the University of New South Wales, Sydney, Australia, for research regarding IgE antibody expression, and then joined Professor Sebastian Johnston's group at Imperial College London as a postdoctoral research associate. In 2007, Dr. Edwards obtained a fellowship from Asthma UK to investigate potential molecular mechanisms of deficient interferon responses in asthmatic bronchial epithelial cells, and he has attracted research funding from Asthma UK, the British Lung Foundation, GlaxoSmithKline, and the MRC. His research interests also include pattern recognition in viral infections, signaling and transcriptional events leading to virus-induced inflammatory molecules, and interferon gene expression.
 Nicolas Regamey received his M.D. at the University of Basel, Switzerland, in 1995. He trained in pediatrics at the university hospitals of Basel and Bern, Switzerland (1996 to 2000), and subspecialized in respiratory medicine at the University of Bern (2001 to 2004). After a fellowship at the Royal Brompton Hospital and Imperial College, London, United Kingdom (2005 to 2007), he moved back to Switzerland to his current post as assistant professor at the Division of Pediatric Respiratory Medicine of the University of Bern. Since graduation as a medical doctor, Professor Regamey has had a particular interest in virology. His research focuses on the role of respiratory viruses in the development and progression of chronic airway diseases, including asthma and cystic fibrosis.
Nicolas Regamey received his M.D. at the University of Basel, Switzerland, in 1995. He trained in pediatrics at the university hospitals of Basel and Bern, Switzerland (1996 to 2000), and subspecialized in respiratory medicine at the University of Bern (2001 to 2004). After a fellowship at the Royal Brompton Hospital and Imperial College, London, United Kingdom (2005 to 2007), he moved back to Switzerland to his current post as assistant professor at the Division of Pediatric Respiratory Medicine of the University of Bern. Since graduation as a medical doctor, Professor Regamey has had a particular interest in virology. His research focuses on the role of respiratory viruses in the development and progression of chronic airway diseases, including asthma and cystic fibrosis.
REFERENCES
- 1.Adamko, D. J., B. L. Yost, G. J. Gleich, A. D. Fryer, and D. B. Jacoby. 1999. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med. 190:1465-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnello, D., C. S. Lankford, J. Bream, A. Morinobu, M. Gadina, J. J. O'Shea, and D. M. Frucht. 2003. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J. Clin. Immunol. 23:147-161. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S. 2009. Pathogen recognition by innate immunity and its signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 85:143-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allakhverdi, Z., M. R. Comeau, H. K. Jessup, B. R. Yoon, A. Brewer, S. Chartier, N. Paquette, S. F. Ziegler, M. Sarfati, and G. Delespesse. 2007. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 204:253-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen, I. C., M. A. Scull, C. B. Moore, E. K. Holl, E. McElvania-TeKippe, D. J. Taxman, E. H. Guthrie, R. J. Pickles, and J. P. Ting. 2009. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30:556-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alwan, W. H., W. J. Kozlowska, and P. J. Openshaw. 1994. Distinct types of lung disease caused by functional subsets of antiviral T cells. J. Exp. Med. 179:81-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin, K., D. Lúdvíksdóttir, C. Janson, O. Nettelbladt, E. Björnsson, G. M. Roomans, G. Boman, L. Sevéus, and P. Venge. 2000. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. BHR Group. Am. J. Respir. Crit. Care Med. 162:2295-2301. [DOI] [PubMed] [Google Scholar]
- 8.Ank, N., and S. R. Paludan. 2009. Type III IFNs: new layers of complexity in innate antiviral immunity. Biofactors 35:82-87. [DOI] [PubMed] [Google Scholar]
- 9.Ank, N., H. West, and S. R. Paludan. 2006. IFN-lambda: novel antiviral cytokines. J. Interferon Cytokine Res. 26:373-379. [DOI] [PubMed] [Google Scholar]
- 10.Arden, K. E., C. E. Faux, N. T. O'Neill, P. McErlean, A. Nitsche, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2010. Molecular characterization and distinguishing features of a novel human rhinovirus (HRV) C, HRVC-QCE, detected in children with fever, cough and wheeze during 2003. J. Clin. Virol. 47:219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avadhanula, V., C. A. Rodriguez, J. P. Devincenzo, Y. Wang, R. J. Webby, G. C. Ulett, and E. E. Adderson. 2006. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J. Virol. 80:1629-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avadhanula, V., Y. Wang, A. Portner, and E. Adderson. 2007. Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein. J. Med. Microbiol. 56:1133-1137. [DOI] [PubMed] [Google Scholar]
- 13.Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 22:59-71. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett, N. W., R. P. Walton, M. R. Edwards, J. Aniscenko, G. Caramori, J. Zhu, N. Glanville, K. J. Choy, P. Jourdan, J. Burnet, T. J. Tuthill, M. S. Pedrick, M. J. Hurle, C. Plumpton, N. A. Sharp, J. N. Bussell, D. M. Swallow, J. Schwarze, B. Guy, J. W. Almond, P. K. Jeffery, C. M. Lloyd, A. Papi, R. A. Killington, D. J. Rowlands, E. D. Blair, N. J. Clarke, and S. L. Johnston. 2008. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat. Med. 14:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basbaum, C. 2002. Mucus hypersecretion in respiratory disease. Chair's introduction. Novartis Found. Symp. 248:1-2. [PubMed] [Google Scholar]
- 16.Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 21:305-337. [DOI] [PubMed] [Google Scholar]
- 17.Bellani, G., L. Guerra, A. Pesenti, and C. Messa. 2010. Imaging of lung inflammation during severe influenza A: H1N1. Intensive Care Med. 36:717-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergelson, J. M. 2009. Intercellular junctional proteins as receptors and barriers to virus infection and spread. Cell Host Microbe 5:517-521. [DOI] [PubMed] [Google Scholar]
- 19.Bergelson, J. M. 2003. Virus interactions with mucosal surfaces: alternative receptors, alternative pathways. Curr. Opin. Microbiol. 6:386-391. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann, M., A. Garcia-Sastre, E. Carnero, H. Pehamberger, K. Wolff, P. Palese, and T. Muster. 2000. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J. Virol. 74:6203-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bermejo-Martin, J. F., R. Ortiz de Lejarazu, T. Pumarola, J. Rello, R. Almansa, P. Ramirez, I. Martin-Loeches, D. Varillas, M. C. Gallegos, C. Seron, D. Micheloud, J. M. Gomez, A. Tenorio-Abreu, M. J. Ramos, M. L. Molina, S. Huidobro, E. Sanchez, M. Gordon, V. Fernandez, A. Del Castillo, M. A. Marcos, B. Villanueva, C. J. Lopez, M. Rodriguez-Dominguez, J. C. Galan, R. Canton, A. Lietor, S. Rojo, J. M. Eiros, C. Hinojosa, I. Gonzalez, N. Torner, D. Banner, A. Leon, P. Cuesta, T. Rowe, and D. J. Kelvin. 2009. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care 13:R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 23.Bisgaard, H., A. Flores-Nunez, A. Goh, P. Azimi, A. Halkas, M. P. Malice, J. L. Marchal, S. B. Dass, T. F. Reiss, and B. A. Knorr. 2008. Study of montelukast for the treatment of respiratory symptoms of post-respiratory syncytial virus bronchiolitis in children. Am. J. Respir. Crit. Care Med. 178:854-860. [DOI] [PubMed] [Google Scholar]
- 24.Bitko, V., A. Velazquez, L. Yang, Y. C. Yang, and S. Barik. 1997. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-kappa B and is inhibited by sodium salicylate and aspirin. Virology 232:369-378. [DOI] [PubMed] [Google Scholar]
- 25.Blaise, G. A., D. Gauvin, M. Gangal, and S. Authier. 2005. Nitric oxide, cell signaling and cell death. Toxicology 208:177-192. [DOI] [PubMed] [Google Scholar]
- 26.Bochkov, Y. A., K. M. Hanson, S. Keles, R. A. Brockman-Schneider, N. N. Jarjour, and J. E. Gern. 2009. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol. 3:69-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossios, A., S. Psarras, D. Gourgiotis, C. L. Skevaki, A. G. Constantopoulos, P. Saxoni-Papageorgiou, and N. G. Papadopoulos. 2005. Rhinovirus infection induces cytotoxicity and delays wound healing in bronchial epithelial cells. Respir. Res. 6:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boukhvalova, M. S., G. A. Prince, L. Soroush, D. C. Harrigan, S. N. Vogel, and J. C. Blanco. 2006. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine 24:5027-5035. [DOI] [PubMed] [Google Scholar]
- 29.Bousquet, J., P. K. Jeffery, W. W. Busse, M. Johnson, and A. M. Vignola. 2000. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am. J. Respir. Crit. Care Med. 161:1720-1745. [DOI] [PubMed] [Google Scholar]
- 30.Bragonzi, A., E. Copreni, S. de Bentzmann, M. Ulrich, and M. Conese. 2004. Airway epithelial cell-pathogen interactions. J. Cyst. Fibros. 3(Suppl. 2):197-201. [DOI] [PubMed] [Google Scholar]
- 31.Caramori, G., K. Ito, M. Contoli, A. Di Stefano, S. L. Johnston, I. M. Adcock, and A. Papi. 2006. Molecular mechanisms of respiratory virus-induced asthma and COPD exacerbations and pneumonia. Curr. Med. Chem. 13:2267-2290. [DOI] [PubMed] [Google Scholar]
- 31a.Centers for Disease Control and Prevention. 2009. Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May-August 2009. MMWR Morb. Mortal. Wkly. Rep. 58:1071-1074. [PubMed] [Google Scholar]
