Abstract
We have identified a clonal complex of Mycobacterium bovis isolated at high frequency from cattle in Uganda, Burundi, Tanzania, and Ethiopia. We have named this related group of M. bovis strains the African 2 (Af2) clonal complex of M. bovis. Af2 strains are defined by a specific chromosomal deletion (RDAf2) and can be identified by the absence of spacers 3 to 7 in their spoligotype patterns. Deletion analysis of M. bovis isolates from Algeria, Mali, Chad, Nigeria, Cameroon, South Africa, and Mozambique did not identify any strains of the Af2 clonal complex, suggesting that this clonal complex of M. bovis is localized in East Africa. The specific spoligotype pattern of the Af2 clonal complex was rarely identified among isolates from outside Africa, and the few isolates that were found and tested were intact at the RDAf2 locus. We conclude that the Af2 clonal complex is localized to cattle in East Africa. We found that strains of the Af2 clonal complex of M. bovis have, in general, four or more copies of the insertion sequence IS6110, in contrast to the majority of M. bovis strains isolated from cattle, which are thought to carry only one or a few copies.
Bovine tuberculosis (TB), caused by Mycobacterium bovis, is mainly a disease of cattle, but it is also a zoonosis infecting humans. Bovine TB has been eradicated in Australia and many European countries; however, it is still believed to be common among cattle throughout the rest of the world. On the African continent, information on the prevalence of bovine TB is scarce and control programs are in place in only a few countries (6, 16). However, a number of reports suggest that the disease is widely spread over the African continent and highly prevalent in several countries (8, 21, 38, 42, 51), with infection present mainly in cattle but also in wildlife (39).
M. bovis is one of seven species constituting the Mycobacterium tuberculosis complex, which includes M. tuberculosis, one of the most devastating bacterial pathogens of humans. There is little or no exchange of chromosomal DNA between cells from the M. tuberculosis complex, making this group of bacteria highly clonal (14, 30, 53-54). In a strictly clonal population, any mutation present in an ancestral strain will be present in all its descendants and can be used to identify clonal complexes. A series of deletions (regions of difference [RD]) within the M. tuberculosis complex have been used to identify phylogenetic relationships between members of the M. tuberculosis complex (11), and for M. tuberculosis, different lineages and sublineages have also been characterized by specific deletions (25, 60). In a similar manner, we are exploring the relationships between lineages of M. bovis that dominate in different geographical regions around the world.
Spoligotyping, a PCR and hybridization technique, is the most common genotyping technique for strains of the M. tuberculosis complex and assays polymorphism in 43 unique spacer sequences found in the direct repeat (DR) region (36, 61). Each spoligotype pattern from strains of the animal-adapted lineage of the M. tuberculosis complex is given a unique identifier by www.Mbovis.org. Several studies of the DR region in closely related strains of M. tuberculosis have concluded that the evolutionary trend of this region is primarily by loss of single or multiple contiguous spacers (23, 29, 63); duplication of direct variable repeat (DVR) units or point mutations in spacer sequences were found to be rare. Although the absence of specific spacers, or groups of spacers, in a spoligotype pattern can be indicative of a closely related group of strains (clonal complex), spacers are frequently lost independently in different lineages (homoplasy). Furthermore, the interpretation of specific spacer loss, such as that of spacers 3 to 7 in the strains described in this article, can be ambiguous if adjacent spacers in the spoligotype pattern are also deleted.
Recently a clonal complex of M. bovis, called African 1 (Af1) (41), that is highly prevalent in several countries of west-central Africa has been identified. In this article, we identify a second M. bovis clonal complex common in East Africa and name this group of strains the African 2 (Af2) clonal complex of M. bovis.
MATERIALS AND METHODS
Bacterial strains.
The majority of all M. bovis strains analyzed in this study were isolated from cattle and are described in more detail in the supplemental material. One hundred twenty strains were collected from six abattoirs in Ethiopia during 2006 to 2008 (8); nine strains were collected at an abattoir in Kampala from cattle originating from seven districts in Uganda (5); ten strains were collected from three sites in or close to the capital Bujumbura in Burundi (48); and fourteen strains were collected from cattle at a Morogoro slaughterhouse in Tanzania (41). Additional population samples of M. bovis isolated from cattle from South Africa (n = 22) (40]), Chad (n = 5) (35]), Mali (n = 20) (42), Cameroon (n = 3), Nigeria (n = 5), Mozambique (n = 14), Algeria (n = 17) (51), Italy (n = 93) (10), and Spain (n = 20) (49]) were analyzed (see the supplemental material). Also, two strains of M. bovis from humans, isolated in Uganda and Sweden, were further investigated for this study.
All isolates were characterized by spoligotyping, and the majority were also deletion typed for regions RD4 and RDAf2. Selected M. bovis isolates were subjected to variable-number tandem repeat (VNTR) typing (24) and RDAf1 deletion typing (41) (see the supplemental material). Isolates of M. tuberculosis H37Rv and M. bovis AF2122/97 were used as controls.
Spoligotyping, VNTR typing, and microarray analysis.
Strains were spoligotyped according to the method of Kamerbeek et al. (36) with minor modifications (12), and the exact tandem repeat (ETR) loci ETR-A to ETR-F were VNTR typed as previously described (12, 24). The VNTR types are displayed as a series of six integers representing the deduced number of repeats present at each locus. All VNTR typing was performed at the VLA, Weybridge, United Kingdom.
For microarray analysis, two isolates (no. BTB0691 and BTB1091; see the supplemental material) were selected from the Ethiopian M. bovis collection. Both isolates lacked spacers 3 to 7 in their spoligotype pattern. Approximately 1 to 2 μg genomic DNA was purified, and deletions were identified by microarray analysis using previously published methods (26). Deletions found in regions associated with repetitive elements and insertion sequences, which are known to be prone to deletion events, were disregarded in this study.
Deletion typing.
The identification of a strain as M. bovis was on the basis of spoligotype signature (56) and growth characteristics; many of the isolates from Uganda, Burundi, Tanzania, and Ethiopia were confirmed as M. bovis by deletion typing of the RD4 region (11). The status of the RDAf2 region (deleted or intact) was assessed by multiplex PCR with a set of three primers (primer set Af2): two primers targeting the flanking regions of RDAf2 (RDAf2_Fw, 5′-ACTGGACCGGCAACGACCTGG, RDAf2_Rev, 5′-CGGGTGACCGTGAACTGCGAC) and one primer hybridizing with the internal region of RDAf2 (RDAf2_IntRev, 5′-CGGATCGCGGTGATCGTCGA). A PCR product of 458 bp (RDAf2 intact) or 707 bp (RDAf2 deleted) was identified by agarose gel electrophoresis. Each PCR mixture contained 1 μl of supernatant of heat-killed mycobacterial cells, a final concentration of 1× HotStartTaq master mix (Qiagen), 1 μM primers RDAf2_FW, RDAf2_Rev, and RDAf2_IntRev, and sterile distilled water to a final volume of 20 μl. Thermal cycling was performed with an initial denaturation step of 15 min at 96°C, 35 cycles of 30 s at 96°C, 30 s at 55°C, and 1 min at 72°C, followed by a final elongation step of 10 min at 72°C. PCR products were separated on a 1% agarose gel. Isolates subjected to RDAf1 typing were examined according to a previously described PCR protocol (41).
