Abstract
Cells of Flavobacterium johnsoniae move rapidly over surfaces by a process known as gliding motility. Gld proteins are thought to comprise the gliding motor that propels cell surface adhesins, such as the 669-kDa SprB. A novel protein secretion apparatus called the Por secretion system (PorSS) is required for assembly of SprB on the cell surface. Genetic and molecular analyses revealed that sprB is part of a seven-gene operon spanning 29.3 kbp of DNA. In addition to sprB, three other genes of this operon (sprC, sprD, and sprF) are involved in gliding. Mutations in sprB, sprC, sprD, and sprF resulted in cells that failed to form spreading colonies on agar but that exhibited some motility on glass in wet mounts. SprF exhibits some similarity to Porphyromonas gingivalis PorP, which is required for secretion of gingipain protease virulence factors via the P. gingivalis PorSS. F. johnsoniae sprF mutants produced SprB protein but were defective in localization of SprB to the cell surface, suggesting a role for SprF in secretion of SprB. The F. johnsoniae PorSS is involved in secretion of extracellular chitinase in addition to its role in secretion of SprB. SprF was not needed for chitinase secretion and may be specifically required for SprB secretion by the PorSS. Cells with nonpolar mutations in sprC or sprD produced and secreted SprB and propelled it rapidly along the cell surface. Multiple paralogs of sprB, sprC, sprD, and sprF are present in the genome, which may explain why mutations in sprB, sprC, sprD, and sprF do not result in complete loss of motility and suggests the possibility that semiredundant SprB-like adhesins may allow movement of cells over different surfaces.
Cells of Flavobacterium johnsoniae crawl over surfaces in a process called gliding motility (18). This type of motility is common among members of the phylum Bacteroidetes, of which F. johnsoniae is a member. Cells of F. johnsoniae move at speeds of approximately 2 μm/s and form colonies that have thin spreading edges.
F. johnsoniae cells lack well-studied motility structures such as flagella or type IV pili, and the mechanism of cell movement appears to be novel. Twelve proteins (GldA, GldB, GldD, GldF, GldG, GldH, GldI, GldJ, GldK, GldL, GldM, and GldN) are required for cell movement (1, 5, 6, 11-13, 19, 20, 28). Disruption of the genes encoding any of these proteins results in the formation of nonspreading colonies and complete absence of cell movement. Several other proteins (SprA, SprB, and SprT) also have roles in motility (23, 24, 29). Mutations in the genes encoding these proteins result in formation of nonspreading colonies on agar that are indistinguishable from those of gld mutants, but cells exhibit limited movements on some surfaces. For sprB mutants the residual motility may be explained by the presence of proteins in the cell that have similar sequences and potentially have overlapping functions. Some of the Gld proteins likely constitute the gliding motor, although the exact nature of the motor is not yet clear. SprB is present on the cell surface and moves rapidly along the surface, apparently propelled by this motor (23). GldK, GldL, GldM, GldN, SprA, and SprT appear to be components of a novel protein secretion system that is only found in members of the phylum Bacteroidetes and has been referred to as the Por secretion system (PorSS) (29). The PorSS is not closely related to the more well-studied type I to VII bacterial protein secretion systems (10). The F. johnsoniae PorSS is required for secretion of SprB to the cell surface and also for secretion of an extracellular chitinase (28, 29).
Multiple sprB paralogs are present in the F. johnsoniae genome (22). Many of these paralogs are flanked by genes that resemble those adjacent to sprB. This conservation of gene order suggests that the proteins encoded by genes near sprB may function with SprB and have roles in motility. This paper describes genetic and molecular experiments that identified three genes, sprC, sprD, and sprF, that are cotranscribed with sprB and are involved in motility and required for formation of spreading colonies. The exact functions of SprC and SprD are not known, but SprF appears to be required for secretion of SprB to the cell surface.
MATERIALS AND METHODS
Bacterial strains, bacteriophages, plasmids, and growth conditions.
F. johnsoniae ATCC 17061 was the wild-type strain used in this study. F. johnsoniae strains were grown in Casitone-yeast extract (CYE) medium at 30°C, as previously described (21). To observe colony spreading, F. johnsoniae was grown on PY2 agar medium (1) at 25°C. Motility medium (MM) was used to observe movement of individual cells in wet mounts (16). The bacteriophages active against F. johnsoniae that were used in this study were φCj1, φCj13, φCj23, φCj28, φCj29, φCj42, φCj48, and φCj54 (7, 27, 32). Sensitivity to bacteriophages was determined essentially as previously described by spotting 5 μl of phage lysate (109 PFU/ml) onto lawns of cells in CYE overlay agar (13). The plates were incubated for 24 h at 25°C to observe lysis. Plasmids used in this study are listed in Table 1, and the primers are listed in Table S1 of the supplemental material. The plasmids used for complementation were all derived from pCP1 and have copy numbers of approximately 10 in F. johnsoniae (1, 14, 21). Antibiotics were used at the following concentrations when needed: ampicillin, 100 μg/ml; cefoxitin, 100 μg/ml; chloramphenicol, 30 μg/ml; erythromycin, 100 μg/ml; kanamycin, 35 μg/ml; tetracycline, 20 μg/ml.
TABLE 1.