- 31b.Centers for Disease Control and Prevention. 2009. Hospitalized patients with novel influenza A (H1N1) virus infection—California, April-May, 2009. MMWR Morb. Mortal. Wkly. Rep. 58:536-541. [PubMed] [Google Scholar]
- 31c.Centers for Disease Control and Prevention. 2009. Intensive-care patients with severe novel influenza A (H1N1) virus infection—Michigan, June 2009. MMWR Morb. Mortal. Wkly. Rep. 58:749-752. [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Chilvers, M. A., and C. O'Callaghan. 2000. Local mucociliary defence mechanisms. Paediatr. Respir. Rev. 1:27-34. [DOI] [PubMed] [Google Scholar]
- 34.Collinson, J., K. G. Nicholson, E. Cancio, J. Ashman, D. C. Ireland, V. Hammersley, J. Kent, and C. O'Callaghan. 1996. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax 51:1115-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Contoli, M., S. D. Message, V. Laza-Stanca, M. R. Edwards, P. A. Wark, N. W. Bartlett, T. Kebadze, P. Mallia, L. A. Stanciu, H. L. Parker, L. Slater, A. Lewis-Antes, O. M. Kon, S. T. Holgate, D. E. Davies, S. V. Kotenko, A. Papi, and S. L. Johnston. 2006. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat. Med. 12:1023-1026. [DOI] [PubMed] [Google Scholar]
- 36.Copenhaver, C. C., J. E. Gern, Z. Li, P. A. Shult, L. A. Rosenthal, L. D. Mikus, C. J. Kirk, K. A. Roberg, E. L. Anderson, C. J. Tisler, D. F. DaSilva, H. J. Hiemke, K. Gentile, R. E. Gangnon, and R. F. Lemanske, Jr. 2004. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am. J. Respir. Crit. Care Med. 170:175-180. [DOI] [PubMed] [Google Scholar]
- 37.Cundell, D. R., N. P. Gerard, C. Gerard, I. Idanpaan-Heikkila, and E. I. Tuomanen. 1995. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377:435-438. [DOI] [PubMed] [Google Scholar]
- 38.Davies, D. E., J. Wicks, R. M. Powell, S. M. Puddicombe, and S. T. Holgate. 2003. Airway remodeling in asthma: new insights. J. Allergy Clin. Immunol. 111:215-225. [DOI] [PubMed] [Google Scholar]
- 39.Davies, J., I. Carlstedt, A. K. Nilsson, A. Hakansson, H. Sabharwal, L. van Alphen, M. van Ham, and C. Svanborg. 1995. Binding of Haemophilus influenzae to purified mucins from the human respiratory tract. Infect. Immun. 63:2485-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies, J. A., and D. R. Garrod. 1997. Molecular aspects of the epithelial phenotype. Bioessays 19:699-704. [DOI] [PubMed] [Google Scholar]
- 41.de Bentzmann, S., C. Plotkowski, and E. Puchelle. 1996. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am. J. Respir. Crit. Care Med. 154:S155-S162. [DOI] [PubMed] [Google Scholar]
- 42.de Gouw, H. W., K. Grunberg, R. Schot, A. C. Kroes, E. C. Dick, and P. J. Sterk. 1998. Relationship between exhaled nitric oxide and airway hyperresponsiveness following experimental rhinovirus infection in asthmatic subjects. Eur. Respir. J. 11:126-132. [DOI] [PubMed] [Google Scholar]
- 43.de Jong, M. D., C. P. Simmons, T. T. Thanh, V. M. Hien, G. J. Smith, T. N. Chau, D. M. Hoang, N. V. Chau, T. H. Khanh, V. C. Dong, P. T. Qui, B. V. Cam, Q. Ha Do, Y. Guan, J. S. Peiris, N. T. Chinh, T. T. Hien, and J. Farrar. 2006. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 12:1203-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Serres, G., N. Lampron, J. La Forge, I. Rouleau, J. Bourbeau, K. Weiss, B. Barret, and G. Boivin. 2009. Importance of viral and bacterial infections in chronic obstructive pulmonary disease exacerbations. J. Clin. Virol. 46:129-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27-38. [DOI] [PubMed] [Google Scholar]
- 46.Didierlaurent, A., J. Goulding, S. Patel, R. Snelgrove, L. Low, M. Bebien, T. Lawrence, L. S. van Rijt, B. N. Lambrecht, J. C. Sirard, and T. Hussell. 2008. Sustained desensitization to bacterial Toll-like receptor ligands after resolution of respiratory influenza infection. J. Exp. Med. 205:323-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dinwiddie, D. L., and K. S. Harrod. 2008. Human metapneumovirus inhibits IFN-alpha signaling through inhibition of STAT1 phosphorylation. Am. J. Respir. Cell. Mol. Biol. 38:661-670. [DOI] [PubMed] [Google Scholar]
- 48.Doherty, T., and D. Broide. 2007. Cytokines and growth factors in airway remodeling in asthma. Curr. Opin. Immunol. 19:676-680. [DOI] [PubMed] [Google Scholar]
- 49.Domachowske, J. B., K. D. Dyer, A. G. Adams, T. L. Leto, and H. F. Rosenberg. 1998. Eosinophil cationic protein/RNase 3 is another RNase A-family ribonuclease with direct antiviral activity. Nucleic Acids Res. 26:3358-3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donnelly, R. P., F. Sheikh, S. V. Kotenko, and H. Dickensheets. 2004. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukoc. Biol. 76:314-321. [DOI] [PubMed] [Google Scholar]
- 51.Dosanjh, A., S. Rednam, and M. Martin. 2003. Respiratory syncytial virus augments production of fibroblast growth factor basic in vitro: implications for a possible mechanism of prolonged wheezing after infection. Pediatr. Allergy Immunol. 14:437-440. [DOI] [PubMed] [Google Scholar]
- 52.Doss, M., M. R. White, T. Tecle, D. Gantz, E. C. Crouch, G. Jung, P. Ruchala, A. J. Waring, R. I. Lehrer, and K. L. Hartshorn. 2009. Interactions of alpha-, beta-, and theta-defensins with influenza A virus and surfactant protein D. J. Immunol. 182:7878-7887. [DOI] [PubMed] [Google Scholar]
- 53.Dowling, R. B., M. Johnson, P. J. Cole, and R. Wilson. 1998. Effect of salmeterol on Haemophilus influenzae infection of respiratory mucosa in vitro. Eur. Respir. J. 11:86-90. [DOI] [PubMed] [Google Scholar]
- 54.Dowling, R. B., C. F. Rayner, A. Rutman, A. D. Jackson, K. Kanthakumar, A. Dewar, G. W. Taylor, P. J. Cole, M. Johnson, and R. Wilson. 1997. Effect of salmeterol on Pseudomonas aeruginosa infection of respiratory mucosa. Am. J. Respir. Crit. Care Med. 155:327-336. [DOI] [PubMed] [Google Scholar]
- 55.Duits, L. A., P. H. Nibbering, E. van Strijen, J. B. Vos, S. P. Mannesse-Lazeroms, M. A. van Sterkenburg, and P. S. Hiemstra. 2003. Rhinovirus increases human beta-defensin-2 and -3 mRNA expression in cultured bronchial epithelial cells. FEMS Immunol. Med. Microbiol. 38:59-64. [DOI] [PubMed] [Google Scholar]
- 56.Edwards, M. R., M. W. Johnson, and S. L. Johnston. 2006. Combination therapy: synergistic suppression of virus-induced chemokines in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 34:616-624. [DOI] [PubMed] [Google Scholar]
- 57.Edwards, M. R., T. Kebadze, M. W. Johnson, and S. L. Johnston. 2006. New treatment regimes for virus-induced exacerbations of asthma. Pulm. Pharmacol. Ther. 19:320-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El Feghaly, R. E., L. McGann, C. A. Bonville, P. J. Branigan, M. Suryadevera, H. F. Rosenberg, and J. B. Domachowske. 2010. Local production of inflammatory mediators during childhood parainfluenza virus infection. Pediatr. Infect. Dis. J. 29:e26-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esposito, E., and S. Cuzzocrea. 2007. The role of nitric oxide synthases in lung inflammation. Curr. Opin. Invest. Drugs 8:899-909. [PubMed] [Google Scholar]
- 60.Fainstein, V., D. M. Musher, and T. R. Cate. 1980. Bacterial adherence to pharyngeal cells during viral infection. J. Infect. Dis. 141:172-176. [DOI] [PubMed] [Google Scholar]
- 61.Fasano, M. B. 2010. Combined airways: impact of upper airway on lower airway. Curr. Opin. Otolaryngol. Head Neck Surg. 18:15-20. [DOI] [PubMed] [Google Scholar]
- 62.Fichorova, R. N., A. O. Cronin, E. Lien, D. J. Anderson, and R. R. Ingalls. 2002. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J. Immunol. 168:2424-2432. [DOI] [PubMed] [Google Scholar]
- 63.Finberg, R. W., J. P. Wang, and E. A. Kurt-Jones. 2007. Toll like receptors and viruses. Rev. Med. Virol. 17:35-43. [DOI] [PubMed] [Google Scholar]
- 64.Fitzgerald, J. M., A. Becker, M. R. Sears, S. Mink, K. Chung, and J. Lee. 2004. Doubling the dose of budesonide versus maintenance treatment in asthma exacerbations. Thorax 59:550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fokkens, W. J., and R. A. Scheeren. 2000. Upper airway defence mechanisms. Paediatr. Respir. Rev. 1:336-341. [DOI] [PubMed] [Google Scholar]
- 66.Folkerts, G., W. W. Busse, F. P. Nijkamp, R. Sorkness, and J. E. Gern. 1998. Virus-induced airway hyperresponsiveness and asthma. Am. J. Respir. Crit. Care Med. 157:1708-1720. [DOI] [PubMed] [Google Scholar]
- 67.Folkerts, G., H. J. van der Linde, and F. P. Nijkamp. 1995. Virus-induced airway hyperresponsiveness in guinea pigs is related to a deficiency in nitric oxide. J. Clin. Invest. 95:26-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fort, M. M., J. Cheung, D. Yen, J. Li, S. M. Zurawski, S. Lo, S. Menon, T. Clifford, B. Hunte, R. Lesley, T. Muchamuel, S. D. Hurst, G. Zurawski, M. W. Leach, D. M. Gorman, and D. M. Rennick. 2001. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15:985-995. [DOI] [PubMed] [Google Scholar]
- 69.Fraenkel, D. J., P. G. Bardin, G. Sanderson, F. Lampe, S. L. Johnston, and S. T. Holgate. 1995. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am. J. Respir. Crit. Care Med. 151:879-886. [DOI] [PubMed] [Google Scholar]
- 70.Fukasawa, C., N. Ishiwada, J. Ogita, H. Hishiki, and Y. Kohno. 2009. The effects of disodium cromoglycate on enhanced adherence of Haemophilus influenzae to A549 cells infected with respiratory syncytial virus. Pediatr. Res. 66:168-173. [DOI] [PubMed] [Google Scholar]
- 71.Gale, M., Jr., and G. C. Sen. 2009. Viral evasion of the interferon system. J. Interferon Cytokine Res. 29:475-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gambotto, A., S. M. Barratt-Boyes, M. D. de Jong, G. Neumann, and Y. Kawaoka. 2008. Human infection with highly pathogenic H5N1 influenza virus. Lancet 371:1464-1475. [DOI] [PubMed] [Google Scholar]