IS6110 RFLP typing.
Genomic DNA was purified from selected M. bovis strains (66), and approximately 2 μg DNA was used for IS6110 restriction fragment length polymorphism (RFLP) analysis according to the internationally standardized protocol (62). In short, DNA was digested with the restriction endonuclease PvuII, separated by agarose gel electrophoresis, and transferred to a nylon membrane by Southern blotting. The membrane was hybridized with a probe targeting the right-hand site of the IS6110 element (62, 65) and subsequently with a 36-bp oligonucleotide targeting the direct repeat region (65). The probes were labeled using the enhanced chemiluminescence detection system (ECL; Amersham). The IS6110 RFLP patterns were analyzed by using the BioNumerics software program (Applied Maths, Sint Martens-Latum, Belgium), and the dendrogram was prepared by using the Dice coefficient and unweighted-pair group method using average linkages (UPGMA).
Nucleotide sequence accession numbers.
The RDAf2 deletion junctions of 10 strains of the Af2 clonal complex from five countries were sequenced using standard methods. The isolate name, country of origin, and GenBank accession numbers for the sequences surrounding the RDAf2 deletion junctions are as follows: BTB0890, Ethiopia, GU004183; BTB1067, Ethiopia, GU004182; BTB1474, Ethiopia, GU004184; JN03, Uganda, GU004178; JN58, Uganda, GU004179; SEA199701128, Somalia, GU004185; 940130, Burundi, GU004186; 940439, Burundi, GU004187; 11, Tanzania, GU004180; and B3, Tanzania, GU004181 (see the supplemental material).
RESULTS
Isolates with spacers 3 to 7 absent.
An extensive slaughterhouse study in Ethiopia of 58 M. bovis strains isolated from six abattoirs dispersed throughout the country showed that many isolates lacked spacers 3 to 7 in their spoligotype pattern, in addition to the absence of spacers 9, 16, and 39 to 43 (8). We supplemented this sample with an additional 62 isolates from the same abattoirs and found that over 75% (n = 91; total = 120) of these Ethiopian isolates had spoligotype patterns that were missing spacers 3 to 7 (Table 1).
TABLE 1.
Spoligotype patterns of M. bovis strains isolated in four east African countriesa
| Country | Pattern designationb | Spoligotype patternc | Frequency [no. (%) of strains] | RDAf2 |
|---|---|---|---|---|
| Uganda | SB1407 | 1100000101111110110000111111111111111100000 | 3 (33.3) | Deleted |
| SB1405 | 0101100101111110111111111111111111101100000 | 2 (22.2) | Intact | |
| SB1406 | 0101100101111110111111111111111011101100000 | 1 (11.1) | Intact | |
| SB0133 | 1100000101111110111111111111111111111100000 | 1 (11.1) | Deleted | |
| SB1404 | 1100000101111000111111111111111111111100000 | 1 (11.1) | Deleted | |
| SB1408 | 1100000101111000110000111111111111101100000 | 1 (11.1) | Deleted | |
| Total | 9 (100.0) | |||
| Burundi | SB0303 | 1100000101110110111111111111111111111100000 | 5 (50.0) | Deleted |
| SB1388 | 1100000101111110111101111111111111111100000 | 4 (40.0) | Deleted | |
| SB0304 | 1100000101111110111100001111111111111100000 | 1 (10.0) | Deleted | |
| Total | 10 (100.0) | |||
| Tanzania | SB0133 | 1100000101111110111111111111111111111100000 | 9 (64.3) | Deleted |
| SB0425 | 0101011101000110111111111111000000101100000 | 4 (28.6) | Intact | |
| SB1446 | 1100000101011110111111111111111111111100000 | 1 (7.1) | Deleted | |
| Total | 14 (100.0) | |||
| Ethiopia | SB1176 | 1100000101111110111111100010000000000100000 | 59 (51.6) | Deleted |
| SB1476 | 1101111101111110111111111111111100000100000 | 21 (16.7) | Intact | |
| SB0133 | 1100000101111110111111111111111111111100000 | 18 (14.3) | Deleted | |
| SB1477 | 1000000101111110111111111111111011111100000 | 8 (6.3) | Deleted | |
| SB0134 | 1100011101111110111111111111111111111100000 | 6 (4.8) | Intact | |
| SB0120 | 1101111101111110111111111111111111111100000 | 2 (1.6) | Intact | |
| SB1942 | 1100000101111110111111111111111111001100000 | 2 (1.6) | Deleted | |
| SB1488 | 1100000101111110111111111001111111111100000 | 1 (0.8) | Deleted | |
| SB1489 | 1100000101111110111111111111111010111100000 | 1 (0.8) | Deleted | |
| SB1941 | 0000000101111110111111111111111110111100000 | 1 (0.8) | Deleted | |
| SB0303 | 1100000101110110111111111111111111111100000 | 1 (0.8) | Deleted | |
| Total | 120 (100.0) |
The presumed ancestral spoligotype pattern of the Af2 clonal complex is shown in bold.
International names for these spoligotype patterns were assigned by Mbovis.org (http://www.Mbovis.org).
The spoligotype pattern is shown as a series of 43 ones and zeros, corresponding to spacers 1 to 43 in spoligotyping, with 1 representing hybridization to the spacer and 0 representing the absence of hybridization.
Furthermore, three separate spoligotype surveys of bovine TB in Ethiopian cattle from Addis Ababa and central/southern Ethiopia showed similar results: over 80% of strains had spacers 3 to 7 deleted (2, 9, 59).
From Uganda, which is situated close to Ethiopia, it was recently shown that six of nine M. bovis isolates from cattle originating from seven districts in both the northwest and southern parts of the country also had spacers 3 to 7 missing in their spoligotype pattern (5). The absence of spacers 3 to 7 in Ugandan isolates was supported by a further spoligotype survey of 19 M. bovis isolates sampled from cattle from similar regions of the country (44).
To further identify the clonal complexes of bovine TB in East Africa, we spoligotyped M. bovis strains previously collected from cattle in Burundi and Tanzania (41, 48). All isolates from Burundi (originating at the city of Bujumbura and nearby in the western parts of the country; n = 10) and 10 out of 14 isolates from cattle sampled at a Morogoro slaughterhouse in south-central Tanzania had spacers 3 to 7 missing in their spoligotype patterns (Table 1).
In total, 117 out of 153 (76%) of M. bovis isolates from these four east African countries had spoligotype patterns missing spacers 3 to 7, and we concluded that, in a manner similar to that of the African 1 clonal complex in west-central Africa (41), these isolates may represent a clonal complex of bovine TB descended from an ancestral cell in which spacers 3 to 7 had been deleted. The commonest spoligotype pattern in this data set was SB0133 (Table 2), which is similar to that of M. bovis BCG (SB0120, which lacks spacers 3, 9, 16, and 39 to 43), with the additional loss of spacers 4 to 7. Spoligotype pattern SB0133 was found at high frequency in the Tanzanian and Ethiopian samples and in a low number in Uganda and was absent from the small sample of strains from Burundi (Table 1).
TABLE 2.