Plasmids used in this study
| Plasmid | Descriptiona | Source or reference |
|---|---|---|
| pBC SK+ | ColE1 ori; Cmr | Stratagene |
| pMAL-c2 | MalE fusion protein expression vector; Apr | New England Biolabs |
| pCP23 | E. coli-F. johnsoniae shuttle plasmid; Apr (Tc)r | 1 |
| pCP29 | E. coli-F. johnsoniae shuttle plasmid; Apr (Cfr Emr) | 14 |
| pLYL03 | Plasmid carrying ermF; Apr (Emr) | 15 |
| pEP4351 | Plasmid carrying Tn4351; Cmr Tcr (Emr) | 9 |
| pHimarEm1 | Plasmid carrying HimarEm1; Kmr (Emr) | 5 |
| pHimarEm2 | Plasmid carrying HimarEm2; Kmr (Emr) | 5 |
| pMM339 | pSN80 with 503-bp BlpI-to-SexAI deletion within sprC; Apr Kmr (Tcr) | This study |
| pRR04 | 1.3-kbp fragment of sprD cloned into BamHI and SphI sites of pLYL03; used for plasmid-mediated disruption of sprD; Apr (Emr) | This study |
| pRR13 | 535-bp fragment of fjoh_0983 cloned into BamHI and PstI sites of pLYL03; used for plasmid-mediated disruption of fjoh_0983; Apr (Emr) | This study |
| pRR46 | 763-bp fragment of sprF cloned into XbaI and SphI sites of pLYL03; used for plasmid-mediated disruption of sprF; Apr (Emr) | This study |
| pRR48 | 1.3-kbp fragment spanning sprF inserted in XbaI and SphI sites of pCP23; Apr (Tcr) | This study |
| pRR49 | 1.2-kbp fragment spanning sprF inserted in MluI and XbaI sites of pSN80; Apr Kmr (Tcr) | This study |
| pRR68 | 2.65-kbp BamHI-SphI fragment of pSP1 spanning fjoh_0984, fjoh_0983, and fjoh_0982 inserted into BamHI and SphI sites of pCP23; Apr (Tcr) | This study |
| pSN19 | Plasmid carrying HimarEm2 insertion FJ121 in sprB and adjacent chromosomal DNA to XbaI sites; Kmr (Emr) | This study |
| pSN60 | pCP29 carrying sprB; Apr (Cfr Emr) | 23 |
| pSN80 | 10.2-kbp BglII fragment of FJ121 chromosomal DNA spanning sprC and sprD and the Kmr cassette inserted in BamHI site of pCP23; Apr Kmr (Tcr) | This study |
| pSN81 | 10.2-kbp BglII fragment of FJ121 chromosomal DNA spanning sprC and sprD and the Kmr cassette inserted in BamHI site of pCP23 (identical to pSN80 except that sprC sprD fragment is in opposite orientation); Apr Kmr (Tcr) | This study |
| pSN92 | 1.3-kbp fragment containing the 3′ end of sprC in EcoRI site of pMAL-c2; Apr | This study |
| pSN94 | 4.8-kbp fragment containing the 3′ end of sprD in SalI site of pMAL-c2; Apr | This study |
| pSP1 | 2.65-kbp fragment upstream of sprC inserted in BamHI and SalI sites of pLYL03; Apr (Emr) | This study |
| pSP2 | 2.7-kbp fragment downstream of sprB inserted in SalI and SphI sites of pSP1; Apr (Emr) | This study |
| pSP24 | 3.5-kbp PstI fragment from pSN80 spanning sprC in pCP23; Apr (Tcr) | This study |
Antibiotic resistance phenotypes: ampicillin, Apr; cefoxitin, Cfr; chloramphenicol, Cmr; erythromycin, Emr; kanamycin, Kmr; tetracycline, Tcr. Unless indicated otherwise, the antibiotic resistance phenotypes are those expressed in E. coli. The antibiotic resistance phenotypes given in parentheses are those expressed in F. johnsoniae but not in E. coli.
Transposon mutagenesis and identification of sites of insertion.
Tn4351 and HimarEm2 were introduced into wild-type F. johnsoniae by conjugation from Escherichia coli as previously described (5, 11, 13). Mutants were selected by plating cells on PY2 agar containing erythromycin and screening for nonspreading colonies. Chromosomal DNA was isolated from mutants, and sites of transposon insertion were determined as previously described (5, 11, 14).
Disruption of sprD, sprF, and fjoh_0983 by plasmid insertion.
For disruption of fjoh_0983, a 535-bp internal fragment was amplified using primers 844 and 845, which were designed with engineered BamHI and PstI restriction sites, respectively. The fragment was digested with BamHI and PstI and inserted into the suicide vector pLYL03 that had been digested with the same enzymes, to generate pRR13. pRR13 was introduced into F. johnsoniae by triparental conjugation, and the mutant CJ1708 possessing the plasmid inserted into fjoh_0983 by recombination was isolated on PY2 agar containing erythromycin. Disruption of fjoh_0983 in CJ1708 was confirmed by PCR using primer 842, which is 1,188 bp upstream of the fjoh_0983 translational start site, and primer 737, which is specific for pLYL03. For disruption of sprD a 1,343-bp fragment near the 5′ end of the gene was amplified using primers 824 and 825, which were designed with BamHI and SphI restriction sites, respectively. The fragment was cloned into pLYL03 that had been digested with BamHI and SphI, to generate pRR04. pRR04 was introduced into F. johnsoniae as described above to obtain the sprD mutant CJ1695. Disruption of sprD in CJ1695 was confirmed by PCR using primer 807, which is 747 bp upstream of the sprD translational start site, and primer 737. For disruption of sprF, a 763-bp internal fragment, was amplified using primers 946 and 952, which were designed with XbaI and SphI restriction sites, respectively. The fragment was inserted into pLYL03 that had been digested with XbaI and SphI, to generate pRR46. pRR46 was introduced into F. johnsoniae as described above to obtain the sprF mutant CJ1814. Disruption of sprF in CJ1814 was confirmed by PCR using primer 650, which is 691 bp upstream of the sprF translational start site, and primer 737.