- 73.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3:710-720. [DOI] [PubMed] [Google Scholar]
- 74.Garbino, J., S. Crespo, J. D. Aubert, T. Rochat, B. Ninet, C. Deffernez, W. Wunderli, J. C. Pache, P. M. Soccal, and L. Kaiser. 2006. A prospective hospital-based study of the clinical impact of non-severe acute respiratory syndrome (non-SARS)-related human coronavirus infection. Clin. Infect. Dis. 43:1009-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garcia, M. A., E. F. Meurs, and M. Esteban. 2007. The dsRNA protein kinase PKR: virus and cell control. Biochimie 89:799-811. [DOI] [PubMed] [Google Scholar]
- 76.Garofalo, R. P., J. Patti, K. A. Hintz, V. Hill, P. L. Ogra, and R. C. Welliver. 2001. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J. Infect. Dis. 184:393-399. [DOI] [PubMed] [Google Scholar]
- 77.Gaston, B., J. M. Drazen, J. Loscalzo, and J. S. Stamler. 1994. The biology of nitrogen oxides in the airways. Am. J. Respir. Crit. Care Med. 149:538-551. [DOI] [PubMed] [Google Scholar]
- 78.Gazit, R., R. Gruda, M. Elboim, T. I. Arnon, G. Katz, H. Achdout, J. Hanna, U. Qimron, G. Landau, E. Greenbaum, Z. Zakay-Rones, A. Porgador, and O. Mandelboim. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7:517-523. [DOI] [PubMed] [Google Scholar]
- 79.Gern, J. E. 2009. Rhinovirus and the initiation of asthma. Curr. Opin. Allergy Clin. Immunol. 9:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gern, J. E., R. Vrtis, K. A. Grindle, C. Swenson, and W. W. Busse. 2000. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am. J. Respir. Crit. Care Med. 162:2226-2231. [DOI] [PubMed] [Google Scholar]
- 81.Gielen, V., S. L. Johnston, and M. R. Edwards. 2010. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 36:646-654. [DOI] [PubMed] [Google Scholar]
- 82.Gil, J., and M. Esteban. 2000. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis 5:107-114. [DOI] [PubMed] [Google Scholar]
- 83.Gleich, G. J. 2000. Mechanisms of eosinophil-associated inflammation. J. Allergy Clin. Immunol. 105:651-663. [DOI] [PubMed] [Google Scholar]
- 84.Grayson, M. H., and M. J. Holtzman. 2007. Emerging role of dendritic cells in respiratory viral infection. J. Mol. Med. 85:1057-1068. [DOI] [PubMed] [Google Scholar]
- 85.Green, K. J., and C. L. Simpson. 2007. Desmosomes: new perspectives on a classic. J. Invest. Dermatol. 127:2499-2515. [DOI] [PubMed] [Google Scholar]
- 86.Greenberg, S. B. 2003. Respiratory consequences of rhinovirus infection. Arch. Intern. Med. 163:278-284. [DOI] [PubMed] [Google Scholar]
- 87.Greenberg, S. B. 2002. Respiratory viral infections in adults. Curr. Opin. Pulm. Med. 8:201-208. [DOI] [PubMed] [Google Scholar]
- 88.Greene, C. M., and N. G. McElvaney. 2005. Toll-like receptor expression and function in airway epithelial cells. Arch. Immunol. Ther. Exp. (Warsaw) 53:418-427. [PubMed] [Google Scholar]
- 89.Groskreutz, D. J., M. M. Monick, L. S. Powers, T. O. Yarovinsky, D. C. Look, and G. W. Hunninghake. 2006. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J. Immunol. 176:1733-1740. [DOI] [PubMed] [Google Scholar]
- 90.Guillot, L., R. Le Goffic, S. Bloch, N. Escriou, S. Akira, M. Chignard, and M. Si-Tahar. 2005. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571-5580. [DOI] [PubMed] [Google Scholar]
- 91.Guo, Z., L. M. Chen, H. Zeng, J. A. Gomez, J. Plowden, T. Fujita, J. M. Katz, R. O. Donis, and S. Sambhara. 2007. NS1 protein of influenza A virus inhibits the function of intracytoplasmic pathogen sensor, RIG-I. Am. J. Respir. Cell Mol. Biol. 36:263-269. [DOI] [PubMed] [Google Scholar]
- 92.Haeberle, H. A., R. Takizawa, A. Casola, A. R. Brasier, H. J. Dieterich, N. Van Rooijen, Z. Gatalica, and R. P. Garofalo. 2002. Respiratory syncytial virus-induced activation of nuclear factor-kappaB in the lung involves alveolar macrophages and Toll-like receptor 4-dependent pathways. J. Infect. Dis. 186:1199-1206. [DOI] [PubMed] [Google Scholar]
- 93.Hakansson, A., A. Kidd, G. Wadell, H. Sabharwal, and C. Svanborg. 1994. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect. Immun. 62:2707-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haller, O., and G. Kochs. 2002. Interferon-induced mx proteins: dynamin-like GTPases with antiviral activity. Traffic 3:710-717. [DOI] [PubMed] [Google Scholar]
- 95.Hament, J. M., P. C. Aerts, A. Fleer, H. van Dijk, T. Harmsen, J. L. Kimpen, and T. F. Wolfs. 2005. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr. Res. 58:1198-1203. [DOI] [PubMed] [Google Scholar]
- 96.Hament, J. M., J. L. Kimpen, A. Fleer, and T. F. Wolfs. 1999. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol. Med. Microbiol. 26:189-195. [DOI] [PubMed] [Google Scholar]
- 97.Hashimoto, K., B. S. Graham, S. B. Ho, K. B. Adler, R. D. Collins, S. J. Olson, W. Zhou, T. Suzutani, P. W. Jones, K. Goleniewska, J. F. O'Neal, and R. S. Peebles, Jr. 2004. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am. J. Respir. Crit. Care Med. 170:306-312. [DOI] [PubMed] [Google Scholar]
- 98.Hassett, D. J., T. R. Korfhagen, R. T. Irvin, M. J. Schurr, K. Sauer, G. W. Lau, M. D. Sutton, H. Yu, and N. Hoiby. 2010. Pseudomonas aeruginosa biofilm infections in cystic fibrosis: insights into pathogenic processes and treatment strategies. Expert Opin. Ther. Targets 14:117-130. [DOI] [PubMed] [Google Scholar]
- 99.Hauber, H. P., S. C. Foley, and Q. Hamid. 2006. Mucin overproduction in chronic inflammatory lung disease. Can. Respir. J. 13:327-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hauber, H. P., T. Goldmann, E. Vollmer, B. Wollenberg, and P. Zabel. 2007. Effect of dexamethasone and ACC on bacteria-induced mucin expression in human airway mucosa. Am. J. Respir. Cell Mol. Biol. 37:606-616. [DOI] [PubMed] [Google Scholar]
- 101.Hauber, H. P., M. K. Tulic, A. Tsicopoulos, B. Wallaert, R. Olivenstein, P. Daigneault, and Q. Hamid. 2005. Toll-like receptors 4 and 2 expression in the bronchial mucosa of patients with cystic fibrosis. Can. Respir. J. 12:13-18. [DOI] [PubMed] [Google Scholar]
- 102.Hayashi, S., and J. C. Hogg. 2007. Adenovirus infections and lung disease. Curr. Opin. Pharmacol. 7:237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haynes, L. M., D. D. Moore, E. A. Kurt-Jones, R. W. Finberg, L. J. Anderson, and R. A. Tripp. 2001. Involvement of Toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 75:10730-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.He, S. H., J. Zheng, and M. K. Duan. 2004. Induction of mucin secretion from human bronchial tissue and epithelial cells by rhinovirus and lipopolysaccharide. Acta Pharmacol. Sin. 25:1176-1181. [PubMed] [Google Scholar]
- 105.Henrickson, K. J., S. Hoover, K. S. Kehl, and W. Hua. 2004. National disease burden of respiratory viruses detected in children by polymerase chain reaction. Pediatr. Infect. Dis. J. 23:S11-S18. [DOI] [PubMed] [Google Scholar]
- 106.Herold, S., W. von Wulffen, M. Steinmueller, S. Pleschka, W. A. Kuziel, M. Mack, M. Srivastava, W. Seeger, U. A. Maus, and J. Lohmeyer. 2006. Alveolar epithelial cells direct monocyte transepithelial migration upon influenza virus infection: impact of chemokines and adhesion molecules. J. Immunol. 177:1817-1824. [DOI] [PubMed] [Google Scholar]
- 107.Hewson, C. A., A. Jardine, M. R. Edwards, V. Laza-Stanca, and S. L. Johnston. 2005. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J. Virol. 79:12273-12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hiemstra, P. S. 2001. Epithelial antimicrobial peptides and proteins: their role in host defence and inflammation. Paediatr. Respir. Rev. 2:306-310. [DOI] [PubMed] [Google Scholar]
- 109.Hogg, J. C. 2000. Chronic bronchitis: the role of viruses. Semin. Respir. Infect. 15:32-40. [DOI] [PubMed] [Google Scholar]
- 110.Holt, P. G., A. Degebrodt, C. O'Leary, K. Krska, and T. Plozza. 1985. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin. Exp. Immunol. 62:586-593. [PMC free article] [PubMed] [Google Scholar]
- 111.Holt, P. G., S. Haining, D. J. Nelson, and J. D. Sedgwick. 1994. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J. Immunol. 153:256-261. [PubMed] [Google Scholar]
- 112.Holt, P. G., J. Oliver, N. Bilyk, C. McMenamin, P. G. McMenamin, G. Kraal, and T. Thepen. 1993. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J. Exp. Med. 177:397-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holt, P. G., D. H. Strickland, M. E. Wikstrom, and F. L. Jahnsen. 2008. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 8:142-152. [DOI] [PubMed] [Google Scholar]
- 114.Hornsleth, A., B. Klug, M. Nir, J. Johansen, K. S. Hansen, L. S. Christensen, and L. B. Larsen. 1998. Severity of respiratory syncytial virus disease related to type and genotype of virus and to cytokine values in nasopharyngeal secretions. Pediatr. Infect. Dis. J. 17:1114-1121. [DOI] [PubMed] [Google Scholar]
- 115.Hurst, S. D., T. Muchamuel, D. M. Gorman, J. M. Gilbert, T. Clifford, S. Kwan, S. Menon, B. Seymour, C. Jackson, T. T. Kung, J. K. Brieland, S. M. Zurawski, R. W. Chapman, G. Zurawski, and R. L. Coffman. 2002. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 169:443-453. [DOI] [PubMed] [Google Scholar]
- 116.Husain, M., and K. S. Harrod. 2009. Influenza A virus-induced caspase-3 cleaves the histone deacetylase 6 in infected epithelial cells. FEBS Lett. 583:2517-2520. [DOI] [PubMed] [Google Scholar]
- 117.Hussell, T., A. Pennycook, and P. J. Openshaw. 2001. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. Eur. J. Immunol. 31:2566-2573. [DOI] [PubMed] [Google Scholar]
- 118.Ichinohe, T., H. K. Lee, Y. Ogura, R. Flavell, and A. Iwasaki. 2009. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ishizuka, S., M. Yamaya, T. Suzuki, H. Takahashi, S. Ida, T. Sasaki, D. Inoue, K. Sekizawa, H. Nishimura, and H. Sasaki. 2003. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J. Infect. Dis. 188:1928-1939. [DOI] [PubMed] [Google Scholar]