Definition and summary of characteristics of the Af2 clonal complex of M. bovis
| Category | Description |
|---|---|
| Definition | Presence of deletion RDAf2 (14.1 kb between Mb0599 and Mb0610) |
| Spoligotype marker | Absence of spacers 3 to 7 |
| Spoligotype signaturea | 1100000101111110111111111111111111111100000 (SB0133) |
| Distribution | At high frequency in East Africa (Uganda, Burundi, Tanzania, and Ethiopia) |
| IS6110 copy no. | 4 or more copies (infrequently less than 4) |
The spoligotype signature represents the assumed spoligotype pattern in the progenitor strain of this clonal complex and is shown as a series of 43 ones and zeros corresponding to spacers 1 to 43 in spoligotyping, with “1” representing hybridization to the spacer and “0” representing absence of hybridization. The international name for this spoligotype pattern was assigned by Mbovis.org (http://www.mbovis.org).
Identification of a specific deletion.
To identify a phylogenetically informative deletion for the east African M. bovis strains lacking spacers 3 to 7, two Ethiopian isolates of spoligotype SB0133, which represents the most complete spoligotype pattern lacking spacers 3 to 7, were tested by microarray analysis using an M. tuberculosis-M. bovis composite amplicon microarray. This analysis showed that these two strains were deleted for all regions that are commonly missing in strains of M. bovis, including RD4 (11). Region RDAf1, which is deleted in members of the African 1 clonal complex of M. bovis, present at high frequency in several countries in west-central Africa, was intact, showing that these strains were not members of the African 1 clonal complex (41), as was a region called RDEu1, which is associated with isolates from cattle originating in Great Britain (unpublished data). However, we identified a unique region of chromosomal DNA of approximately 14 kb that was deleted in both Ethiopian isolates. The endpoints of this deletion were characterized by nucleotide sequencing and compared to the whole genome sequence of M. bovis AF2122/97 (27). We determined that 14,094 bp were deleted, and to our knowledge, this deletion has not previously been described. We named this deletion Region of Difference African 2 (RDAf2).
The RDAf2 deletion removed the entire coding sequences of 10 genes from Mb0600c to Mb0609 and parts of Mb0599 and Mb0610 (corresponding to the genes Rv0585c to Rv0593 and parts of Rv0584 and Rv0594 in M. tuberculosis H37Rv). The regions surrounding the RDAf2 deletion junction in the genome of the sequenced strain M. bovis AF2122/97 showed no evidence of the common M. tuberculosis complex insertion sequences or repetitive DNA and were not GC rich. No significant inverted or direct repeats could be identified at either side of the deletion junctions in the RDAf2 region of the AF2122/97 chromosome sequence. We therefore concluded that this region of the chromosome is not prone to generate homoplastic deletions and hence the RDAf2 deletion could be a suitable phylogenetic marker to identify strains of a clonal complex of M. bovis strains which descended from an ancestral cell in which RDAf2 was deleted in addition to spacers 3 to 7 in the spoligotype pattern.
Distribution of RDAf2 among cattle isolates in East Africa.
To rapidly identify strains with the RDAf2 deletion, we developed a simple PCR method using two primers targeting both flanking regions of RDAf2 and one primer targeting an RDAf2 internal sequence. All 120 M. bovis isolates from cattle from Ethiopia were tested by this deletion assay, and 91 of these were deleted for RDAf2. Furthermore, we tested samples of M. bovis collected from cattle from Uganda (5), Burundi (48), and Tanzania (41) for the status of the RDAf2 region. The RDAf2 region was deleted in 6 out of 9 isolates from Uganda, in all isolates sampled from Burundi (n = 10), and in 10 of the 14 isolates from Tanzania (Table 1). All strains in these samples and throughout this article that were deleted for RDAf2 were also missing spacers 3 to 7 in their spoligotype pattern.
To provide supportive evidence that the RDAf2 deletion was identical by descent, we sequenced across the RDAf2 deletion junction in nine M. bovis isolates of different spoligotypes (at least two from each of the four east African countries). The RDAf2 deletion endpoints were the same in all nine strains, suggesting that in these strains the RDAf2 deletion is identical by descent.
We concluded that a clonal complex of M. bovis strains, defined by the deletion RDAf2 and marked by the loss of spoligotype spacers 3 to 7, was present at high frequency in Uganda, Burundi, Tanzania, and Ethiopia (Table 2). We named this M. bovis clonal complex African 2.
Af2 in west-central Africa.
The M. bovis Af1 clonal complex has previously been identified at high frequency in samples of strains from Mali, Nigeria, Chad, and Cameroon (41). In a manner similar to that of Af2, described here, all isolates of the Af1 clonal complex have a specific deletion, RDAf1, and have spacer 30 missing in their spoligotype. To determine the phylogenetic relationship between Af1 and Af2 strains, a selection of isolates which represent the population of Af1 strains present in each of these west-central African countries were deletion typed for the RDAf2 deletion. All Af1 strains from Mali (n = 13), Nigeria (n = 5), Chad (n = 5), and Cameroon (n = 3) were intact for RDAf2 (see the supplemental material). Reciprocal deletion analysis showed that strains of Af2 are not deleted for RDAf1 (n = 27), and we concluded that Af1 and Af2 were phylogenetically distinct. We also performed RDAf2 deletion typing of seven strains from Mali of spoligotypes SB0134 and SB0991; these patterns represent non-Af1 isolates found in that country (42); these strains were intact at the RDAf2 and RDAf1 loci (41). We concluded, based on the dominance of the Af1 clonal complex in west-central Africa (53), that the Af2 clonal complex was rare or absent from Mali, Nigeria, Chad, and Cameroon.
Af2 elsewhere in Africa.
Spoligotype surveys of M. bovis from Algeria, Zambia, and South Africa suggest that strains with spacers 3 to 7 deleted are absent or present at low frequency in these countries (40, 43, 47, 51). Furthermore, a spoligotype survey of over 100 strains from Madagascar did not disclose any patterns with spacers 3 to 7 missing (47). To determine the prevalence of Af2 in other African countries, we RDAf2 deletion typed samples representing previously published spoligotype surveys of M. bovis from Algeria (n = 17) (51) and South Africa (n = 20) (40), as well as an unpublished set of 14 strains from Mozambique; all were intact for the RDAf2 region.
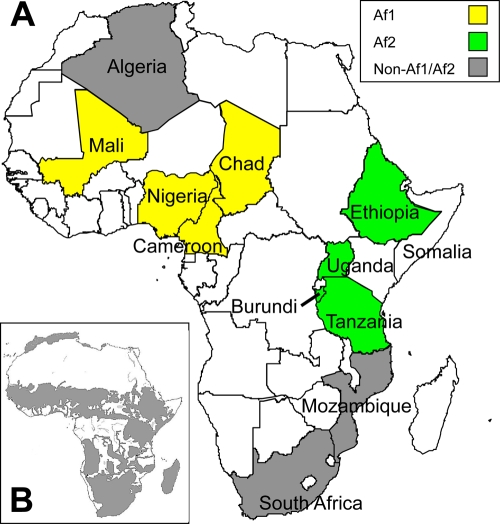
From these spoligotype and deletion surveys of M. bovis in African nations, we concluded that strains of the Af2 clonal complex were present at high frequency in some east African countries (Uganda, Burundi, Tanzania, and Ethiopia) but were rare or absent in Algeria, Mali, Nigeria, Chad, Cameroon, Zambia, South Africa, Mozambique, and Madagascar. This observation echoes the localization of the Af1 clonal complex to west-central Africa (41) (Fig. 1).