Sequence analysis.
Sequences were analyzed with MacVector software (MacVector, Inc., Cary, NC), and comparisons to database sequences were made using the BLAST algorithm (2). Predictions regarding cellular localization were made using SignalP (4), PSORTb (34), and CELLO (33), and potential coiled-coil structures were identified using COILS (17) and Multicoil (30).
Cloning of genes of the sprB operon.
F. johnsoniae Fj121 has a HimarEm2 insertion within sprB, 2,058 bp downstream of the sprD stop codon. Fj121 chromosomal DNA was digested with BglII, generating a 10,285-bp fragment that spanned fjoh_0982, sprC, and sprD. This fragment included 616 bp upstream of the fjoh_0982 start codon and the kanamycin resistance gene of the transposon. The fragment was ligated into the BamHI site of pCP23 in both orientations to generate pSN80 and pSN81. To construct a plasmid that contained only fjoh_0982 and sprC, the 3.5-kbp PstI fragment of pSN80 spanning sprC was inserted into the PstI site of pCP23, resulting in pSP24. To generate a plasmid that expressed sprD but not sprC, pSN80 was cut with BlpI and SexAI, removing a 503-bp piece of DNA internal to sprC. The ends of the larger fragment were filled in using Klenow polymerase and ligated, resulting in pMM339. To construct a plasmid carrying only sprF, primers 954 and 955, with engineered XbaI and SphI restriction sites, respectively, were used to amplify a 1.3-kbp fragment spanning sprF. This fragment was digested with XbaI and SphI and inserted into pCP23 that had been digested with the same enzymes, to generate pRR48. To construct a plasmid expressing fjoh_0982, sprC, sprD, and sprF, primers 966 and 967, with engineered MluI and XbaI sites, respectively, were used to amplify a 1.2-kbp fragment spanning sprF. The fragment was digested with MluI and XbaI and inserted into the same sites of pSN80 to generate pRR49. To generate a plasmid that carried fjoh_0984, fjoh_0983, and fjoh_0982, a 2.65-kbp fragment spanning these genes was amplified from wild-type chromosomal DNA using primers 718 and 719. The resulting fragment was digested with BamHI and SalI and ligated into pLYL03 that had been digested with the same enzymes, resulting in pSP1. pSP1 was digested with BamHI and SphI, and the 2.65-kbp fragment spanning fjoh_0984, fjoh_0983, and fjoh_0982 was ligated into pCP23 which had been digested with the same enzymes, generating pRR68.
Deletion of sprC, sprD, and sprB.
A 2.78-kbp fragment spanning pgk, sprF, and the last 223 bp of sprB was amplified from wild-type chromosomal DNA using primers 720 and 727. The fragment was digested with SalI and SphI and ligated into pSP1 at the SalI and SphI sites to form pSP2. pSP2, which carries a 2.65-kbp region upstream of sprC and a 2.78-kbp region downstream of sprB, was introduced into wild-type F. johnsoniae by conjugation, and erythromycin-resistant colonies were obtained following integration of the plasmid into the genome by a single recombination event. Bacteriophage resistance was used for selection, and loss of erythromycin resistance was used as a screening tool, to obtain a strain (CJ1584) in which a second recombination event had resulted in loss of sprC, sprD, and sprB, essentially as previously described (28). Bacteriophage φCj13 was used for this selection, since it was previously demonstrated that sprB mutants are resistant to this phage (23). Strains with pSP2 inserted into the genome were selected for phage resistance by plating on CYE agar (without erythromycin) overlaid with top agar containing bacteriophage φCj13 (4 × 108 phage per plate) to kill cells that had not lost sprB. Bacteriophage φCj13-resistant colonies were screened for erythromycin sensitivity to confirm loss of the integrated plasmid. A 2.1-kbp fragment was amplified from chromosomal DNA using primers 678 and 669 and sequenced to confirm the deletion of sprC, sprD, and sprB in CJ1584.
Reverse transcriptase PCR (RT-PCR) and 5′-random amplification of cDNA ends (5′-RACE) to characterize the sprB transcriptional unit.
RNA was extracted from wild-type F. johnsoniae cells grown in 25 ml of MM overnight at 25°C without shaking. Cells were harvested by centrifugation at 4,000 × g for 10 min, and the pellet was suspended in 450 μl of MM. One milliliter of Bacteria RNAProtect (Qiagen, Valencia, CA) was added, mixed by vortexing, and incubated at room temperature for 5 min. Cells were collected by centrifugation at 5,000 × g for 10 min, the supernatant was removed, and the pellet was stored at −80°C for subsequent RNA extraction. RNA was extracted and purified using the RNeasy minikit (Qiagen) according to the manufacturer's instructions, except that 40 units of RNasin (Promega Corp., Madison, WI) was added after extraction, and the sample was treated twice for 1 h at 37°C with 5 units of RQ1 RNase-free DNase (Promega) to remove contaminating genomic DNA. RNA concentrations (based on the optical density at 260 nm [OD260]) and purity (OD260/280) were determined by UV spectroscopy, and RNA integrity was verified by visualizing the intensity of the 16S and 23S rRNA bands on a 1% agarose gel containing ethidium bromide.