- 120.Iwamura, C., and T. Nakayama. 2008. Toll-like receptors in the respiratory system: their roles in inflammation. Curr. Allergy Asthma Rep. 8:7-13. [DOI] [PubMed] [Google Scholar]
- 121.Jacques, J., H. Moret, D. Minette, N. Leveque, N. Jovenin, G. Deslee, F. Lebargy, J. Motte, and L. Andreoletti. 2008. Epidemiological, molecular, and clinical features of enterovirus respiratory infections in French children between 1999 and 2005. J. Clin. Microbiol. 46:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jain, R., and R. D. Goldman. 2009. Novel influenza A(H1N1): clinical presentation, diagnosis, and management. Pediatr. Emerg. Care 25:791-796. [DOI] [PubMed] [Google Scholar]
- 123.Jamaluddin, M., R. Garofalo, P. L. Ogra, and A. R. Brasier. 1996. Inducible translational regulation of the NF-IL6 transcription factor by respiratory syncytial virus infection in pulmonary epithelial cells. J. Virol. 70:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jiang, Z., N. Nagata, E. Molina, L. O. Bakaletz, H. Hawkins, and J. A. Patel. 1999. Fimbria-mediated enhanced attachment of nontypeable Haemophilus influenzae to respiratory syncytial virus-infected respiratory epithelial cells. Infect. Immun. 67:187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin, Y., X. H. Yuan, Z. P. Xie, H. C. Gao, J. R. Song, R. F. Zhang, Z. Q. Xu, L. S. Zheng, Y. D. Hou, and Z. J. Duan. 2009. Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections. J. Clin. Microbiol. 47:2895-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johansen, H. K., and N. Hoiby. 1992. Seasonal onset of initial colonisation and chronic infection with Pseudomonas aeruginosa in patients with cystic fibrosis in Denmark. Thorax 47:109-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johnston, N. W., S. L. Johnston, J. M. Duncan, J. M. Greene, T. Kebadze, P. K. Keith, M. Roy, S. Waserman, and M. R. Sears. 2005. The September epidemic of asthma exacerbations in children: a search for etiology. J. Allergy Clin. Immunol. 115:132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Johnston, S. L., F. Blasi, P. N. Black, R. J. Martin, D. J. Farrell, and R. B. Nieman. 2006. The effect of telithromycin in acute exacerbations of asthma. N. Engl. J. Med. 354:1589-1600. [DOI] [PubMed] [Google Scholar]
- 129.Johnston, S. L., A. Papi, P. J. Bates, J. G. Mastronarde, M. M. Monick, and G. W. Hunninghake. 1998. Low grade rhinovirus infection induces a prolonged release of IL-8 in pulmonary epithelium. J. Immunol. 160:6172-6181. [PubMed] [Google Scholar]
- 130.Johnstone, J., S. R. Majumdar, J. D. Fox, and T. J. Marrie. 2008. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest 134:1141-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones, W. T., and J. H. Menna. 1982. Influenza type A virus-mediated adherence of type 1a group B streptococci to mouse tracheal tissue in vivo. Infect. Immun. 38:791-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kadowaki, N., S. Ho, S. Antonenko, R. W. Malefyt, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kahn, J. 2008. Human bocavirus: clinical significance and implications. Curr. Opin. Pediatr. 20:62-66. [DOI] [PubMed] [Google Scholar]
- 134.Kahn, J. S. 2006. Epidemiology of human metapneumovirus. Clin. Microbiol. Rev. 19:546-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kampf, C., A. J. Relova, S. Sandler, and G. M. Roomans. 1999. Effects of TNF-alpha, IFN-gamma and IL-beta on normal human bronchial epithelial cells. Eur. Respir. J. 14:84-91. [DOI] [PubMed] [Google Scholar]
- 136.Kao, Y. J., P. A. Piedra, G. L. Larsen, and G. N. Colasurdo. 2001. Induction and regulation of nitric oxide synthase in airway epithelial cells by respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 163:532-539. [DOI] [PubMed] [Google Scholar]
- 137.Kato, A., S. Favoreto, Jr., P. C. Avila, and R. P. Schleimer. 2007. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J. Immunol. 179:1080-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 139.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nat. Rev. Immunol. 2:675-687. [DOI] [PubMed] [Google Scholar]
- 140.Kawai, T., and S. Akira. 2007. TLR signaling. Semin. Immunol. 19:24-32. [DOI] [PubMed] [Google Scholar]
- 141.Kawai, T., and S. Akira. 2008. Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 1143:1-20. [DOI] [PubMed] [Google Scholar]
- 142.Kishimoto, T. K., M. A. Jutila, E. L. Berg, and E. C. Butcher. 1989. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science 245:1238-1241. [DOI] [PubMed] [Google Scholar]
- 143.Klotman, M. E., and T. L. Chang. 2006. Defensins in innate antiviral immunity. Nat. Rev. Immunol. 6:447-456. [DOI] [PubMed] [Google Scholar]
- 144.Knight, D. A., and S. T. Holgate. 2003. The airway epithelium: structural and functional properties in health and disease. Respirology 8:432-446. [DOI] [PubMed] [Google Scholar]
- 145.Knobil, K., A. M. Choi, G. W. Weigand, and D. B. Jacoby. 1998. Role of oxidants in influenza virus-induced gene expression. Am. J. Physiol. 274:L134-L142. [DOI] [PubMed] [Google Scholar]
- 146.Koetzler, R., R. S. Zaheer, R. Newton, and D. Proud. 2009. Nitric oxide inhibits IFN regulatory factor 1 and nuclear factor-kappaB pathways in rhinovirus-infected epithelial cells. J. Allergy Clin. Immunol. 124:551-557. [DOI] [PubMed] [Google Scholar]
- 147.Kohlmeier, J. E., and D. L. Woodland. 2009. Immunity to respiratory viruses. Annu. Rev. Immunol. 27:61-82. [DOI] [PubMed] [Google Scholar]
- 148.Kota, S., A. Sabbah, T. H. Chang, R. Harnack, Y. Xiang, X. Meng, and S. Bose. 2008. Role of human beta-defensin-2 during tumor necrosis factor-alpha/NF-kappaB-mediated innate antiviral response against human respiratory syncytial virus. J. Biol. Chem. 283:22417-22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kotenko, S. V., G. Gallagher, V. V. Baurin, A. Lewis-Antes, M. Shen, N. K. Shah, J. A. Langer, F. Sheikh, H. Dickensheets, and R. P. Donnelly. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69-77. [DOI] [PubMed] [Google Scholar]
- 150.Kuiken, T., and J. K. Taubenberger. 2008. Pathology of human influenza revisited. Vaccine 26(Suppl. 4):D59-D66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kumar, H., T. Kawai, and S. Akira. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388:621-625. [DOI] [PubMed] [Google Scholar]
- 152.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 153.Lambrecht, B. N., and H. Hammad. 2003. Taking our breath away: dendritic cells in the pathogenesis of asthma. Nat. Rev. Immunol. 3:994-1003. [DOI] [PubMed] [Google Scholar]
- 154.Lechapt-Zalcman, E., V. Pruliere-Escabasse, D. Advenier, S. Galiacy, C. Charriere-Bertrand, A. Coste, A. Harf, M. P. d'Ortho, and E. Escudier. 2006. Transforming growth factor-beta1 increases airway wound repair via MMP-2 upregulation: a new pathway for epithelial wound repair? Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L1277-L1282. [DOI] [PubMed] [Google Scholar]
- 155.Le Goffic, R., J. Pothlichet, D. Vitour, T. Fujita, E. Meurs, M. Chignard, and M. Si-Tahar. 2007. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 178:3368-3372. [DOI] [PubMed] [Google Scholar]
- 156.Leigh, R., W. Oyelusi, S. Wiehler, R. Koetzler, R. S. Zaheer, R. Newton, and D. Proud. 2008. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J. Allergy Clin. Immunol. 121:1238-1245. [DOI] [PubMed] [Google Scholar]
- 157.Lemanske, R. F., Jr., D. J. Jackson, R. E. Gangnon, M. D. Evans, Z. Li, P. A. Shult, C. J. Kirk, E. Reisdorf, K. A. Roberg, E. L. Anderson, K. T. Carlson-Dakes, K. J. Adler, S. Gilbertson-White, T. E. Pappas, D. F. Dasilva, C. J. Tisler, and J. E. Gern. 2005. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J. Allergy Clin. Immunol. 116:571-577. [DOI] [PubMed] [Google Scholar]
- 158.Levy, D. E., and A. Garcia-Sastre. 2001. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 12:143-156. [DOI] [PubMed] [Google Scholar]
- 159.Li, M., X. Liu, Y. Zhou, and S. B. Su. 2009. Interferon-lambdas: the modulators of antivirus, antitumor, and immune responses. J. Leukoc. Biol. 86:23-32. [DOI] [PubMed] [Google Scholar]
- 160.Li, W., and H. H. Shen. 2007. Effect of respiratory syncytial virus on the activity of matrix metalloproteinase in mice. Chin. Med. J. (England) 120:5-11. [PubMed] [Google Scholar]
- 161.Ling, Z., K. C. Tran, and M. N. Teng. 2009. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of beta interferon transcription by interacting with RIG-I. J. Virol. 83:3734-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lo, M. S., R. M. Brazas, and M. J. Holtzman. 2005. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J. Virol. 79:9315-9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lopez-Souza, N., S. Favoreto, H. Wong, T. Ward, S. Yagi, D. Schnurr, W. E. Finkbeiner, G. M. Dolganov, J. H. Widdicombe, H. A. Boushey, and P. C. Avila. 2009. In vitro susceptibility to rhinovirus infection is greater for bronchial than for nasal airway epithelial cells in human subjects. J. Allergy Clin. Immunol. 123:1384-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Louie, J. K., A. E. Kajon, M. Holodniy, L. Guardia-LaBar, B. Lee, A. M. Petru, J. K. Hacker, and D. P. Schnurr. 2008. Severe pneumonia due to adenovirus serotype 14: a new respiratory threat? Clin. Infect. Dis. 46:421-425. [DOI] [PubMed] [Google Scholar]
