Abstract
Few therapeutic alternatives remain for the treatment of infections due to multiresistant Mycobacterium abscessus. Here we show that the peptidoglycans of the “rough” and “smooth” morphotypes contain predominantly 3→3 cross-links generated by l,d-transpeptidases, indicating that these enzymes are attractive targets for the development of efficient drugs.
Mycobacterium abscessus has emerged in the last decades as a serious pathogen (16, 18) responsible for a wide spectrum of infections in both immunocompetent and immunosuppressed patients (3). The most frequent infection involves the lung (20), and M. abscessus infection has become a major issue in cystic fibrosis patients (6, 11). The pathogenicity and virulence of M. abscessus appear dependent on its morphotype (5, 8). M. abscessus may switch from a “smooth” to a “rough” morphotype due to the absence of a glycopeptidolipid at its surface. The rough morphotype is associated with greater virulence than the smooth morphotype (4, 5, 8). Variants with the smooth biofilm-forming phenotype are less able to survive inside macrophages or in a murine model of infection than the rough, non-biofilm-forming phenotype, which can penetrate and persist for long periods of time inside its host (4, 5, 8).
M. abscessus is known to be one of the most drug-resistant mycobacteria. Like other rapidly growing mycobacteria, M. abscessus is naturally resistant to first-line antituberculous drugs, leaving limited therapeutic options (3). M. abscessus is normally susceptible in vitro to amikacin, cefoxitin, imipenem, and clarithromycin. However, the in vivo efficiency of these antibiotics is currently questioned (1). In addition, most strains of M. abscessus harbor a gene conferring inducible resistance to clarithromycin (17). Novel therapeutic approaches are needed since clinical failures are frequent, leaving surgical treatment of lung abscesses the only option (1).
Although cefoxitin and imipenem inhibit the last cross-linking step of peptidoglycan synthesis, little is known about the structure of the polymer in M. abscessus. These drugs can potentially inhibit two classes of transpeptidases, the classical d,d-transpeptidases, which belong to the penicillin-binding protein (PBP) family and catalyze formation of 4→3 cross-links (19), and the l,d-transpeptidases, originally detected in Enterococcus faecium, which form 3→3 cross-links (13, 14). To assess the respective contributions of the two types of transpeptidases in cell wall formation, we determined the structure of the peptidoglycan using mass spectrometry of the smooth and rough morphotypes of M. abscessus in different phases of growth.
Structure of the peptidoglycan of M. abscessus.
Initial structure determination was performed with a rough variant of M. abscessus strain CIP104536T (5) grown to the stationary phase. Bacteria were grown for 96 h (optical density at 600 nm [OD600], 7.8) at 37°C with shaking (150 rpm) in Middlebrook 7H9 broth (Becton Dickinson) supplemented with 10% (vol/vol) OADC (oleic acid, bovine albumin, dextrose, catalase) medium (Becton Dickinson) and 0.5% (vol/vol) Tween 80. Cells were collected by centrifugation (7,500 × g for 20 min at 4°C) and resuspended in 10 mM phosphate buffer (pH 7.0). Bacteria were disrupted with glass beads (150 to 212 μm; 5 g/5 ml [wt/vol]) for 16 h at 4°C in a cell disintegrator (Mickle Laboratory Engineering Co.). Peptidoglycans were treated with proteases and digested with mutanolysin and lysozyme as previously described (9).