FIG. 1.
Localization of the M. bovis Af1 and Af2 clonal complexes in Africa. (A) The four west-central African countries where Af1 strains were found to be dominant are shown in yellow, and the four east African countries where Af2 strains are highly prevalent are shown in green. Isolates of the Af1 clonal complex are very rare or not present in countries with the Af2 complex and vice versa. Countries where no Af1 or Af2 strains have been identified are labeled in gray, and countries where no isolates were studied are white. (B) Cattle distribution on the African continent (gray shaded area). (Reprinted from reference 32 with permission from the publisher.).
Global distribution of Af2.
In unpublished data, we have shown that a globally distributed clonal complex of M. bovis called European 1 (Eu1), which is defined by a specific deletion called RDEu1 and the loss of spoligotype spacer 11 (spacers 4 to 7 are usually present), is present at high frequency in many parts of the world. RDAf2 deletion analysis of a sample of 21 Eu1 strains from Great Britain (see the supplemental material) showed that they were intact at the RDAf2 locus, and reciprocal deletion analysis showed that a sample of RDAf2 strains was intact at the RDEu1 locus. We concluded that strains of the Eu1 clonal complex are not members of the Af2 clonal complex and vice versa. The phylogenetic independence of Af2 and Eu1 implies that Af2 strains are rare or absent on the British Isles, most of the New World, Australia, and New Zealand, where the Eu1 clonal complex is virtually at fixation (unpublished data).
In mainland Europe, where Eu1 is rare, we inspected previously published spoligotype surveys of cattle isolates of M. bovis from France, Italy, Spain, Belgium, and Portugal for isolates showing the spoligotype signature of Af2 strains: the loss of spacers 3 to 7. We identified small numbers of isolates with the Af2 spoligotype signature from France (<2%), Italy (<2%), and Spain (<1%) (3, 10, 31, 49, 52). Sixteen (2%) of 747 M. bovis isolates from northern Italy had either spoligotype SB0133 (the most common Af2 spoligotype pattern in East Africa) or SB1584. RDAf2 deletion typing showed that none of the Italian strains were deleted for this region. Furthermore, in a large survey of 5,585 M. bovis isolates from Spanish cattle, 20 isolates were, by spoligotype pattern, possible members of the Af2 clonal complex (49). However, none of these Spanish strains were deleted for RDAf2 (see the supplemental material). We concluded that strains of the Af2 clonal complex were rare or absent in cattle outside East Africa. In this respect, Af2 again mimics the Af1 clonal complex, which has not been found in cattle outside west-central Africa (41).
Human isolates of Af2.
The prevalence of bovine TB in humans is unknown in most African countries, and M. bovis is isolated only occasionally from humans. However, previously published studies from Uganda identified three human M. bovis isolates with spoligotype patterns identical to those of the commonest Af2 strains found in that country (44-45). One of these strains was deletion typed for RDAf2, and we confirmed the region to be deleted. A previously unpublished M. bovis strain, collected in Sweden from a patient born in Somalia, had a spoligotype identical to the commonest spoligotype found in cattle in the neighboring country Ethiopia (SB1176) and was also deleted for RDAf2, with deletion boundaries identical to those observed in strains isolated from cattle (see the supplemental material).
IS6110 copy number.
It has frequently been suggested that M. bovis isolates from cattle have only one, or a few, copies of the insertion element IS6110 (4, 7, 15, 17-18, 50, 64). However, cattle isolates of M. bovis from Burundi and Uganda have been shown to have multiple copies of IS6110 (5, 48). All 10 isolates from Burundi that we have characterized as Af2 had four or more IS6110 copies, and five of the Af2 isolates from Uganda had six or more copies of IS6110. In contrast, two strains from Uganda, previously shown to have only one copy of IS6110 (5), were found to be intact at RDAf2 and therefore were not members of the Af2 clonal complex.
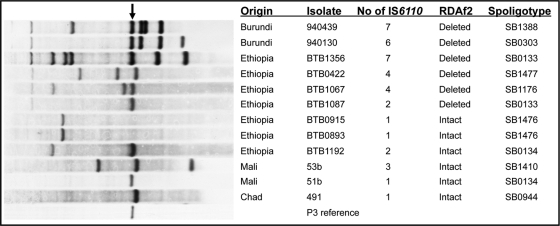
To further explore the IS6110 copy number in Af2 strains, we subjected four isolates of the Af2 clonal complex from Ethiopia to IS6110 RFLP typing. Three of the Af2 isolates contained between four and seven IS6110 copies, while a single Af2 strain from Ethiopia had only two copies of IS6110 (Fig. 2). We also IS6110 RFLP typed six strains that were intact at the RDAf2 locus (not members of the Af2 clonal complex) from Ethiopia, Mali, and Chad; four of these isolates had only one copy of IS6110; however, two strains, from Ethiopia and Mali, had two and three copies, respectively (Fig. 2). In total, including previously published IS6110 RFLP data (5, 48) on strains identified here by deletion typing as Af2, 20 of 21 Af2 strains had four or more copies of IS6110.
FIG. 2.
IS6110 RFLP patterns, IS6110 copy numbers, RDAf2 deletion types, and spoligotypes of 12 M. bovis isolates from Africa and of the reference M. bovis BCG strain P3. The arrow marks a ∼1.9-kb restriction fragment commonly representing an IS6110 copy found in the DR region of M. bovis strains.
DISCUSSION
We have identified an epidemiologically important clonal complex of M. bovis which is found at high frequency in Uganda, Burundi, Tanzania, and Ethiopia and have named this clonal complex African 2 (Af2). The Af2 clonal complex is epidemiologically important because it is commonly recovered from cattle in these four countries, but we do not yet know how phylogenetically distinct this clonal complex is from other clonal complexes of M. bovis. Members of the Af2 clonal complex are defined by a 14.1-kb deletion of chromosomal DNA which we have named Region of Difference Af2 (RDAf2). Sequencing of the RDAf2 region in nine isolates from the four countries has shown that the deletion boundaries are identical; in the absence of repetitive elements, or other features, flanking the RDAf2 deletion that can promote homoplastic deletions and the apparent strict clonality of M. bovis, we conclude that this deletion is identical by descent in strains from each of these four countries. That is, RDAf2 was deleted in the most recent common ancestor of this clonal complex, and this region is therefore deleted in all of its descendants. A definition and summary of the Af2 clonal complex are shown in Table 2.
Strains of the Af2 clonal complex can be identified by the loss of spacers 3 to 7 in the DR locus; however, this characteristic is not necessarily specific. It is theoretically possible for strains with the RDAf2 deletion to have these spacers present, although we have not yet identified such an isolate. Furthermore, because the loss of spoligotype spacers can be subject to homoplasy (54, 67), strains that are not members of the Af2 clonal complex (RDAf2 region intact) may also lack spacers 3 to 7; for example, two Nigerian strains of the African 1 clonal complex (deleted for RDAf1) (41) had also lost spacers 3 to 7, as well as the signature loss of spacer 30, and were shown to be intact for RDAf2.