- 165.Lu, L. L., M. Puri, C. M. Horvath, and G. C. Sen. 2008. Select paramyxoviral V proteins inhibit IRF3 activation by acting as alternative substrates for inhibitor of kappaB kinase epsilon (IKKe)/TBK1. J. Biol. Chem. 283:14269-14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lukens, M. V., D. Kruijsen, F. E. Coenjaerts, J. L. Kimpen, and G. M. van Bleek. 2009. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J. Virol. 83:7235-7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mackay, I. M. 2008. Human rhinoviruses: the cold wars resume. J. Clin. Virol. 42:297-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mall, M. A. 2008. Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models. J. Aerosol Med. Pulm. Drug Deliv. 21:13-24. [DOI] [PubMed] [Google Scholar]
- 170.Mallia, P., M. Contoli, G. Caramori, A. Pandit, S. L. Johnston, and A. Papi. 2007. Exacerbations of asthma and chronic obstructive pulmonary disease (COPD): focus on virus induced exacerbations. Curr. Pharm. Des. 13:73-97. [DOI] [PubMed] [Google Scholar]
- 171.McCray, P. B., Jr., and L. Bentley. 1997. Human airway epithelia express a beta-defensin. Am. J. Respir. Cell Mol. Biol. 16:343-349. [DOI] [PubMed] [Google Scholar]
- 172.McCullers, J. A., A. R. Iverson, R. McKeon, and P. J. Murray. 2008. The platelet activating factor receptor is not required for exacerbation of bacterial pneumonia following influenza. Scand. J. Infect. Dis. 40:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McNamara, P. S., B. F. Flanagan, C. A. Hart, and R. L. Smyth. 2005. Production of chemokines in the lungs of infants with severe respiratory syncytial virus bronchiolitis. J. Infect. Dis. 191:1225-1232. [DOI] [PubMed] [Google Scholar]
- 174.McNamara, P. S., B. F. Flanagan, A. M. Selby, C. A. Hart, and R. L. Smyth. 2004. Pro- and anti-inflammatory responses in respiratory syncytial virus bronchiolitis. Eur. Respir. J. 23:106-112. [DOI] [PubMed] [Google Scholar]
- 175.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 176.Melmed, G., L. S. Thomas, N. Lee, S. Y. Tesfay, K. Lukasek, K. S. Michelsen, Y. Zhou, B. Hu, M. Arditi, and M. T. Abreu. 2003. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J. Immunol. 170:1406-1415. [DOI] [PubMed] [Google Scholar]
- 177.Mese, G., G. Richard, and T. W. White. 2007. Gap junctions: basic structure and function. J. Invest. Dermatol. 127:2516-2524. [DOI] [PubMed] [Google Scholar]
- 178.Message, S. D., and S. L. Johnston. 2004. Host defense function of the airway epithelium in health and disease: clinical background. J. Leukoc. Biol. 75:5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Message, S. D., V. Laza-Stanca, P. Mallia, H. L. Parker, J. Zhu, T. Kebadze, M. Contoli, G. Sanderson, O. M. Kon, A. Papi, P. K. Jeffery, L. A. Stanciu, and S. L. Johnston. 2008. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc. Natl. Acad. Sci. U. S. A. 105:13562-13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mizgerd, J. P. 2006. Lung infection—a public health priority. PLoS Med. 3:e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mogensen, T. H., and S. R. Paludan. 2001. Molecular pathways in virus-induced cytokine production. Microbiol. Mol. Biol. Rev. 65:131-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Reference deleted.
- 183.Reference deleted.
- 184.Reference deleted.
- 185.Morens, D. M., J. K. Taubenberger, and A. S. Fauci. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198:962-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mori, I., T. Komatsu, K. Takeuchi, K. Nakakuki, M. Sudo, and Y. Kimura. 1995. In vivo induction of apoptosis by influenza virus. J. Gen. Virol. 76:2869-2873. [DOI] [PubMed] [Google Scholar]
- 187.Morris, D. P. 2007. Bacterial biofilm in upper respiratory tract infections. Curr. Infect. Dis. Rep. 9:186-192. [DOI] [PubMed] [Google Scholar]
- 188.Muir, A., G. Soong, S. Sokol, B. Reddy, M. I. Gomez, A. Van Heeckeren, and A. Prince. 2004. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 30:777-783. [DOI] [PubMed] [Google Scholar]
- 189.Mukherjee, A., S. A. Morosky, L. Shen, C. R. Weber, J. R. Turner, K. S. Kim, T. Wang, and C. B. Coyne. 2009. Retinoic acid-induced gene-1 (RIG-I) associates with the actin cytoskeleton via caspase activation and recruitment domain-dependent interactions. J. Biol. Chem. 284:6486-6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Murawski, M. R., G. N. Bowen, A. M. Cerny, L. J. Anderson, L. M. Haynes, R. A. Tripp, E. A. Kurt-Jones, and R. W. Finberg. 2009. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J. Virol. 83:1492-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Murphy, A. W., T. A. Platts-Mills, M. Lobo, and F. Hayden. 1998. Respiratory nitric oxide levels in experimental human influenza. Chest 114:452-456. [DOI] [PubMed] [Google Scholar]
- 192.Muruve, D. A., V. Petrilli, A. K. Zaiss, L. R. White, S. A. Clark, P. J. Ross, R. J. Parks, and J. Tschopp. 2008. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452:103-107. [DOI] [PubMed] [Google Scholar]
- 193.Nagarkar, D. R., Q. Wang, J. Shim, Y. Zhao, W. C. Tsai, N. W. Lukacs, U. Sajjan, and M. B. Hershenson. 2009. CXCR2 is required for neutrophilic airway inflammation and hyperresponsiveness in a mouse model of human rhinovirus infection. J. Immunol. 183:6698-6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nair, H., D. J. Nokes, B. D. Gessner, M. Dherani, S. A. Madhi, R. J. Singleton, K. L. O'Brien, A. Roca, P. F. Wright, N. Bruce, A. Chandran, E. Theodoratou, A. Sutanto, E. R. Sedyaningsih, M. Ngama, P. K. Munywoki, C. Kartasasmita, E. A. Simoes, I. Rudan, M. W. Weber, and H. Campbell. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nicholas, B., P. Skipp, R. Mould, S. Rennard, D. E. Davies, C. D. O'Connor, and R. Djukanovic. 2006. Shotgun proteomic analysis of human-induced sputum. Proteomics 6:4390-4401. [DOI] [PubMed] [Google Scholar]
- 196.Novelli, F., and J. L. Casanova. 2004. The role of IL-12, IL-23 and IFN-gamma in immunity to viruses. Cytokine Growth Factor Rev. 15:367-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.O'Byrne, P. M., H. Bisgaard, P. P. Godard, M. Pistolesi, M. Palmqvist, Y. Zhu, T. Ekstrom, and E. D. Bateman. 2005. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am. J. Respir. Crit. Care Med. 171:129-136. [DOI] [PubMed] [Google Scholar]
- 198.Onomoto, K., M. Yoneyama, and T. Fujita. 2007. Regulation of antiviral innate immune responses by RIG-I family of RNA helicases. Curr. Top. Microbiol. Immunol. 316:193-205. [DOI] [PubMed] [Google Scholar]
- 199.Oshansky, C. M., W. Zhang, E. Moore, and R. A. Tripp. 2009. The host response and molecular pathogenesis associated with respiratory syncytial virus infection. Future Microbiol. 4:279-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Osterlund, P., J. Pirhonen, N. Ikonen, E. Ronkko, M. Strengell, S. M. Makela, M. Broman, O. J. Hamming, R. Hartmann, T. Ziegler, and I. Julkunen. 2010. Pandemic H1N1 2009 influenza A virus induces weak cytokine responses in human macrophages and dendritic cells and is highly sensitive to the antiviral actions of interferons. J. Virol. 84:1414-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Osterlund, P. I., T. E. Pietila, V. Veckman, S. V. Kotenko, and I. Julkunen. 2007. IFN regulatory factor family members differentially regulate the expression of type III IFN (IFN-lambda) genes. J. Immunol. 179:3434-3442. [DOI] [PubMed] [Google Scholar]
- 202.Papadopoulos, N. G., P. Xepapadaki, P. Mallia, G. Brusselle, J. B. Watelet, M. Xatzipsalti, G. Foteinos, C. M. van Drunen, W. J. Fokkens, C. D'Ambrosio, S. Bonini, A. Bossios, J. Lotvall, P. van Cauwenberge, S. T. Holgate, G. W. Canonica, A. Szczeklik, G. Rohde, J. Kimpen, A. Pitkaranta, M. Makela, P. Chanez, J. Ring, and S. L. Johnston. 2007. Mechanisms of virus-induced asthma exacerbations: state-of-the-art. A GA2LEN and InterAirways document. Allergy 62:457-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Papi, A., and S. L. Johnston. 1999. Rhinovirus infection induces expression of its own receptor intercellular adhesion molecule 1 (ICAM-1) via increased NF-kappaB-mediated transcription. J. Biol. Chem. 274:9707-9720. [DOI] [PubMed] [Google Scholar]
- 204.Patel, J., H. Faden, S. Sharma, and P. L. Ogra. 1992. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. Int. J. Pediatr. Otorhinolaryngol. 23:15-23. [DOI] [PubMed] [Google Scholar]
- 205.Peiris, J. S., C. Y. Cheung, C. Y. Leung, and J. M. Nicholls. 2009. Innate immune responses to influenza A H5N1: friend or foe? Trends Immunol. 30:574-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Peiris, J. S., W. C. Yu, C. W. Leung, C. Y. Cheung, W. F. Ng, J. M. Nicholls, T. K. Ng, K. H. Chan, S. T. Lai, W. L. Lim, K. Y. Yuen, and Y. Guan. 2004. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet 363:617-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Peltola, V. T., and J. A. McCullers. 2004. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr. Infect. Dis. J. 23:S87-S97. [DOI] [PubMed] [Google Scholar]
- 208.Peltola, V. T., K. G. Murti, and J. A. McCullers. 2005. Influenza virus neuraminidase contributes to secondary bacterial pneumonia. J. Infect. Dis. 192:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Perrone, L. A., J. K. Plowden, A. Garcia-Sastre, J. M. Katz, and T. M. Tumpey. 2008. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 4:e1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Peterhans, E. 1997. Oxidants and antioxidants in viral diseases: disease mechanisms and metabolic regulation. J. Nutr. 127:962S-965S. [DOI] [PubMed] [Google Scholar]
- 211.Petersen, N. T., N. Hoiby, C. H. Mordhorst, K. Lind, E. W. Flensborg, and B. Bruun. 1981. Respiratory infections in cystic fibrosis patients caused by virus, chlamydia and mycoplasma—possible synergism with Pseudomonas aeruginosa. Acta Paediatr. Scand. 70:623-628. [DOI] [PubMed] [Google Scholar]
- 212.Reference deleted.