RT-PCR was used to determine the transcriptional organization of sprB and flanking genes. cDNA was generated using the SuperScript first-strand synthesis for RT-PCR kit (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions. Briefly, 1 μg of RNA was reverse transcribed with SuperScript III RT enzyme at 50°C for 50 min using gene-specific primers 829, 831, 947, and 986. The RT enzyme was heat inactivated, and RNA was degraded from RNA-cDNA hybrids using RNase H. A separate no-RT reaction was run with each gene-specific primer to confirm that the RNA was not contaminated with chromosomal DNA. For PCR, antisense primers used to generate the cDNA were used in various combinations with sense primers to obtain products spanning the junction of adjacent genes. Three reaction mixtures were prepared for each primer set, and the following templates were added: (i) 40 ng of wild-type F. johnsoniae chromosomal DNA as a positive control, (ii) 2 μl of the appropriate no-RT negative-control reaction mixture, and (iii) 2 μl of the appropriate cDNA reaction mixture. Templates were amplified using ExTaq (Takara Bio Inc., Otsu, Japan) with a thermal cycle consisting of initial denaturation for 5 min at 94°C, followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 to 3 min (depending on the size of the expected product). This was followed by a final extension at 72°C for 10 min. PCR products were separated on a 1% agarose gel and visualized using ethidium bromide.
The transcriptional start site for the sprB operon was determined using the 2nd Generation 5′/3′ RACE kit (Roche Applied Science, Mannheim, Germany). Briefly, first-strand cDNA synthesis was performed with 1 μg of total RNA using antisense primer 858, which lies within fjoh_0983. The cDNA reaction mixture was purified using the High Pure PCR product purification kit (Roche Applied Science), divided into two reaction tubes, and tailed with A or G by using recombinant terminal deoxynucleotidyl transferase and dATP or dGTP. A PCR product was obtained from each tailed cDNA by using the appropriate anchor primer [oligo(dT)-anchor primer or oligo(dC)-anchor primer] and the nested gene-specific primer 857. The sequences of the PCR products were used to determine the transcriptional start site. Promoter analysis was carried out by comparison of the region upstream of the transcriptional start site with that of the previously described F. johnsoniae promoter consensus sequence (8).
Protein expression and antibody production.
A 1,347-bp fragment encoding the C-terminal 426 amino acids of SprC was amplified using FideliTaq DNA polymerase (USB Corporation, Cleveland, OH) and primers 700 and 701. The fragment was cloned into the EcoRI site of pMalC2 (New England Biolabs, Ispwich, MA), generating pSN92. pSN92 was introduced into E. coli Rosetta 2(DE3) cells (Novagen, Madison, WI), which expressed seven rare tRNAs required for efficient expression of SprC. To isolate recombinant SprC, cells were grown to mid-log phase at 37°C in rich medium plus glucose (10 g tryptone, 5 g yeast extract, 5 g NaCl, 2 g glucose/liter), induced by the addition of 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and incubated for an additional 48 h at 37°C. Cells were disrupted using a French press, insoluble material was pelleted by centrifugation at 6,415 × g for 30 min, and the supernatant was applied to amylose resin (New England Biolabs). Recombinant SprC was eluted with column buffer (200 mM NaCl, 1 mM EDTA, 20 mM Tris-HCl; pH 7.0) containing 10 mM maltose. SprC was further purified by SDS-PAGE, visualized by CuCl2 staining, and electro-eluted as previously described (23), except that 7.5% acrylamide gels were used.
A 4,800-bp fragment encoding the C-terminal 1,562 amino acids of SprD was amplified using FideliTaq DNA polymerase and primers 702 and 703 and inserted into pMalC2 at the SalI site to generate pSN94. pSN94 was introduced into E. coli Rosetta 2(DE3) cells, as described above. To isolate recombinant SprD, cells were grown in rich medium plus glucose to mid-log phase at 37°C, induced with 0.3 mM IPTG, and incubated for an additional 48 h at 25°C. Cells were disrupted with a French press, and insoluble material was removed by centrifugation at 6,415 × g for 30 min. Recombinant SprD was purified from the supernatant on 5% acrylamide gels by SDS-PAGE, as described above. Polyclonal antibodies against recombinant SprC and SprD were produced and affinity purified using the recombinant proteins of Proteintech Group, Inc. (Chicago, IL).
Western blot analyses and cell fractionation.
F. johnsoniae cells were grown to mid-log phase in MM at 25°C without shaking. Cells were pelleted at 4,000 × g, disrupted with a French pressure cell, and solubilized in SDS-PAGE loading buffer by boiling for 5 min. Proteins (40 μg) were separated by SDS-PAGE (7.5% acrylamide running gel for SprC, 5% or 7.5% acrylamide running gel for SprD, and 3 to 8% Criterion XT Tris-acetate-acrylamide gradient gels [Bio-Rad, Hercules, CA] for SprB), and Western blot analyses were performed as previously described (28).
Microscopic observations of cell movement and attachment.
Wild-type and mutant cells of F. johnsoniae were examined for attachment to glass by using a Petroff-Hausser counting chamber, as previously described (24). Cells were also examined for movement over glass by phase-contrast microscopy. Cells in MM were spotted onto a glass microscope slide and were covered with a glass coverslip, incubated for 1 min, and observed for motility by using an Olympus BH-2 phase-contrast microscope with a heated stage set at 25°C. For analyses of cell tracks, cell movement was observed using Palmer counting cells (Wildlife Supply Company, Saginaw, MI) as previously described (23), except that 22-mm2 glass coverslips were used. Images were recorded using a Photometrics CoolSNAPcf2 camera and were analyzed using MetaMorph software (Molecular Devices, Downingtown, PA). Tracks illustrating the movements of cells were obtained by superimposing individual digital video frames using the “Logical AND” operation of MetaMorph.