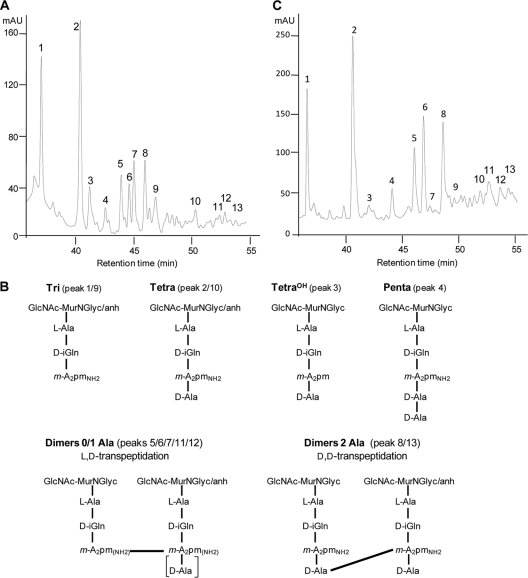
In order to analyze the sugar moieties of muropeptides, peptidoglycan fragments were reduced with sodium borohydride, purified by reverse-phase high-performance liquid chromatography (RP-HPLC) (Fig. 1A), and analyzed by mass spectrometry (MS) and tandem mass spectrometry (MS/MS) as previously described (2). The disaccharide moiety of the resulting muropeptides mainly contained N-glycolylmuramic acid (MurNGlyc) and N-acetylglucosamine (GlcNAc) (Fig. 1B). MS analysis also revealed the presence of N-acetylmuramic (MurNAc) acid instead of MurNGlyc. The corresponding muropeptides were present in very small amounts and could not be assigned to any specific peak in the HPLC profile shown in Fig. 1, whereas minor peaks corresponding to MurNAc-containing muropeptides were detected in other peptidoglycan preparations (data not shown). Anhydro MurNGlyc, present at the extremities of glycan chains (21), was detected in ca. 15% of the muropeptides, revealing an average chain length of ca. 7 disaccharide-peptide units (Table 1). The most abundant monomers (peak 2; 26%) contained a linear stem tetrapeptide, l-Ala1-d-iGlu2NH2-m-A2pm3NH2-d-Ala4, where d-iGlu is d-isoglutamine, m-A2pm is meso-diaminopimelic acid, and NH2 is the amidated ɛ-carboxyl group of m-A2pm (Fig. 1; Table 1). The α-carboxyl of d-Glu was amidated in all muropeptides, and the ɛ-carboxyl group of m-A2pm3 was partially amidated (18%). Additional polymorphism resulted from the presence of tripeptide (e.g., peak 1; 15%) and pentapeptide (e.g., peak 4; 3%) stems.
FIG. 1.
Structure of the peptidoglycan of M. abscessus. (A) RP-HPLC profile of peptidoglycan fragments (muropeptides) obtained by digestion of peptidoglycan by muramidases and treatment with sodium borohydride. Peptidoglycan was extracted from a stationary-phase culture of a rough variant of strain CIP104536T. (B) Structures of monomers and dimers. anh, anhydro; d-iGln, d-iso-glutamine; GlcNAc-MurNGly, N-acetylglucosamine-N-glycolylmuramic acid; m-A2pm, meso-diaminopimelic acid; NH2, amidated ɛ-carboxyl group of m-A2pm. (C) RP-HPLC profile of muropeptides from an exponentially growing rough variant of CIP104536T. mAU, absorbance unit (×103) at 210 nm.
TABLE 1.
Relative abundances (%) of muropeptides from stationary- and exponential-phase cultures of rough and smooth variants of M. abscessus CIP104536T
| Muropeptideb [peak] | Massc | Cross-linkd | Relative abundance (%)a in indicated phase |
|||
|---|---|---|---|---|---|---|
| Rough variant |
Smooth variant |
|||||
| Stationary | Exponential | Stationary | Exponential | |||
| Monomers | ||||||
| Tri [1] | 884.40 | NA | 15 | 19 | 19 | 15 |
| Tetra [2] | 955.45 | NA | 26 | 27 | 12 | 22 |
| TetraOH [3] | 956.44 | NA | 8 | 2 | 3 | 2 |
| Penta [4] | 1,026.47 | NA | 3 | 4 | 5 | 6 |
| Tri(anh) [9] | 864.38 | NA | 7 | 1 | 10 | 2 |
| Tetra(anh) [10] | 864.38 | NA | 4 | 2 | 5 | 3 |
| Total | 63 | 55 | 54 | 50 | ||
| Dimers | ||||||
| Tri-Tri [5] | 1,750.78 | 3→3 | 8 | 9 | 13 | 9 |
| Tri-Tetra [6] | 1,821.21 | 3→3 | 6 | 11 | 10 | 11 |
| Tri-TetraOH [7] | 1,822.85 | 3→3 | 10 | 1 | 1 | 8 |
| Tetra-Tetra [8] | 1,892.86 | 4→3 | 9 | 14 | 11 | 9 |
| Tri-Tri(anh) [11] | 1,730.76 | 3→3 | 1 | 3 | 5 | 4 |
| Tri-Tetra(anh) [12] | 1,801.79 | 3→3 | 2 | 5 | 4 | 5 |
| Tetra-Tetra(anh) [13] | 1,872.83 | 4→3 | 1 | 2 | 2 | 4 |
| Total | 37 | 45 | 46 | 50 | ||
| Cross-links | ||||||
| 3→3 | 73 | 64 | 72 | 74 | ||
| 4→3 | 27 | 36 | 28 | 26 | ||
Relative abundances were estimated by integration of the peak areas of RP-HPLC profiles.