Af2 in Africa.
We showed by reciprocal deletion analysis that strains of the Af2 clonal complex are not members of the African 1 (Af1) clonal complex, which is virtually fixed in Nigeria, Chad, and Cameroon and represents 65% of the isolates from Mali (41). Isolates from Mali that were not members of the dominant Af1 clonal complex have been given the preliminary name African 5 (Af5) based on the common loss of spacers 3 to 5 in their spoligotype pattern. RDAf2 deletion analysis of Af5 strains from Mali showed they were not members of the Af2 clonal complex, and we conclude that Af2 is rare or absent in all four of these west-central African countries.
We have also subjected strains from Algeria, South Africa, and Mozambique to RDAf2 deletion typing, and although the number of strains sampled in each of these countries was small, this supported our conclusion that the Af2 clonal complex is not uniformly distributed throughout Africa. In general, spoligotype surveys showed that strains with spacers 3 to 7 deleted are rare in these African nations, reinforcing the suggestion of localization of Af2 to cattle in East Africa (Fig. 1).
Af2 in the rest of the world.
An inspection of previously published spoligotype surveys from countries throughout the world did not identify any country with more than 2% of strains with spacers 3 to 7 missing in their spoligotype patterns. In unpublished data, we have observed that another clonal complex of M. bovis, European 1, is virtually fixed in the British Isles, most of the New World, Australia, and New Zealand and therefore the Af2 clonal complex is rare or absent from these countries. Furthermore, large spoligotype surveys of strains from mainland Europe (10, 22, 49) and Iran (58) and RDAf2 deletion analysis of the few strains with the Af2 spoligotype signature from Italy and Spain did not identify any Af2 strains in cattle outside Africa.
We conclude that among the countries sampled, strains of the Af2 clonal complex were common in cattle only in the four east African nations; in this respect, Af2 resembles the Af1 clonal complex, which is apparently confined to cattle in west-central Africa (41). Strains of the Af2 clonal complex represent over 70% of the isolates from Uganda, Burundi, Tanzania, and Ethiopia and have not been isolated from cattle elsewhere in the world.
Localization of Af2 genotypes.
If we assume that spoligotype spacers are lost and never regained, then all the Af2 spoligotype patterns described here can be derived from an ancestral spoligotype pattern equivalent to that of the vaccine strain BCG (SB0120, missing spacers 3, 9, 16, and 39 to 43), with the additional deletion of spacers 4 to 7 (SB0133). Although the number of strains sampled here from Uganda, Burundi, and Tanzania is small, the population structure of Af2 in these countries showed remarkable differences; the most common Af2 spoligotype pattern in each of the four east African countries surveyed was different. Spoligotype patterns SB0133 and SB0303 (a single spacer 13 loss derivative of spoligotype pattern SB0133) were the only Af2 patterns found in more than one country in our data set. However, the frequency of strains with these two patterns varied remarkably between countries. SB0133 was most common in our sample from Tanzania (64%) but was at much lower frequencies in the three other east African nations (from 0 to 14%), and spoligotype pattern SB0303 was common in Burundi (50%) but was only found in a single isolate of the 120 strains from Ethiopia. This observation contrasts with the spoligotype distribution of the Af1 clonal complex, for which a single ancestral-type spoligotype pattern was dominant in three of four west-central African nations (41).
To further investigate the national differences between Af2 clones, we six-locus VNTR typed a sample of eight strains with spoligotype pattern SB0133 from Tanzania and nine strains with the same spoligotype pattern from Ethiopia (Table 3). The Tanzanian strains were all of the same genotype (spoligotype plus VNTR type); however, this genotype was not found among the nine SB0133 strains from Ethiopia. Finally, a single isolate of spoligotype SB0303 from Ethiopia differed from the genotypes found in five strains with that spoligotype from Burundi (Table 3).
TABLE 3.
Countries of isolation, spoligotypes, VNTR types, and frequencies of M. bovis strains of the Af2 clonal complex with spoligotype patterns that were found in multiple countries
| Country | Spoligotype | ETR-VNTRa | Frequency, no. of strains (source) |
|---|---|---|---|
| Ethiopia | SB 0133 | 4 2 5 4* 2 3.1 | 6 |
| Ethiopia | SB 0133 | 3 2 5 4* 2 3.1 | 1 |
| Ethiopia | SB 0133 | 5 2 4 3* 3 3.1 | 1 |
| Ethiopia | SB 0133 | 5 2 4 3* 3 2.1 | 1 |
| Tanzania | SB 0133 | 3 2 5 4* 3 3.1 | 8 |
| Ethiopia | SB 0303 | 5 2 4 3* 3 3.1 | 1 |
| Burundi | SB 0303 | 2 2 5 4* 3 3.1 | 3 |
| Burundi | SB 0303 | 2 2 5 6* 3 3.1 | 2 |
| Ethiopia | SB 1176 | 5 2 5 4* 3 3.1 | 6 |
| Somaliab | SB 1176 | 5 2 5 4* 3 3.1 | 1 (human) |
Allele call for the ETR-A to -F loci (12).
Strain isolated in Sweden.
Both the spoligotype surveys and the genotype comparisons suggest that the population of Af2 strains in each of these four east African countries is unique. That is, for any isolate of Af2, it should be possible, with reasonable accuracy, to determine from its genotype its country of origin. This conclusion reinforces a continuing theme of national localization of M. bovis genotypes initially described for Af1 strains in west-central Africa (41). However, the genotype data presented here for Af2 and, to a lesser extent, the spoligotype data must be interpreted with care. Apart from Ethiopia, the sample sizes of strains from the other three countries were very small and, more significantly, isolated in only a few localized areas. Intracountry geographical localization of M. bovis genotypes, as is commonly seen in the United Kingdom (54) and as was discussed in a previous study (41), could confound the observation of national localization of the Af2 genotypes presented here.
Human isolates of Af2.
Support for geographical localization of Af2 genotypes comes from M. bovis strains isolated from humans. In Uganda, several human isolates were shown to have the same spoligotype pattern as those found in local cattle (5, 45). Furthermore, a Somali immigrant was diagnosed with abdominal TB shortly after arrival in Sweden, and the infection was confirmed as bovine TB (unpublished data). This M. bovis isolate was deleted for RDAf2 and had a genotype identical to those found at high frequency in Ethiopia (SB1176; 5 2 5 4* 3 3.1) (Table 3). We do not know where this patient contracted this disease, but it is possible that the original source of infection was cattle in Somalia. This epidemiological link to Somalia implies that the Af2 clonal complex may be more widely distributed across the Horn of Africa than the present study shows. However, it is possible that the Somali patient migrated via Ethiopia and was infected during transit.
Evolution of the Af2 clonal complex.