Immunofluorescent localization of SprB.
Cells were grown overnight in MM at 25°C. Twenty-microliter aliquots of cells were diluted in 130 μl of MM and fixed with 1% formaldehyde for 15 min. Cells were collected on 0.4-μm Isopore membrane filters (Millipore, Billerica, MA) by filtration. Cells were washed three times with 200 μl of phosphate-buffered saline (PBS) and were blocked with 0.1% bovine serum albumin (BSA) in PBS for 30 min. After removal of the blocking solution by filtration, cells were exposed to 200 μl of a 1:200 dilution of purified anti-SprB in PBS with 0.1% BSA for 90 min. Cells were washed five times with 200 μl of PBS and exposed to 200 μl of F(ab[prime]) fragment of goat anti-rabbit IgG conjugated to Alexa-488 (0.4 μg/ml; Invitrogen) in PBS plus 0.1% BSA. Cells were incubated for 60 min in the dark, the liquid was removed, and cells were washed five times with PBS. During the final PBS wash, 1 μl of 0.3% InSpeck relative intensity fluorescent beads (Invitrogen-Molecular Probes, Eugene, OR) was added as a control. The final wash was removed by filtration, the filters were mounted on glass slides with 6 μl of VectaShield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA), coverslips were applied, and samples were observed using a Nikon Eclipse 50i microscope. Images were captured with a Photometrics CoolSNAPES camera with exposure times of 500 to 700 ms (DAPI) and 500 ms (Alexa-488).
Binding and movement of protein G-coated polystyrene spheres.
Movement of surface-localized SprB was detected as previously described (23). Cells were grown overnight at 25°C in MM without shaking. Purified anti-SprB (1 μl of a 1:10 dilution of a 300-mg/liter stock), 0.5-μm-diameter protein G-coated polystyrene spheres (1 μl of a 0.1% stock preparation; Spherotech Inc., Libertyville, IL), and bovine serum albumin (1 μl of a 1% solution) were added to 7 μl of cells (approximately 5 × 108 cells per ml) in MM. The cells were spotted on a glass slide, covered with a glass coverslip, and examined by phase-contrast microscopy at 25°C. Samples were examined 1 min after spotting, and images were captured for 30 s. Similar experiments were performed using purified anti-SprC and anti-SprD instead of anti-SprB.
Measurements of chitin digestion.
Chitin utilization on plates was observed as previously described (19, 28). Chitinase activity in culture medium and in cell extracts was measured as previously described (28) by using the synthetic substrate 4-methylumbelliferyl β-d-N,N′-diacetyl-chitobioside [4-MU-(GlcNAc)2; Sigma-Aldrich, St. Louis, MO]. Release of fluorescent 4-methylumbelliferone was detected using an excitation wavelength of 360 nm and an emission wavelength of 460 nm. Standard curves using authentic 4-methylumbelliferone were used to determine pmol of substrate hydrolyzed. Enzyme assays were performed in duplicate and averages were calculated. Means and standard errors were determined from three independent experiments. Activities in the cell-free supernatants (secreted chitinase) and in cell extracts are indicated as pmol of 4-methylumbelliferone released per μg of total protein in the original cell suspension. Protein concentrations were determined with the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Waltham, MA).
RESULTS
Mutations in genes near sprB result in motility defects.
Strains with mutations in or near sprB were isolated and characterized (Fig. 1). The strains with Tn4351, HimarEm1, and HimarEm2 insertions in sprB were previously described (23). Additional mutants identified in this study by random transposon mutagenesis followed by screening for mutants that formed nonspreading colonies included CJ996, which has a Tn4351 insertion in sprC, FJ105, which has a HimarEm2 insertion in sprC, and FJ162, which has a HimarEm2 insertion in sprD. Plasmid insertions disrupting fjoh_0983 (strain CJ1708), sprD (strain CJ1695), and sprF (strain CJ1814) were obtained by cloning internal fragments of the genes into the suicide vector pLYL03, introducing the plasmids into F. johnsoniae by conjugation, and selecting for erythromycin-resistant colonies resulting from insertion of the plasmid into the genome by recombination. Strain UW102-91, which has a premature stop codon in sprC as the result of an A-to-T substitution at position 574 (numbered from the A of the sprC start codon), was isolated by Pate and colleagues as a spontaneous nonspreading mutant (7, 26), and the site of the mutation was identified by complementation analysis (28) and DNA sequencing. The resulting mutants each displayed motility defects and produced nonspreading colonies on agar, in contrast to the thin spreading colonies formed by wild-type cells (Fig. 2, columns 1 to 3).
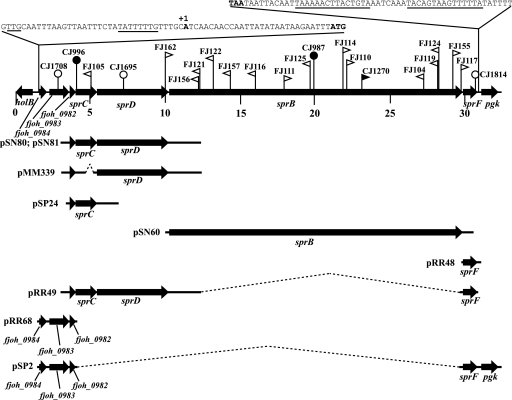
FIG. 1.