Abbreviations: anh, anhydro; OH, unamidated m-A2pm.
Monoisotopic mass.
NA, not applicable.
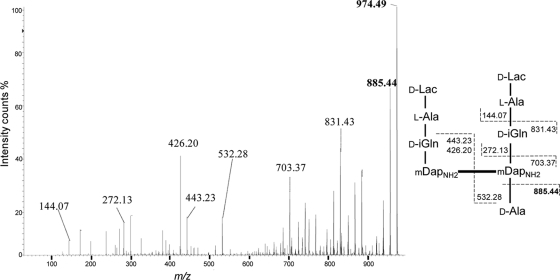
Monomers and dimers accounted for 63% and 37%, respectively, of all muropeptides (Table 1), indicating that the peptidoglycan of M. abscessus is moderately cross-linked. Accordingly, trimers could be detected only following ammonium hydroxide treatment (see below) and accounted for less than 3% of the total muropeptides (data not shown). The sequences of the cross-links were determined by MS/MS (2, 9, 10) and allowed assessment of the contributions of transpeptidases of the d,d and l,d specificities to peptidoglycan polymerization. For this purpose, the muropeptides were treated with ammonium hydroxide to generate lactoyl peptides, which are easily sequenced following removal of the disaccharide moieties (2, 9, 10). The molecular mass, 973.49, was compatible with those of two alternative structures containing either a donor tetrapeptide stem and an acceptor tripeptide stem with a d-Ala4→m-A2pmNH2 (4→3) cross-link or a donor tripeptide stem and an acceptor tetrapeptide stem with an m-A2pmNH2→m-A2pmNH2 (3→3) cross-link. The two structures were differentiated by MS/MS, which allowed detection of the presence (3→3 cross-link) of an alanyl residue at the C terminus of the acceptor stem peptide. The fragmentation pattern (Fig. 2) indicated a 3→3 cross-link, since one alanine residue (−89.05) was lost from the C-terminal end of the acceptor tetrapeptide stem (peaks at 974.49 and 885.44 m/z; [M+H]+ ions). In MS/MS analysis, muropeptides containing 3→3 (peaks 5, 6, 7, 11, and 12) and 4→3 (peaks 8 and 13) cross-links accounted for 73% and 27% of the dimers, respectively (Table 1), indicating that l,d-transpeptidases are the major contributors to peptidoglycan cross-linking in M. abscessus.
FIG. 2.
Sequencing of a dimer generated by l,d-transpeptidation. Tandem mass spectrometry was performed on the [M+H]+ ion at 974.49 m/z (peak 6). Dashed lines indicate cleavage at single peptide bonds.
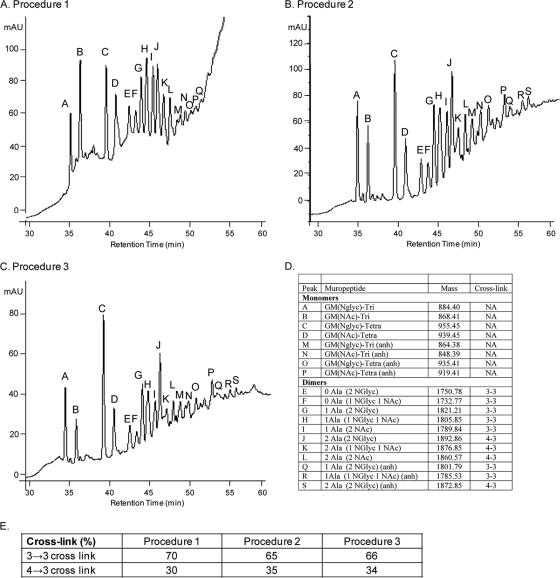
To ensure that the peptidoglycan profile obtained was representative of the whole peptidoglycan structure, we performed a novel peptidoglycan purification procedure based on the procedure described by Mahapatra et al. (12) to chemically remove the arabinogalactan-mycolic acid complex (Fig. 3A). In parallel, we also reproduced our usual procedure with (Fig. 3B) and without (Fig. 3C) an additional fluorhydric acid treatment to cleave the phosphodiester bonds at C-6 of the muramic acid residues and to release arabinogalactan (15). As determined for the rough variant grown to stationary phase, the three procedures provided essentially the same results (Fig. 3D and E).