- 213.Pittet, L. A., L. Hall-Stoodley, M. R. Rutkowski, and A. G. Harmsen. 2010. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 42:450-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Player, M. R., and P. F. Torrence. 1998. The 2-5A system: modulation of viral and cellular processes through acceleration of RNA degradation. Pharmacol. Ther. 78:55-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Plotkowski, M. C., E. Puchelle, G. Beck, J. Jacquot, and C. Hannoun. 1986. Adherence of type I Streptococcus pneumoniae to tracheal epithelium of mice infected with influenza A/PR8 virus. Am. Rev. Respir. Dis. 134:1040-1044. [DOI] [PubMed] [Google Scholar]
- 216.Potena, A., G. Caramori, P. Casolari, M. Contoli, S. L. Johnston, and A. Papi. 2007. Pathophysiology of viral-induced exacerbations of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2:477-483. [PMC free article] [PubMed] [Google Scholar]
- 217.Power, C. A., D. J. Church, A. Meyer, S. Alouani, A. E. Proudfoot, I. Clark-Lewis, S. Sozzani, A. Mantovani, and T. N. Wells. 1997. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J. Exp. Med. 186:825-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Proud, D., S. P. Sanders, and S. Wiehler. 2004. Human rhinovirus infection induces airway epithelial cell production of human beta-defensin 2 both in vitro and in vivo. J. Immunol. 172:4637-4645. [DOI] [PubMed] [Google Scholar]
- 219.Proud, D., R. B. Turner, B. Winther, S. Wiehler, J. P. Tiesman, T. D. Reichling, K. D. Juhlin, A. W. Fulmer, B. Y. Ho, A. A. Walanski, C. L. Poore, H. Mizoguchi, L. Jump, M. L. Moore, C. K. Zukowski, and J. W. Clymer. 2008. Gene expression profiles during in vivo human rhinovirus infection: insights into the host response. Am. J. Respir. Crit. Care Med. 178:962-968. [DOI] [PubMed] [Google Scholar]
- 220.Psarras, S., E. Volonaki, C. L. Skevaki, M. Xatzipsalti, A. Bossios, H. Pratsinis, S. Tsigkos, D. Gourgiotis, A. G. Constantopoulos, A. Papapetropoulos, P. Saxoni-Papageorgiou, and N. G. Papadopoulos. 2006. Vascular endothelial growth factor-mediated induction of angiogenesis by human rhinoviruses. J. Allergy Clin. Immunol. 117:291-297. [DOI] [PubMed] [Google Scholar]
- 221.Puchelle, E., J. M. Zahm, J. M. Tournier, and C. Coraux. 2006. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 3:726-733. [DOI] [PubMed] [Google Scholar]
- 222.Qi, B., Y. Huang, D. Rowe, and G. Halliday. 2007. VISA—a pass to innate immunity. Int. J. Biochem. Cell Biol. 39:287-291. [DOI] [PubMed] [Google Scholar]
- 223.Qiu, Y., J. Zhu, V. Bandi, K. K. Guntupalli, and P. K. Jeffery. 2007. Bronchial mucosal inflammation and upregulation of CXC chemoattractants and receptors in severe exacerbations of asthma. Thorax 62:475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ramphal, R., P. M. Small, J. W. Shands, Jr., W. Fischlschweiger, and P. A. Small, Jr. 1980. Adherence of Pseudomonas aeruginosa to tracheal cells injured by influenza infection or by endotracheal intubation. Infect. Immun. 27:614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ramshaw, I. A., A. J. Ramsay, G. Karupiah, M. S. Rolph, S. Mahalingam, and J. C. Ruby. 1997. Cytokines and immunity to viral infections. Immunol. Rev. 159:119-135. [DOI] [PubMed] [Google Scholar]
- 226.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1-47. [DOI] [PubMed] [Google Scholar]
- 227.Rate, A., J. W. Upham, A. Bosco, K. L. McKenna, and P. G. Holt. 2009. Airway epithelial cells regulate the functional phenotype of locally differentiating dendritic cells: implications for the pathogenesis of infectious and allergic airway disease. J. Immunol. 182:72-83. [DOI] [PubMed] [Google Scholar]
- 228.Reddy, M. S. 1992. Human tracheobronchial mucin: purification and binding to Pseudomonas aeruginosa. Infect. Immun. 60:1530-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Regamey, N., L. Kaiser, H. L. Roiha, C. Deffernez, C. E. Kuehni, P. Latzin, C. Aebi, and U. Frey. 2008. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr. Infect. Dis. J. 27:100-105. [DOI] [PubMed] [Google Scholar]
- 230.Regamey, N., C. Obregon, S. Ferrari-Lacraz, C. van Leer, M. Chanson, L. P. Nicod, and T. Geiser. 2007. Airway epithelial IL-15 transforms monocytes into dendritic cells. Am. J. Respir. Cell Mol. Biol. 37:75-84. [DOI] [PubMed] [Google Scholar]
- 231.Rehwinkel, J., and C. Reis e Sousa. 2010. RIGorous detection: exposing virus through RNA sensing. Science 327:284-286. [DOI] [PubMed] [Google Scholar]
- 232.Reibman, J., Y. Hsu, L. C. Chen, B. Bleck, and T. Gordon. 2003. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am. J. Respir. Cell Mol. Biol. 28:648-654. [DOI] [PubMed] [Google Scholar]
- 233.Reiss, C. S., and T. Komatsu. 1998. Does nitric oxide play a critical role in viral infections? J. Virol. 72:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Roche, W. R., S. Montefort, J. Baker, and S. T. Holgate. 1993. Cell adhesion molecules and the bronchial epithelium. Am. Rev. Respir. Dis. 148:S79-S82. [DOI] [PubMed] [Google Scholar]
- 235.Rogers, D. F. 2004. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr. Opin. Pharmacol. 4:241-250. [DOI] [PubMed] [Google Scholar]
- 236.Rogers, D. F. 2000. Mucus pathophysiology in COPD: differences to asthma, and pharmacotherapy. Monaldi Arch. Chest Dis. 55:324-332. [PubMed] [Google Scholar]
- 237.Rohde, G. 2009. Drug targets in rhinoviral infections. Infect. Disord. Drug Targets 9:126-132. [DOI] [PubMed] [Google Scholar]
- 238.Rose, M. C., T. J. Nickola, and J. A. Voynow. 2001. Airway mucus obstruction: mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am. J. Respir. Cell Mol. Biol. 25:533-537. [DOI] [PubMed] [Google Scholar]
- 239.Rose, M. C., and J. A. Voynow. 2006. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 86:245-278. [DOI] [PubMed] [Google Scholar]
- 240.Saadi, A. T., C. C. Blackwell, S. D. Essery, M. W. Raza, O. R. el Ahmer, D. A. MacKenzie, V. S. James, D. M. Weir, M. M. Ogilvie, R. A. Elton, A. Busuttil, and J. W. Keeling. 1996. Developmental and environmental factors that enhance binding of Bordetella pertussis to human epithelial cells in relation to sudden infant death syndrome (SIDS). FEMS Immunol. Med. Microbiol. 16:51-59. [DOI] [PubMed] [Google Scholar]
- 241.Saadi, A. T., C. C. Blackwell, M. W. Raza, V. S. James, J. Stewart, R. A. Elton, and D. M. Weir. 1993. Factors enhancing adherence of toxigenic Staphylococcus aureus to epithelial cells and their possible role in sudden infant death syndrome. Epidemiol. Infect. 110:507-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sacco, O., M. Silvestri, F. Sabatini, R. Sale, A. C. Defilippi, and G. A. Rossi. 2004. Epithelial cells and fibroblasts: structural repair and remodelling in the airways. Paediatr. Respir. Rev. 5(Suppl. A):S35-S40. [DOI] [PubMed] [Google Scholar]
- 243.Saeki, H., A. M. Moore, M. J. Brown, and S. T. Hwang. 1999. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J. Immunol. 162:2472-2475. [PubMed] [Google Scholar]
- 244.Sajjan, U., Q. Wang, Y. Zhao, D. C. Gruenert, and M. B. Hershenson. 2008. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am. J. Respir. Crit. Care Med. 178:1271-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sanders, S. P., J. Kim, K. R. Connolly, J. D. Porter, E. S. Siekierski, and D. Proud. 2001. Nitric oxide inhibits rhinovirus-induced granulocyte macrophage colony-stimulating factor production in bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 24:317-325. [DOI] [PubMed] [Google Scholar]
- 247.Sanders, S. P., E. S. Siekierski, J. D. Porter, S. M. Richards, and D. Proud. 1998. Nitric oxide inhibits rhinovirus-induced cytokine production and viral replication in a human respiratory epithelial cell line. J. Virol. 72:934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Sanders, S. P., E. S. Siekierski, S. M. Richards, J. D. Porter, F. Imani, and D. Proud. 2001. Rhinovirus infection induces expression of type 2 nitric oxide synthase in human respiratory epithelial cells in vitro and in vivo. J. Allergy Clin. Immunol. 107:235-243. [DOI] [PubMed] [Google Scholar]
- 249.Sanford, B. A., V. E. Davison, and M. A. Ramsay. 1982. Fibrinogen-mediated adherence of group A Streptococcus to influenza A virus-infected cell cultures. Infect. Immun. 38:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sanford, B. A., and M. A. Ramsay. 1987. Bacterial adherence to the upper respiratory tract of ferrets infected with influenza A virus. Proc. Soc. Exp. Biol. Med. 185:120-128. [DOI] [PubMed] [Google Scholar]
- 251.Sanford, B. A., A. Shelokov, and M. A. Ramsay. 1978. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J. Infect. Dis. 137:176-181. [DOI] [PubMed] [Google Scholar]
- 252.Sano, H., K. Nagai, H. Tsutsumi, and Y. Kuroki. 2003. Lactoferrin and surfactant protein A exhibit distinct binding specificity to F protein and differently modulate respiratory syncytial virus infection. Eur. J. Immunol. 33:2894-2902. [DOI] [PubMed] [Google Scholar]