Map of the sprB operon. Numbers below the map refer to kilobase pairs of the sequence. The sites of HimarEm1, HimarEm2, Tn4351, and pLYL03 insertions are indicated by filled triangles, open triangles, filled circles, and open circles, respectively. Orientations of HimarEm insertions are indicated by the direction in which the triangles are pointing. Triangles pointing to the right (FJ114, for example) have IR2 on the right side and the kanamycin resistance gene of the transposon reading toward the right, and they typically result in nonpolar mutations. The regions of DNA carried by plasmids used in this study are indicated beneath the map.
FIG. 2.
Photomicrographs of F. johnsoniae colonies. Colonies were grown for 48 h at 25°C on PY2 agar medium containing the appropriate antibiotics. Photomicrographs were taken with a Photometrics Cool-SNAPcf2 camera mounted on an Olympus IMT-2 phase-contrast microscope. Bar (at lower right; applies to all panels), 1 mm. Rows (A to J) indicate different strains of F. johnsoniae, and columns (1 to 12) indicate different plasmids introduced into these strains. Rows: A, wild-type F. johnsoniae UW101; B, fjoh_0983 insertion mutant CJ1708; C, sprC mutant UW102-91; D, sprC HimarEm2 insertion mutant FJ105; E, sprD HimarEm2 insertion mutant FJ162; F, sprD insertion mutant CJ1695; G, sprB HimarEm2 insertion mutant FJ114; H, sprB HimarEm2 insertion mutant FJ156; I, sprCDB deletion mutant CJ1584; J, sprF insertion mutant CJ1814. Columns: 1, control vector pCP23; 2, control vector pCP29; 3, control vectors pCP23 and pCP29; 4, pSN80 expressing sprC and sprD; 5, pSN81 carrying sprC and sprD in the opposite orientation as pSN80; 6, pMM339 expressing sprD; 7, pSN60 expressing sprB; 8, pSN80 (sprC sprD) and pSN60 (sprB); 9, pRR48 expressing sprF; 10, pRR48 (sprF) and pSN60 (sprB); 11, pRR49 expressing sprC, sprD, and sprF; 12, pRR49 (sprC sprD sprF) and pSN60 (sprB).
The genetic arrangement shown in Fig. 1 suggested the possibility that sprB might be cotranscribed with nearby genes and that some of the motility defects associated with mutations in fjoh_0983, sprC, and sprD could be the result of polar effects on expression of sprB. Complementation analyses were performed to determine which genes are required for colony spreading and to determine whether any of the mutations result in polar effects on downstream genes. Attempts to clone the entire 29.3-kbp region in a single plasmid for complementation analyses were not successful. Instead, smaller overlapping regions (Fig. 1) were cloned into separate shuttle vectors, and the genes were expressed using vector promoters. Individual plasmids, or combinations of plasmids, were used to determine the genes required for complementation of each mutant.
The sprF mutant CJ1814 was complemented by pRR48, which carries sprF downstream of a vector promoter, indicating that sprF is required for formation of spreading colonies (Fig. 2, row J). CJ1814 contains pLYL03, which carries an internal fragment of sprF integrated into the genome, which disrupts sprF. Similar pLYL03 gene disruption mutations have polar effects on expression of downstream genes (13). Since CJ1814 was complemented by a plasmid that carries just sprF, there did not appear to be motility genes downstream of and cotranscribed with sprF.
sprB mutants with HimarEm1, HimarEm2, and Tn4351 insertions were examined to determine whether these insertions had polar effects on sprF. For the HimarEm insertions, the degree of polarity appeared to depend on the orientation of insertion of the transposon. Each of the insertions with the kanamycin resistance gene and inverted repeat 2 (IR2) oriented toward the left in Fig. 1 (triangle pointed left) resulted in apparent polar effects on sprF. Introduction of pSN60 (which carries sprB) into six of these mutants (FJ156, FJ121, FJ122, FJ157, FJ116, and FJ125) failed to restore colony spreading, whereas introduction of pSN60 plus pRR48 (carrying sprF) resulted in spreading colonies (Fig. 2, row H). The remaining three mutants with Himar insertions in sprB in this orientation (FJ104, FJ119, and FJ124) also exhibited polar effects, but they were slightly less severe. Introduction of pSN60 into these mutants resulted in colonies that exhibited very slight flares, whereas introduction of pSN60 plus pRR48 resulted in more complete complementation. The insertions in these three mutants are closer to the 3′ end of sprB, and the reduced polarity may indicate the presence of a weak promoter oriented toward IR1 that results in a small amount of expression of sprF in these mutants. Five strains with HimarEm insertions in sprB in the opposite orientation (FJ114, FJ110, CJ1270, FJ155, and FJ117) resulted in nonpolar mutations. These mutants were complemented by pSN60, suggesting that a promoter reading toward IR2 of HimarEm2 (and of the closely related HimarEm1 in CJ1270) allowed expression of sprF (Fig. 2, row G). The complemented colonies spread on agar but did not spread as well as those formed by wild-type cells, perhaps because of differences in the level of expression of sprB or of sprF, which appear to be driven by vector or Himar promoters rather than by their native promoter(s). Surprisingly, FJ111, which had a Himar insertion in sprB in the same orientation as the nonpolar mutants described above, exhibited polarity on sprF. FJ111 was not complemented by pSN60 but was complemented by the introduction of pSN60 and pRR48 together (data not shown). We do not know the reason for this result, but possibilities include the presence of a second mutation (perhaps in sprF), decreased expression of the Himar promoter because of nearby sequences on the genome, or premature termination or transcript instability.