FIG. 3.
Peptidoglycan structure of a rough variant of M. abscessus strain CIP104536T grown to stationary phase based on three peptidoglycan purification procedures. (A) RP-HPLC profile of peptidoglycan fragments (muropeptides) obtained by following the protocol described by Mahapatra et al. (12) (procedure 1). (B) RP-HPLC profile of peptidoglycan fragments obtained by digestion of peptidoglycan by muramidases and treatment with sodium borohydride after additional fluorhydric acid treatment (procedure 2). (C) RP-HPLC profile of peptidoglycan fragments obtained by digestion of peptidoglycan by muramidases and treatment with sodium borohydride (procedure 3). (D) Peptidoglycan composition. Mass, observed monoisotopic mass; NA, not applicable. (E) Relative abundances (%) of 3→3 and 4→3 cross-links among muropeptide dimers obtained with the three different procedures.
Comparisons of peptidoglycan structures at different phases of growth.
Peptidoglycan was extracted from a culture of the rough variant of M. abscessus CIP104536T grown to mid-log exponential phase (38 h; OD600, 0.8). The muropeptide profiles of peptidoglycans extracted from the stationary (Fig. 1A) and exponential (Fig. 1C) phases of growth were similar. In comparison to that of the stationary phase, the peptidoglycan from exponentially growing bacteria appears more cross-linked (45% versus 37% of dimers) (Table 1) and to contain less unamidated m-A2pm (2% versus 8% for monomers and 1% versus 10% for dimers, respectively) (Table 1). Among dimers, the proportions of 3→3 cross-links were slightly decreased during the exponential phase (64% versus 73%) (Table 1).
Comparisons of peptidoglycans from variants of the rough and smooth morphotypes.
In order to evaluate if the switch between high and low levels of glycopeptidolipid production is associated with any modification of the peptidoglycan structure, we prepared muropeptides from a smooth variant of M. abscessus CIP104536T grown to both the exponential (OD600, 0.6) and stationary (OD600, 10) phases of growth. A minor difference in the proportions of monomers containing tripeptide stems was observed (Table 1), as this type of monomer appeared to be more abundant in the smooth morphotype than in the rough morphotype during the stationary phase (29% versus 22%). The proportions of 3→3 cross-links in stationary-phase cultures of the smooth and rough variants were similar (72% versus 73%, respectively) (Table 1), but the proportion in the smooth variant (74%) was slightly increased during the exponential phase compared to that of the rough variant (64%) (Table 1). Thus, the respective contributions of transpeptidases of the l,d and d,d specificities to peptidoglycan cross-linking appeared to be similar for the two morphotypes. Overall, changes in the glycopeptidolipid content were not associated with any profound modification of peptidoglycan structure.
Concluding remarks.
We have previously shown that M. tuberculosis peptidoglycan from a stationary-phase culture contained a large amount (80%) of 3→3 linkages generated by l,d-transpeptidation (7, 10). Analysis of the peptidoglycan structure of M. abscessus revealed that cross-links of the 3→3 type are also predominant (64% to 74%) in the smooth and rough variants during both the exponential and stationary phases of growth. Analysis of the M. abscessus genome revealed five putative l,d-transpeptidases; the major contribution of l,d-transpeptidases to peptidoglycan cross-linking indicates that these enzymes could be a target for β-lactam antibiotics in addition to the classical PBPs, as was recently proposed for M. tuberculosis (7, 10). The large amount of 3→3 cross-links in the two morphotypes suggests that inhibitors of l,d-transpeptidases could be active on both planktonic and biofilm-forming variants of M. abscessus.