The simplest explanation for the observed distribution and population structure of the Af2 clonal complex throughout these east African countries is that this clonal complex of M. bovis spread between these four countries. The progenitor strain, originating in one place, would have had spoligotype pattern SB0133 (spacers 3 to 7 missing) and carried the RDAf2 deletion; all Af2 spoligotype patterns described here can be generated from SB0133 by spacer loss. The country-specific population structures could have evolved by drift during the spread of the Af2 clonal complex between countries in a series of founder events or subsequently as the population expanded in each country. This explanation is similar to that used to explain the distribution of Af1 strains throughout west-central African nations (41), but with one important difference. The Af1 clonal complex of M. bovis was fixed in three of the four west-central African countries in which it was sampled, and therefore it was suggested that the Af1 clonal complex was transmitted through cattle naive to bovine TB. The spoligotype surveys of strains from East Africa (Uganda, Burundi, Tanzania, and Ethiopia) showed that in three of these countries non-Af2 strains of M. bovis are present at a reasonably high frequency (between 5 and 33%). There is no obvious relationship between the spoligotype patterns of non-Af2 strains identified in Uganda, Tanzania, and Ethiopia, and we do not know the temporal relationship between the origin of these non-Af2 strains and the Af2 clonal complex; the non-Af2 strains may have been present before the introduction of the Af2 clonal complex or may have been introduced recently from neighboring countries that have not yet been surveyed for bovine TB. This question may be resolved as more countries in Africa are surveyed for genotypes of bovine TB.
Whether the progenitor of the Af2 clonal complex evolved in Africa or evolved elsewhere and was subsequently imported to Africa is unknown, although this may be resolved when the phylogenetic relationship of this clonal complex to strains from other countries is determined by whole-genome sequencing.
The RDAf2 deletion.
The large RDAf2 deletion (14.1 kb) affects 12 open reading frames on the M. bovis chromosome, of which 9 belong to an operon, mce2. M. tuberculosis has four homologous mce operons, mce1 to mce4 (14), whereas all M. bovis strains are missing the entire mce3 operon due to a deletion, RD7, which was lost early in the lineage of animal-adapted strains that leads from the recent common ancestor with M. tuberculosis to M. bovis (55). All members of the Af2 clonal complex have, in addition, lost the mce2 operon as a consequence of the RDAf2 deletion.
Each mce operon includes two yrbE and six mce genes, which show homology to ABC transporter permeases and substrate-binding proteins, respectively (13), and are believed to be involved in transport across the cell envelope. The mce4 operon in M. tuberculosis has been identified as a cholesterol import system (46), while the functions of the other three mce operons are still unclear. However, several studies have shown that M. tuberculosis isolates deleted for mce2 are attenuated in mice (1, 28, 37). It remains to be seen if strains of the M. bovis Af2 clonal complex, with a naturally deleted mce2 operon, would also be attenuated in mice. However, isolates of the Af2 clonal complex collected from cattle for this study were, in general, isolated from typical tubercle lesions, suggesting that RDAf2 isolates are capable of causing typical tuberculosis-like pathology in cattle. Further work is needed to assess any differences in pathogenicity between strains of the Af2 clonal complex and those of other clonal complexes of M. bovis.
IS6110 copy number.
It has been suggested that M. bovis from cattle has only one or two copies of the transposable element IS6110 (4, 7, 15, 17-18, 39-40, 50, 64). Most other species (or ecotypes [56]) of the M. tuberculosis complex have multiple copies of IS6110, with the exception of some M. tuberculosis lineages; for example, strains of the TbD1 intact or “ancestral” lineage are frequently found to have low copy numbers of IS6110 (20, 57). We here show that the Af2 clonal complex of M. bovis is, in general, multicopy (four or more) for IS6110. However, this is not a reliable characteristic of Af2 strains; isolate BTB1087 in this study was deleted for RDAf2 and contained only two copies of IS6110.
We suspect that the association between M. bovis and a low copy number of IS6110 has developed because of the global distribution of an M. bovis clonal complex which has, in general, only one (or rarely two) copies of IS6110 (the European 1 clonal complex; unpublished data) and is present in many developed nations with the technology to carry out IS6110 RFLP analysis.
Strains of almost all species within the M. tuberculosis complex carry an IS6110 element in the direct repeat (DR) region, which is a hot spot integration region for insertion elements (34). For M. bovis strains, this IS6110 copy in the DR region is usually visualized as a ∼1.9-kb PvuII restriction fragment in IS6110 RFLP analysis (15, 19, 64). It has previously been suggested that small variations in the size of this fragment correlate with variation in the number of spoligotype spacers in the DR region that are flanking the IS6110 DNA (64). We observed in our RFLP analysis that the single copy of IS6110 in the two Ethiopian strains, BTB0893 and BTB0915 (both intact for RDAf2), was integrated in a much larger restriction fragment of ∼4.2 kb. We suggest that this much larger fragment may be explained by the loss of the PvuII restriction site that is commonly found in spacer 36 (63); spacer 36 is absent from the spoligotype pattern of strains BTB0893 and BTB0915. Using a DR probe in RFLP analysis, we verified that a single IS6110 copy was located in the DR region in those two strains and in the other 10 isolates examined. We concluded that all M. bovis strains investigated by RFLP typing in this study carried an IS6110 copy in the DR region.
Conclusions.
We have identified a second clonal complex of M. bovis in Africa, found at high frequency in east African cattle and with a distribution that does not overlap with the previously identified west-central African clonal complex, African 1. The geographical localization of the Af2 clonal complex to these four east African countries, and perhaps to some additional neighboring countries not yet surveyed, may have been governed by geographical features that affect cattle density, trading, and movement in this part of Africa. For example, Fig. 1B shows the cattle distribution in Africa (32), and it is interesting to note the limited links between regions of high cattle density in East Africa, where Af2 is prevalent, and regions in west-central Africa, where Af1 dominates. The uneven distribution of cattle in Africa may have contributed to localized dominance of clonal complexes of M. bovis in different regions of Africa.
However, these two clonal complexes, Af1 and Af2, may represent groups of strains with different selective advantages or behaviors, and comparing and contrasting the phenotypic differences between these distinct divisions within M. bovis may elucidate the molecular mechanisms of these differences and identify the selective forces operating on both bovine TB and its cattle host. For example, Bos taurus (European cattle) is common in West Africa, where the African 1 clonal complex of M. bovis dominates, whereas Bos indicus (Zebu; Asian cattle) is common in East Africa, where African 2 dominates (32-33). It will require further work to determine if the African 1 and African 2 clonal complexes are specifically adapted to these two different types of cattle or if the relationship merely represents demographic happenstance.
On a more practical level, the results presented here show that the development of simple genotype schemes for M. bovis within these east African countries is worthwhile and will aid eradication schemes by identifying strains imported from neighboring countries (41). Furthermore, now that the African 1 and African 2 clonal complexes have been identified, it is a simple matter to sequence chromosomes of representative isolates and gather a rich harvest of specific molecular polymorphisms to use in local epidemiological analysis. Comparative genome sequence analysis will also resolve the phylogenetic status of these clonal complexes and may show that the majority of bovine TB found in Africa originated elsewhere and has been imported to the continent relatively recently. This, in turn, will develop our understanding of the historical and phylogeographical basis of bovine TB in Africa and inform our understanding of the disease in Europe and throughout the world.
Supplementary Material
Acknowledgments
We thank A. Mulder at RIVM and M. Okker and K. Gover at the VLA for excellent technical assistance.