- 253.Sasai, M., M. Shingai, K. Funami, M. Yoneyama, T. Fujita, M. Matsumoto, and T. Seya. 2006. NAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J. Immunol. 177:8676-8683. [DOI] [PubMed] [Google Scholar]
- 254.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 255.Satoh, T., H. Kato, Y. Kumagai, M. Yoneyama, S. Sato, K. Matsushita, T. Tsujimura, T. Fujita, S. Akira, and O. Takeuchi. 2010. LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. U. S. A. 107:1512-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schildgen, O., A. Muller, T. Allander, I. M. Mackay, S. Volz, B. Kupfer, and A. Simon. 2008. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin. Microbiol. Rev. 21:291-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Schutte, B. C., and P. B. McCray, Jr. 2002. Beta-defensins in lung host defense. Annu. Rev. Physiol. 64:709-748. [DOI] [PubMed] [Google Scholar]
- 258.See, H., and P. Wark. 2008. Innate immune response to viral infection of the lungs. Paediatr. Respir. Rev. 9:243-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Selinger, D. S., W. P. Reed, and L. C. McLaren. 1981. Model for studying bacterial adherence to epithelial cells infected with viruses. Infect. Immun. 32:941-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sha, Q., A. Q. Truong-Tran, J. R. Plitt, L. A. Beck, and R. P. Schleimer. 2004. Activation of airway epithelial cells by Toll-like receptor agonists. Am. J. Respir. Cell Mol. Biol. 31:358-364. [DOI] [PubMed] [Google Scholar]
- 261.Sheppard, P., W. Kindsvogel, W. Xu, K. Henderson, S. Schlutsmeyer, T. E. Whitmore, R. Kuestner, U. Garrigues, C. Birks, J. Roraback, C. Ostrander, D. Dong, J. Shin, S. Presnell, B. Fox, B. Haldeman, E. Cooper, D. Taft, T. Gilbert, F. J. Grant, M. Tackett, W. Krivan, G. McKnight, C. Clegg, D. Foster, and K. M. Klucher. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63-68. [DOI] [PubMed] [Google Scholar]
- 262.Shuter, J., V. B. Hatcher, and F. D. Lowy. 1996. Staphylococcus aureus binding to human nasal mucin. Infect. Immun. 64:310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 264.Sigurs, N., R. Bjarnason, F. Sigurbergsson, and B. Kjellman. 2000. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 161:1501-1507. [DOI] [PubMed] [Google Scholar]
- 265.Simon, H. U., H. Seelbach, R. Ehmann, and M. Schmitz. 2003. Clinical and immunological effects of low-dose IFN-alpha treatment in patients with corticosteroid-resistant asthma. Allergy 58:1250-1255. [DOI] [PubMed] [Google Scholar]
- 266.Singh, D., K. L. McCann, and F. Imani. 2007. MAPK and heat shock protein 27 activation are associated with respiratory syncytial virus induction of human bronchial epithelial monolayer disruption. Am. J. Physiol. Lung Cell. Mol. Physiol. 293:L436-L445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. A. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. McCray, Jr. 1998. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. U. S. A. 95:14961-14966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Skevaki, C. L., I. Christodoulou, I. S. Spyridaki, I. Tiniakou, V. Georgiou, P. Xepapadaki, D. A. Kafetzis, and N. G. Papadopoulos. 2009. Budesonide and formoterol inhibit inflammatory mediator production by bronchial epithelial cells infected with rhinovirus. Clin. Exp. Allergy 39:1700-1710. [DOI] [PubMed] [Google Scholar]
- 269.Sloots, T. P., D. M. Whiley, S. B. Lambert, and M. D. Nissen. 2008. Emerging respiratory agents: new viruses for old diseases? J. Clin. Virol. 42:233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Smit, J. J., D. M. Lindell, L. Boon, M. Kool, B. N. Lambrecht, and N. W. Lukacs. 2008. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One 3:e1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Smyth, R. L., K. J. Mobbs, U. O'Hea, D. Ashby, and C. A. Hart. 2002. Respiratory syncytial virus bronchiolitis: disease severity, interleukin-8, and virus genotype. Pediatr. Pulmonol. 33:339-346. [DOI] [PubMed] [Google Scholar]
- 272.Soumelis, V., P. A. Reche, H. Kanzler, W. Yuan, G. Edward, B. Homey, M. Gilliet, S. Ho, S. Antonenko, A. Lauerma, K. Smith, D. Gorman, S. Zurawski, J. Abrams, S. Menon, T. McClanahan, R. de Waal-Malefyt Rd, F. Bazan, R. A. Kastelein, and Y. J. Liu. 2002. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3:673-680. [DOI] [PubMed] [Google Scholar]
- 273.Spann, K. M., K. C. Tran, and P. L. Collins. 2005. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J. Virol. 79:5353-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 275.Swain, S. L., R. W. Dutton, and D. L. Woodland. 2004. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol. 17:197-209. [DOI] [PubMed] [Google Scholar]
- 276.Sykes, A., and S. L. Johnston. 2008. Etiology of asthma exacerbations. J. Allergy Clin. Immunol. 122:685-688. [DOI] [PubMed] [Google Scholar]
- 277.Takaoka, A., S. Hayakawa, H. Yanai, D. Stoiber, H. Negishi, H. Kikuchi, S. Sasaki, K. Imai, T. Shibue, K. Honda, and T. Taniguchi. 2003. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature 424:516-523. [DOI] [PubMed] [Google Scholar]
- 278.Tamura, S., and T. Kurata. 2004. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn. J. Infect. Dis. 57:236-247. [PubMed] [Google Scholar]
- 279.Taubenberger, J. K., and D. M. Morens. 2008. The pathology of influenza virus infections. Annu. Rev. Pathol. 3:499-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Thomas, P. G., P. Dash, J. R. Aldridge, Jr., A. H. Ellebedy, C. Reynolds, A. J. Funk, W. J. Martin, M. Lamkanfi, R. J. Webby, K. L. Boyd, P. C. Doherty, and T. D. Kanneganti. 2009. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30:566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Thornton, D. J., K. Rousseau, and M. A. McGuckin. 2008. Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 70:459-486. [DOI] [PubMed] [Google Scholar]
- 282.Ting, J. P., J. A. Duncan, and Y. Lei. 2010. How the noninflammasome NLRs function in the innate immune system. Science 327:286-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.To, K. K., I. F. Hung, I. W. Li, K. L. Lee, C. K. Koo, W. W. Yan, R. Liu, K. Y. Ho, K. H. Chu, C. L. Watt, W. K. Luk, K. Y. Lai, F. L. Chow, T. Mok, T. Buckley, J. F. Chan, S. S. Wong, B. Zheng, H. Chen, C. C. Lau, H. Tse, V. C. Cheng, K. H. Chan, and K. Y. Yuen. 2010. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin. Infect. Dis. 50:850-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tregoning, J. S., and J. Schwarze. 2010. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 23:74-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Trinchieri, G. 1989. Biology of natural killer cells. Adv. Immunol. 47:187-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Uematsu, S., and S. Akira. 2006. Toll-like receptors and innate immunity. J. Mol. Med. 84:712-725. [DOI] [PubMed] [Google Scholar]
- 287.Uetani, K., S. D. Der, M. Zamanian-Daryoush, C. de La Motte, B. Y. Lieberman, B. R. Williams, and S. C. Erzurum. 2000. Central role of double-stranded RNA-activated protein kinase in microbial induction of nitric oxide synthase. J. Immunol. 165:988-996. [DOI] [PubMed] [Google Scholar]
- 288.Van Alphen, L. 1996. Interaction of bacteria and airway epithelial cells. Eur. Respir. J. 9:1342-1343. [DOI] [PubMed] [Google Scholar]
- 289.Van der Sluijs, K. F., C. Obregon, T. K. Geiser, K. Muhlemann, and L. P. Nicod. 2010. Monocyte differentiation towards regulatory dendritic cells is not affected by RSV-induced inflammatory mediators. Am. J. Respir. Cell Mol. Biol. [Epub ahead of print.] doi: 10.1165/rcmb.2010-0136OC. [DOI] [PubMed]
- 290.Van der Strate, B. W., L. Beljaars, G. Molema, M. C. Harmsen, and D. K. Meijer. 2001. Antiviral activities of lactoferrin. Antiviral Res. 52:225-239. [DOI] [PubMed] [Google Scholar]
- 291.Van der Zalm, M. M., B. E. van Ewijk, B. Wilbrink, C. S. Uiterwaal, T. F. Wolfs, and C. K. van der Ent. 2009. Respiratory pathogens in children with and without respiratory symptoms. J. Pediatr. 154:396-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Van Ewijk, B. E., T. F. Wolfs, P. C. Aerts, K. P. Van Kessel, A. Fleer, J. L. Kimpen, and C. K. Van der Ent. 2007. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr. Res. 61:398-403. [DOI] [PubMed] [Google Scholar]
- 293.Vermaelen, K., and R. Pauwels. 2005. Pulmonary dendritic cells. Am. J. Respir. Crit. Care Med. 172:530-551. [DOI] [PubMed] [Google Scholar]
- 294.Visse, R., and H. Nagase. 2003. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ. Res. 92:827-839. [DOI] [PubMed] [Google Scholar]
- 295.Vogelmeier, C., A. D'Urzo, R. Pauwels, J. M. Merino, M. Jaspal, S. Boutet, I. Naya, and D. Price. 2005. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur. Respir. J. 26:819-828. [DOI] [PubMed] [Google Scholar]