Analysis of transposon and plasmid insertion mutations in sprC and sprD were consistent with the results described above regarding orientations of HimarEm insertions and polarity. FJ162, which has a HimarEm2 insertion in sprD oriented in the nonpolar orientation (IR2 on right side of Himar in Fig. 1) was complemented by pSN80, which spans fjoh_0982, sprC, and sprD, and by pMM339, which expresses sprD but not sprC (Fig. 2, row E). In contrast, CJ1695, which carries a pLYL03 insertion in sprD, exhibited polar effects and was not complemented by pSN80 or by pMM339 (Fig. 2, row F). Partial complementation of this mutant required the introduction of pRR49, which carries fjoh_0982, sprC, sprD, and sprF, and pSN60, which carries sprB. FJ105, which carries a HimarEm2 insertion in sprC in the polar orientation (IR2 facing upstream) was not complemented by pSN80 but was partially complemented by the introduction of pRR49 (fjoh_0982, sprC, sprD, and sprF) and pSN60 (sprB) together (Fig. 2, row D). In contrast, UW102-91, which has a point mutation resulting in an internal stop codon in sprC, was complemented by pSN80 (Fig. 2, row C). pSN81, which carries the same region as pSN80 but in the opposite orientation, failed to complement any of the mutants (Fig. 2, column 5, and data not shown). This suggests that the native promoter for expression of sprC and sprD lies further upstream and that sprC and sprD are expressed from the pCP1 orf1 vector promoter in pSN80. The pCP1 orf1 promoter is in the correct orientation in pSN80 to allow expression of sprC and sprD, and it has been shown to express other genes cloned into pCP23 (3, 5, 28).
Analysis of CJ1708, which has a pLYL03 insertion in fjoh_0983, supports the idea that the native promoter for sprC, sprD, sprB, and sprF lies further upstream. Disruption of fjoh_0983 resulted in the formation of nonspreading colonies (Fig. 2, row B). The mutant was not complemented by pRR68 (fjoh_0984, fjoh_0983, and fjoh_0982), pRR49 (fjoh_0982, sprC, sprD, and sprF), or pSN60 (sprB) but was partially complemented by introduction of pRR49 and pSN60 together (Fig. 2, row B, and data not shown). These results suggest that fjoh_0983 is part of an operon that spans fjoh_0982, sprC, sprD, sprB, and sprF. Partial complementation of CJ1708 by plasmids that do not carry fjoh_0983 also suggests that fjoh_0983 is not required for formation of spreading colonies. None of the mutants complemented by the introduction of pRR49 and pSN60 together spread as well as the wild type (Fig. 2, column 12). This may be the result of abnormal expression levels of some of the gene products from these constructs or deleterious effects of maintaining multiple related plasmids in the same cell. The fact that the nonpolar sprC mutant UW102-91 and the nonpolar sprD mutant FJ162 were complemented well by pSN80 (Fig. 2, column 4, rows C and E) but were complemented less well by pRR49 (Fig. 2, column 11, rows C and E) and much less well by pRR49 and pSN60 together (Fig. 2, column 12, rows C and E) supports these ideas. The formation of nonspreading colonies by nonpolar sprC (UW102-91), sprD (FJ162), and sprB (FJ114) mutants indicates that each of these genes is required for formation of spreading colonies. CJ1584, which carries a deletion spanning sprC, sprD, and sprB, also formed nonspreading colonies on agar. Introduction of pSN60 and pSN80 together into CJ1584 resulted in complementation (Fig. 2, row I).
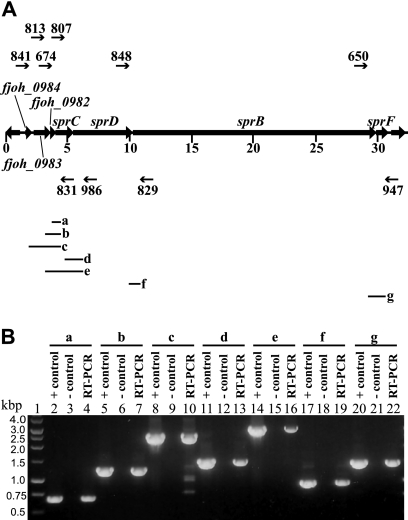
The boundaries of the sprB operon are not unambiguously defined by the complementation experiments described above. Inspection of the sequence revealed an inverted repeat sequence followed by a string of T's downstream of sprF, which may be a transcription terminator at the 3′ end of the transcript (Fig. 1), and a putative Flavobacterium promoter that was nearly identical to the consensus −33/−7 promoter sequence TTTG/TANNTTTG (8) upstream of fjoh_0984. RT-PCR analyses suggested that fjoh_0984, fjoh_0983, fjoh_0982, sprC, sprD, sprB, and sprF are cotranscribed (Fig. 3), and 5′-RACE was used to identify a transcriptional start site 29 bp upstream of the fjoh_0984 start codon (Fig. 1). The combination of complementation and molecular analyses described above suggests that the operon spans 29.3 kbp and includes seven genes. This large operon may have internal promoter sites, but if so they are probably weak, since strains with polar mutations, including CJ1708 (fjoh_0983 mutant), FJ105 (sprC mutant), CJ1695 (sprD mutant), and FJ156 (sprB mutant) required the introduction of plasmids containing the distal gene, sprF, for complementation (Fig. 2).