This work was funded by the Wellcome Trust Livestock for Life and Animal Health in the Developing World initiatives, the Swiss National Science Foundation, the Swedish Heart-Lung Foundation, the Swedish Research Council, the Swedish International Development Cooperation Agency, the Damien Foundation (Belgium), the South African Medical Research Council and National Research Foundation, the MacArthur Foundation/University of Ibadan, the European Union Seventh Framework Program (Integrated Control of Neglected Zoonoses), and the Department of Environment, Food and Rural Affairs, United Kingdom.
Footnotes
Published ahead of print on 19 November 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Aguilar, L. D., et al. 2006. Immunogenicity and protection induced by Mycobacterium tuberculosis mce-2 and mce-3 mutants in a Balb/c mouse model of progressive pulmonary tuberculosis. Vaccine 24:2333-2342. [DOI] [PubMed] [Google Scholar]
- 2.Ameni, G., et al. 2007. Effect of skin testing and segregation on the prevalence of bovine tuberculosis, and molecular typing of Mycobacterium bovis, in Ethiopia. Vet. Rec. 161:782-786. [PMC free article] [PubMed] [Google Scholar]
- 3.Aranaz, A., et al. 2004. Bovine tuberculosis (Mycobacterium bovis) in wildlife in Spain. J. Clin. Microbiol. 42:2602-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aranaz, A., E. Liebana, A. Mateos, L. Dominguez, and D. Cousins. 1998. Restriction fragment length polymorphism and spacer oligonucleotide typing: a comparative analysis of fingerprinting strategies for Mycobacterium bovis. Vet. Microbiol. 61:311-324. [DOI] [PubMed] [Google Scholar]
- 5.Asiimwe, B. B., et al. 2009. Molecular characterisation of Mycobacterium bovis isolates from cattle carcases at a city slaughterhouse in Uganda. Vet. Rec. 164:655-658. [DOI] [PubMed] [Google Scholar]
- 6.Ayele, W. Y., S. D. Neill, J. Zinsstag, M. G. Weiss, and I. Pavlik. 2004. Bovine tuberculosis: an old disease but a new threat to Africa. Int. J. Tuber. Lung Dis. 8:924-937. [PubMed] [Google Scholar]
- 7.Bauer, J., A. B. Andersen, K. Kremer, and H. Miorner. 1999. Usefulness of spoligotyping to discriminate IS6110 low-copy-number Mycobacterium tuberculosis complex strains cultured in Denmark. J. Clin. Microbiol. 37:2602-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg, S., et al. 2009. The burden of mycobacterial disease in Ethiopian cattle: implications for public health. PLoS One 4:e5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biffa, D., et al. 2010. Molecular characterization of Mycobacterium bovis isolates from Ethiopian cattle. BMC Vet. Res. 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boniotti, M. B., et al. 2009. Molecular typing of Mycobacterium bovis strains isolated in Italy from 2000 to 2006 and evaluation of variable-number tandem repeats for geographically optimized genotyping. J. Clin. Microbiol. 47:636-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brosch, R., et al. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. U. S. A. 99:3684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cadmus, S., et al. 2006. Molecular analysis of human and bovine tubercle bacilli from a local setting in Nigeria. J. Clin. Microbiol. 44:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casali, N., and L. W. Riley. 2007. A phylogenomic analysis of the Actinomycetales mce operons. BMC Genomics 8:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole, S. T., et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 15.Collins, D. M., S. K. Erasmuson, D. M. Stephens, G. F. Yates, and G. W. De Lisle. 1993. DNA fingerprinting of Mycobacterium bovis strains by restriction fragment analysis and hybridization with insertion elements IS1081 and IS6110. J. Clin. Microbiol. 31:1143-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosivi, O., et al. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costello, E., et al. 1999. Study of restriction fragment length polymorphism analysis and spoligotyping for epidemiological investigation of Mycobacterium bovis infection. J. Clin. Microbiol. 37:3217-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cousins, D., et al. 1998. Evaluation of four DNA typing techniques in epidemiological investigations of bovine tuberculosis. J. Clin. Microbiol. 36:168-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cousins, D. V., S. N. Williams, B. C. Ross, and T. M. Ellis. 1993. Use of a repetitive element isolated from Mycobacterium tuberculosis in hybridization studies with Mycobacterium bovis: a new tool for epidemiological studies of bovine tuberculosis. Vet. Microbiol. 37:1-17. [DOI] [PubMed] [Google Scholar]
- 20.Das, S., C. N. Paramasivan, D. B. Lowrie, R. Prabhakar, and P. R. Narayanan. 1995. IS6110 restriction fragment length polymorphism typing of clinical isolates of Mycobacterium tuberculosis from patients with pulmonary tuberculosis in Madras, south India. Tuber. Lung Dis. 76:550-554. [DOI] [PubMed] [Google Scholar]
- 21.Diguimbaye-Djaibe, C., et al. 2006. Mycobacterium bovis isolates from tuberculous lesions in Chadian zebu carcasses. Emerg. Infect. Dis. 12:769-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duarte, E. L., M. Domingos, A. Amado, and A. Botelho. 2008. Spoligotype diversity of Mycobacterium bovis and Mycobacterium caprae animal isolates. Vet. Microbiol. 130:415-421. [DOI] [PubMed] [Google Scholar]
- 23.Fang, Z., N. Morrison, B. Watt, C. Doig, and K. J. Forbes. 1998. IS6110 transposition and evolutionary scenario of the direct repeat locus in a group of closely related Mycobacterium tuberculosis strains. J. Bacteriol. 180:2102-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frothingham, R., and W. A. Meeker-O'Connell. 1998. Genetic diversity in the Mycobacterium tuberculosis complex based on variable numbers of tandem DNA repeats. Microbiology 144(Pt. 5):1189-1196. [DOI] [PubMed] [Google Scholar]
- 25.Gagneux, S., et al. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 103:2869-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Pelayo, M. C., et al. 2004. Microarray analysis of Mycobacterium microti reveals deletion of genes encoding PE-PPE proteins and ESAT-6 family antigens. Tuberculosis (Edinb.) 84:159-166. [DOI] [PubMed] [Google Scholar]
- 27.Garnier, T., et al. 2003. The complete genome sequence of Mycobacterium bovis. Proc. Natl. Acad. Sci. U. S. A. 100:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gioffre, A., et al. 2005. Mutation in mce operons attenuates Mycobacterium tuberculosis virulence. Microbes Infect. 7:325-334. [DOI] [PubMed] [Google Scholar]
- 29.Groenen, P. M., A. E. Bunschoten, D. van Soolingen, and J. D. van Embden. 1993. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Mol. Microbiol. 10:1057-1065. [DOI] [PubMed] [Google Scholar]
- 30.Gutacker, M. M., et al. 2002. Genome-wide analysis of synonymous single nucleotide polymorphisms in Mycobacterium tuberculosis complex organisms: resolution of genetic relationships among closely related microbial strains. Genetics 162:1533-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haddad, N., et al. 2001. Spoligotype diversity of Mycobacterium bovis strains isolated in France from 1979 to 2000. J. Clin. Microbiol. 39:3623-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanotte, O., et al. 2002. African pastoralism: genetic imprints of origins and migrations. Science 296:336-339. [DOI] [PubMed] [Google Scholar]