- 296.Volonaki, E., S. Psarras, P. Xepapadaki, D. Psomali, D. Gourgiotis, and N. G. Papadopoulos. 2006. Synergistic effects of fluticasone propionate and salmeterol on inhibiting rhinovirus-induced epithelial production of remodelling-associated growth factors. Clin. Exp. Allergy 36:1268-1273. [DOI] [PubMed] [Google Scholar]
- 297.Von Boehmer, H. 2005. Mechanisms of suppression by suppressor T cells. Nat. Immunol. 6:338-344. [DOI] [PubMed] [Google Scholar]
- 298.Voynow, J. A., S. J. Gendler, and M. C. Rose. 2006. Regulation of mucin genes in chronic inflammatory airway diseases. Am. J. Respir. Cell Mol. Biol. 34:661-665. [DOI] [PubMed] [Google Scholar]
- 299.Voynow, J. A., and B. K. Rubin. 2009. Mucins, mucus, and sputum. Chest 135:505-512. [DOI] [PubMed] [Google Scholar]
- 300.Wang, H., N. Peters, and J. Schwarze. 2006. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J. Immunol. 177:6263-6270. [DOI] [PubMed] [Google Scholar]
- 301.Wang, H., Z. Su, and J. Schwarze. 2009. Healthy but not RSV-infected lung epithelial cells profoundly inhibit T cell activation. Thorax 64:283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wang, J. H., H. J. Kwon, and Y. J. Jang. 2009. Rhinovirus enhances various bacterial adhesions to nasal epithelial cells simultaneously. Laryngoscope 119:1406-1411. [DOI] [PubMed] [Google Scholar]
- 303.Wang, J. H., H. J. Kwon, and Y. J. Jang. 2009. Rhinovirus upregulates matrix metalloproteinase-2, matrix metalloproteinase-9, and vascular endothelial growth factor expression in nasal polyp fibroblasts. Laryngoscope 119:1834-1838. [DOI] [PubMed] [Google Scholar]
- 304.Wang, Q., D. R. Nagarkar, E. R. Bowman, D. Schneider, B. Gosangi, J. Lei, Y. Zhao, C. L. McHenry, R. V. Burgens, D. J. Miller, U. Sajjan, and M. B. Hershenson. 2009. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J. Immunol. 183:6989-6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wang, S. Z., H. Xu, A. Wraith, J. J. Bowden, J. H. Alpers, and K. D. Forsyth. 1998. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur. Respir. J. 12:612-618. [DOI] [PubMed] [Google Scholar]
- 306.Wang, X., E. R. Hinson, and P. Cresswell. 2007. The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe 2:96-105. [DOI] [PubMed] [Google Scholar]
- 307.Ward, P. P., E. Paz, and O. M. Conneely. 2005. Multifunctional roles of lactoferrin: a critical overview. Cell. Mol. Life Sci. 62:2540-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wareing, M. D., A. B. Lyon, B. Lu, C. Gerard, and S. R. Sarawar. 2004. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J. Leukoc. Biol. 76:886-895. [DOI] [PubMed] [Google Scholar]
- 309.Wareing, M. D., A. L. Shea, C. A. Inglis, P. B. Dias, and S. R. Sarawar. 2007. CXCR2 is required for neutrophil recruitment to the lung during influenza virus infection, but is not essential for viral clearance. Viral Immunol. 20:369-378. [DOI] [PubMed] [Google Scholar]
- 310.Wark, P. A., S. L. Johnston, F. Bucchieri, R. Powell, S. Puddicombe, V. Laza-Stanca, S. T. Holgate, and D. E. Davies. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201:937-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wat, D., C. Gelder, S. Hibbitts, F. Cafferty, I. Bowler, M. Pierrepoint, R. Evans, and I. Doull. 2008. The role of respiratory viruses in cystic fibrosis. J. Cyst. Fibros. 7:320-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wattier, R. L., M. Vazquez, C. Weibel, E. D. Shapiro, D. Ferguson, M. L. Landry, and J. S. Kahn. 2008. Role of human polyomaviruses in respiratory tract disease in young children. Emerg. Infect. Dis. 14:1766-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Weinberg, G. A. 2006. Parainfluenza viruses: an underappreciated cause of pediatric respiratory morbidity. Pediatr. Infect. Dis. J. 25:447-448. [DOI] [PubMed] [Google Scholar]
- 314.Weiss, S. R., and S. Navas-Martin. 2005. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 69:635-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Welliver, R. C., Sr. 2008. The immune response to respiratory syncytial virus infection: friend or foe? Clin. Rev. Allergy Immunol. 34:163-173. [DOI] [PubMed] [Google Scholar]
- 316.Whiteman, S. C., A. Bianco, R. A. Knight, and M. A. Spiteri. 2003. Human rhinovirus selectively modulates membranous and soluble forms of its intercellular adhesion molecule-1 (ICAM-1) receptor to promote epithelial cell infectivity. J. Biol. Chem. 278:11954-11961. [DOI] [PubMed] [Google Scholar]
- 317.Wilson, R., R. B. Dowling, and A. D. Jackson. 1996. The biology of bacterial colonization and invasion of the respiratory mucosa. Eur. Respir. J. 9:1523-1530. [DOI] [PubMed] [Google Scholar]
- 318.Winther, B., J. M. Gwaltney, and J. O. Hendley. 1990. Respiratory virus infection of monolayer cultures of human nasal epithelial cells. Am. Rev. Respir. Dis. 141:839-845. [DOI] [PubMed] [Google Scholar]
- 319.Woo, P. C., E. T. Tung, K. H. Chan, C. C. Lau, S. K. Lau, and K. Y. Yuen. 2010. Cytokine profiles induced by the novel swine-origin influenza A/H1N1 virus: implications for treatment strategies. J. Infect. Dis. 201:346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Wu, L., and A. Dakic. 2004. Development of dendritic cell system. Cell. Mol. Immunol. 1:112-118. [PubMed] [Google Scholar]
- 321.Xatzipsalti, M., F. Psarros, G. Konstantinou, M. Gaga, D. Gourgiotis, P. Saxoni-Papageorgiou, and N. G. Papadopoulos. 2008. Modulation of the epithelial inflammatory response to rhinovirus in an atopic environment. Clin. Exp. Allergy 38:466-472. [DOI] [PubMed] [Google Scholar]
- 322.Xepapadaki, P., and N. G. Papadopoulos. 2007. Viral infections and allergies. Immunobiology 212:453-459. [DOI] [PubMed] [Google Scholar]
- 323.Yamaguchi, T., K. Kawabata, N. Koizumi, F. Sakurai, K. Nakashima, H. Sakurai, T. Sasaki, N. Okada, K. Yamanishi, and H. Mizuguchi. 2007. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum. Gene Ther. 18:753-762. [DOI] [PubMed] [Google Scholar]
- 324.Yanagi, S., J. Ashitani, H. Ishimoto, Y. Date, H. Mukae, N. Chino, and M. Nakazato. 2005. Isolation of human beta-defensin-4 in lung tissue and its increase in lower respiratory tract infection. Respir. Res. 6:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yang, D., A. Biragyn, L. W. Kwak, and J. J. Oppenheim. 2002. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23:291-296. [DOI] [PubMed] [Google Scholar]
- 326.Yang, L., Y. Luo, J. Wei, and S. He. 2010. Integrative genomic analyses on IL28RA, the common receptor of interferon-lambda1, -lambda2 and -lambda3. Int. J. Mol. Med. 25:807-812. [PubMed] [Google Scholar]
- 327.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Yoshikawa, T., T. Hill, K. Li, C. J. Peters, and C. T. Tseng. 2009. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J. Virol. 83:3039-3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Young, D. F., T. S. Carlos, K. Hagmaier, L. Fan, and R. E. Randall. 2007. AGS and other tissue culture cells can unknowingly be persistently infected with PIV5; a virus that blocks interferon signalling by degrading STAT1. Virology 365:238-240. [DOI] [PubMed] [Google Scholar]
- 330.Yu, D., L. Wei, L. Zhengxiu, L. Jian, W. Lijia, L. Wei, Y. Xiqiang, Z. Xiaodong, F. Zhou, and L. Enmei. 2009. Impact of bacterial colonization on the severity and airway inflammation of children with virus induced wheezing. Clin. Microbiol. Infect. [Epub ahead of print.] doi: 10.1111/j.1469-0691.2009.03147.x. [DOI] [PMC free article] [PubMed]
- 331.Zarember, K. A., and P. J. Godowski. 2002. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J. Immunol. 168:554-561. [DOI] [PubMed] [Google Scholar]
- 332.Zhang, L., A. Bukreyev, C. I. Thompson, B. Watson, M. E. Peeples, P. L. Collins, and R. J. Pickles. 2005. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 79:1113-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zheng, S., B. P. De, S. Choudhary, S. A. Comhair, T. Goggans, R. Slee, B. R. Williams, J. Pilewski, S. J. Haque, and S. C. Erzurum. 2003. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity 18:619-630. [DOI] [PubMed] [Google Scholar]
- 334.Zheng, S., W. Xu, S. Bose, A. K. Banerjee, S. J. Haque, and S. C. Erzurum. 2004. Impaired nitric oxide synthase-2 signaling pathway in cystic fibrosis airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 287:L374-L381. [DOI] [PubMed] [Google Scholar]
- 335.Zhou, Z., O. J. Hamming, N. Ank, S. R. Paludan, A. L. Nielsen, and R. Hartmann. 2007. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 81:7749-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhu, L., P. K. Lee, W. M. Lee, Y. Zhao, D. Yu, and Y. Chen. 2009. Rhinovirus-induced major airway mucin production involves a novel TLR3-EGFR-dependent pathway. Am. J. Respir. Cell Mol. Biol. 40:610-619. [DOI] [PMC free article] [PubMed] [Google Scholar]