Acknowledgments
This work was supported by the Fondation pour la Recherche Médicale (Equipe FRM 2006; DEQ200661107918).
Footnotes
Published ahead of print on 19 November 2010.
REFERENCES
- 1.American Thoracic Society. 1997. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am. J. Respir. Crit. Care Med. 156:S1-S25. [DOI] [PubMed] [Google Scholar]
- 2.Arbeloa, A., et al. 2004. Synthesis of mosaic peptidoglycan cross-bridges by hybrid peptidoglycan assembly pathways in Gram-positive bacteria. J. Biol. Chem. 279:41546-41556. [DOI] [PubMed] [Google Scholar]
- 3.Brown-Elliott, B. A., and R. J. Wallace, Jr. 2002. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15:716-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd, T. F., and C. R. Lyons. 1999. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 67:4700-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Catherinot, E., et al. 2007. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect. Immun. 75:1055-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith, D. E. 2003. Emergence of nontuberculous mycobacteria as pathogens in cystic fibrosis. Am. J. Respir. Crit. Care Med. 167:810-812. [DOI] [PubMed] [Google Scholar]
- 7.Gupta, R., et al. 2010. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat. Med. 16:466-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard, S. T., et al. 2006. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 152:1581-1590. [DOI] [PubMed] [Google Scholar]
- 9.Lavollay, M., et al. 2009. The β-lactam-sensitive d,d-carboxypeptidase activity of Pbp4 controls the l,d and d,d transpeptidation pathways in Corynebacterium jeikeium. Mol. Microbiol. 74:650-661. [DOI] [PubMed] [Google Scholar]
- 10.Lavollay, M., et al. 2008. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by l,d-transpeptidation. J. Bacteriol. 190:4360-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy, I., et al. 2008. Multicenter cross-sectional study of nontuberculous mycobacterial infections among cystic fibrosis patients, Israel. Emerg. Infect. Dis. 14:378-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahapatra, S., D. C. Crick, M. R. McNeil, and P. J. Brennan. 2008. Unique structural features of the peptidoglycan of Mycobacterium leprae. J. Bacteriol. 190:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mainardi, J. L., et al. 2005. A novel peptidoglycan cross-linking enzyme for a beta-lactam-resistant transpeptidation pathway. J. Biol. Chem. 280:38146-38152. [DOI] [PubMed] [Google Scholar]
- 14.Mainardi, J. L., R. Villet, T. D. Bugg, C. Mayer, and M. Arthur. 2008. Evolution of peptidoglycan biosynthesis under the selective pressure of antibiotics in Gram-positive bacteria. FEMS Microbiol. Rev. 32:386-408. [DOI] [PubMed] [Google Scholar]
- 15.McNeil, M., M. Daffe, and P. J. Brennan. 1990. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J. Biol. Chem. 265:18200-18206. [PubMed] [Google Scholar]
- 16.Medjahed, H., and J. M. Reyrat. 2009. Construction of Mycobacterium abscessus defined glycopeptidolipid mutants: comparison of genetic tools. Appl. Environ. Microbiol. 75:1331-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nash, K. A., B. A. Brown-Elliott, and R. J. Wallace, Jr. 2009. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob. Agents Chemother. 53:1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petrini, B. 2006. Mycobacterium abscessus: an emerging rapid-growing potential pathogen. APMIS 114:319-328. [DOI] [PubMed] [Google Scholar]
- 19.Sauvage, E., F. Kerff, M. Terrak, J. A. Ayala, and P. Charlier. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32:234-258. [DOI] [PubMed] [Google Scholar]
- 20.Sermet-Gaudelus, I., et al. 2003. Mycobacterium abscessus and children with cystic fibrosis. Emerg. Infect. Dis. 9:1587-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollmer, W. 2008. Structural variation in the glycan strands of bacterial peptidoglycan. FEMS Microbiol. Rev. 32:287-306. [DOI] [PubMed] [Google Scholar]