- 33.Hanotte, O., et al. 2000. Geographic distribution and frequency of a taurine Bos taurus and an indicine Bos indicus Y specific allele amongst sub-saharan African cattle breeds. Mol. Ecol. 9:387-396. [DOI] [PubMed] [Google Scholar]
- 34.Hermans, P. W., et al. 1991. Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect. Immun. 59:2695-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilty, M., et al. 2005. Evaluation of the discriminatory power of variable number tandem repeat (VNTR) typing of Mycobacterium bovis strains. Vet. Microbiol. 109:217-222. [DOI] [PubMed] [Google Scholar]
- 36.Kamerbeek, J., et al. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marjanovic, O., T. Miyata, A. Goodridge, L. V. Kendall, and L. W. Riley. 2010. Mce2 operon mutant strain of Mycobacterium tuberculosis is attenuated in C57BL/6 mice. Tuberculosis (Edinb.) 90:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mfinanga, S. G., et al. 2004. Mycobacterial adenitis: role of Mycobacterium bovis, non-tuberculous mycobacteria, HIV infection, and risk factors in Arusha, Tanzania. East Afr. Med. J. 81:171-178. [DOI] [PubMed] [Google Scholar]
- 39.Michel, A. L., et al. 2009. Molecular epidemiology of Mycobacterium bovis isolates from free-ranging wildlife in South African game reserves. Vet. Microbiol. 133:335-343. [DOI] [PubMed] [Google Scholar]
- 40.Michel, A. L., et al. 2008. High Mycobacterium bovis genetic diversity in a low prevalence setting. Vet. Microbiol. 126:151-159. [DOI] [PubMed] [Google Scholar]
- 41.Muller, B., et al. 2009. African 1, an epidemiologically important clonal complex of Mycobacterium bovis dominant in Mali, Nigeria, Cameroon, and Chad. J. Bacteriol. 191:1951-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller, B., et al. 2008. Molecular characterisation of Mycobacterium bovis isolated from cattle slaughtered at the Bamako abattoir in Mali. BMC Vet. Res. 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munyeme, M., et al. 2009. Isolation and characterization of Mycobacterium bovis strains from indigenous Zambian cattle using spacer oligonucleotide typing technique. BMC Microbiol. 9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oloya, J., et al. 2007. Characterisation of mycobacteria isolated from slaughter cattle in pastoral regions of Uganda. BMC Microbiol. 7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oloya, J., et al. 2008. Mycobacteria causing human cervical lymphadenitis in pastoral communities in the Karamoja region of Uganda. Epidemiol. Infect. 136:636-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pandey, A. K., and C. M. Sassetti. 2008. Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U. S. A. 105:4376-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rasolofo Razanamparany, V., et al. 2006. Usefulness of restriction fragment length polymorphism and spoligotyping for epidemiological studies of Mycobacterium bovis in Madagascar: description of new genotypes. Vet. Microbiol. 114:115-122. [DOI] [PubMed] [Google Scholar]
- 48.Rigouts, L., et al. 1996. Use of DNA restriction fragment typing in the differentiation of Mycobacterium tuberculosis complex isolates from animals and humans in Burundi. Tuber. Lung Dis. 77:264-268. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez, S., et al. 2010. High spoligotype diversity within a Mycobacterium bovis population: clues to understanding the demography of the pathogen in Europe. Vet. Microbiol. 141:89-95. [DOI] [PubMed] [Google Scholar]
- 50.Romano, M. I., et al. 1996. Comparison of different genetic markers for molecular epidemiology of bovine tuberculosis. Vet. Microbiol. 50:59-71. [DOI] [PubMed] [Google Scholar]
- 51.Sahraoui, N., et al. 2009. Molecular characterization of Mycobacterium bovis strains isolated from cattle slaughtered at two abattoirs in Algeria. BMC Vet. Res. 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serraino, A., et al. 1999. Monitoring of transmission of tuberculosis between wild boars and cattle: genotypical analysis of strains by molecular epidemiology techniques. J. Clin. Microbiol. 37:2766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith, N. H., et al. 2003. The population structure of Mycobacterium bovis in Great Britain: clonal expansion. Proc. Natl. Acad. Sci. U. S. A. 100:15271-15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith, N. H., S. V. Gordon, R. de la Rua-Domenech, R. S. Clifton-Hadley, and R. G. Hewinson. 2006. Bottlenecks and broomsticks: the molecular evolution of Mycobacterium bovis. Nat. Rev. Microbiol. 4:670-681. [DOI] [PubMed] [Google Scholar]
- 55.Smith, N. H., R. G. Hewinson, K. Kremer, R. Brosch, and S. V. Gordon. 2009. Myths and misconceptions: the origin and evolution of Mycobacterium tuberculosis. Nat. Rev. Microbiol. 7:537-544. [DOI] [PubMed] [Google Scholar]
- 56.Smith, N. H., et al. 2006. Ecotypes of the Mycobacterium tuberculosis complex. J. Theor. Biol. 239:220-225. [DOI] [PubMed] [Google Scholar]
- 57.Sun, Y. J., et al. 2004. Characterization of ancestral Mycobacterium tuberculosis by multiple genetic markers and proposal of genotyping strategy. J. Clin. Microbiol. 42:5058-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tadayon, K., N. Mosavari, F. Sadeghi, and K. J. Forbes. 2008. Mycobacterium bovis infection in Holstein Friesian cattle, Iran. Emerg. Infect. Dis. 14:1919-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsegaye, A., et al. 2010. Conventional and molecular epidemiology of bovine tuberculosis in dairy farms in Addis Ababa City, the capital of Ethiopia. J. Appl. Res. Vet. Med. 8:143-151. [Google Scholar]
- 60.Tsolaki, A. G., et al. 2005. Genomic deletions classify the Beijing/W strains as a distinct genetic lineage of Mycobacterium tuberculosis. J. Clin. Microbiol. 43:3185-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Zanden, A. G., et al. 1998. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis complex in paraffin wax embedded tissues and in stained microscopic preparations. Mol. Pathol. 51:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Embden, J. D., et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Embden, J. D., et al. 2000. Genetic variation and evolutionary origin of the direct repeat locus of Mycobacterium tuberculosis complex bacteria. J. Bacteriol. 182:2393-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Soolingen, D., et al. 1994. Use of various genetic markers in differentiation of Mycobacterium bovis strains from animals and humans and for studying epidemiology of bovine tuberculosis. J. Clin. Microbiol. 32:2425-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Soolingen, D., P. E. W. de Haas, and K. Kremer. 2001. Restriction fragment length polymorphism typing of mycobacteria, p. 165-203. In T. Parish and N. G. Stoker (ed.), Mycobacterium tuberculosis protocols. Humana Press Inc., Totowa, NJ. [DOI] [PubMed]
- 66.van Soolingen, D., P. W. Hermans, P. E. de Haas, D. R. Soll, and J. D. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warren, R. M., et al. 2002. Microevolution of the direct repeat region of Mycobacterium tuberculosis: implications for interpretation of spoligotyping data. J. Clin. Microbiol. 40:4457-4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.