FIG. 3.
RT-PCR of the sprB operon. (A) Map of the sprB operon with primers used for RT-PCR (numbered arrows) and predicted PCR products (a to g; diagramed below in Fig. 3A). (B) RT-PCR of F. johnsoniae wild-type RNA to determine the sprB transcriptional unit. Antisense primer 831 and sense primers 674 (lane 4), 813 (lane 7), and 841 (lane 10) were used to amplify across the junctions spanning fjoh_0984 to sprC. Antisense primer 986 and sense primers 807 (lane 13) and 813 (lane 16) were used to amplify across the junctions spanning fjoh_0983 to sprD. Antisense primer 829 was used with sense primer 848 to amplify across the sprD-sprB junction (lane 19), and antisense primer 947 was used with sense primer 650 to amplify across the sprB-sprF junction (lane 22). For each primer pair, three reaction mixtures were loaded on the gel: a positive control PCR using F. johnsoniae chromosomal DNA as template (lanes 2, 5, 8, 11, 14, 17, and 20), a no-RT control reaction mixture (lanes 3, 6, 9, 12, 15, 18, and 21), and a RT-PCR mixture. A 1-kb ladder was used as a size standard (lane 1).
Immunodetection of SprB, SprC, and SprD.
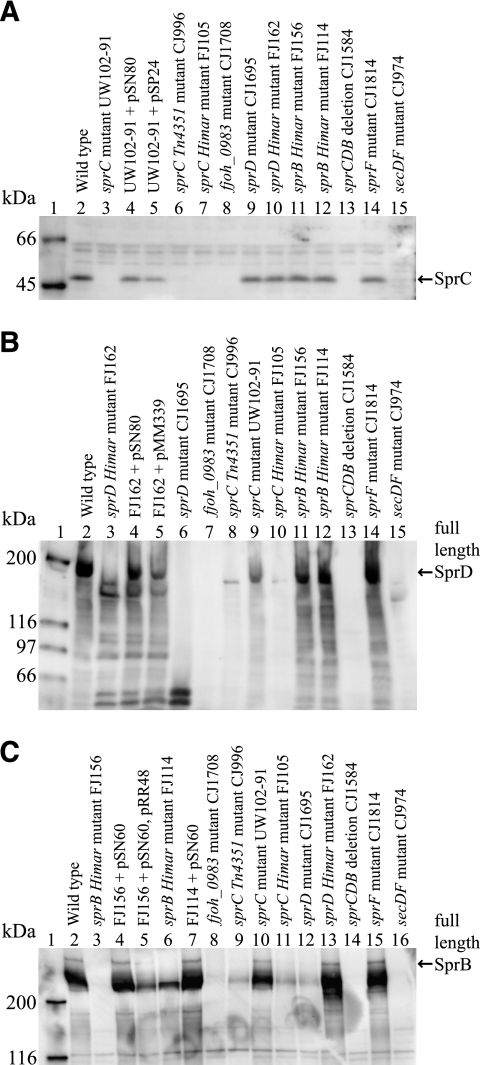
Recombinant SprC and SprD were produced, and polyclonal antisera recognizing these proteins were obtained and used to detect SprC and SprD in whole-cell extracts (Fig. 4 ). SprC, which migrated with an apparent molecular mass of approximately 48 kDa, was present in wild-type cells (Fig. 4A, lane 2) but was absent in the sprC mutants (Fig. 4A, lanes 3, 6, 7, and 13). Addition of sprC on pSN80 or pSP24 restored SprC production (Fig. 4A, lanes 4 and 5). SprD migrated with an apparent molecular mass of approximately 170 kDa in whole-cell extracts of wild-type cells (Fig. 4B, lane 2). Numerous shorter fragments of SprD were also observed, which may be functionally important. A similar result was previously observed for the large repetitive SprB protein (23). SprD was absent in the sprCDB deletion mutant (Fig. 4B, lane 13). The sprD insertion mutant CJ1695 failed to produce full-length SprD but did produce truncated forms of the protein (Fig. 4B, lane 6). FJ162, which has a nonpolar HimarEm2 insertion 152 bp from the end of sprD, produced a nearly full-length, but apparently nonfunctional, SprD protein (Fig. 4B, lane 3). Addition of sprD on pSN80 restored production of full-length SprD (Fig. 4B, lane 4).
FIG. 4.
Western immunoblot detection of SprC, SprD, and SprB in whole-cell extracts of wild-type and mutant strains of F. johnsoniae. (A) Detection of SprC. Lane 1, molecular mass markers; lane 2, wild-type F. johnsoniae UW101; lane 3, sprC point mutant UW102-91; lane 4, UW102-91 complemented with pSN80; lane 5, UW102-91 with pSP24; lane 6, sprC Tn4351 insertion mutant CJ996; lane 7, sprC HimarEm2 insertion mutant FJ105; lane 8, fjoh_0983 insertion mutant CJ1708; lane 9, sprD insertion mutant CJ1695; lane 10, sprD HimarEm2 insertion mutant FJ162; lane 11, sprB HimarEm2 insertion mutant FJ156; lane 12, sprB HimarEm2 insertion mutant FJ114; lane 13, sprCDB deletion mutant CJ1584; lane 14, sprF insertion mutant CJ1814; lane 15, secDF mutant CJ974. (B) Detection of SprD. Lane 1, molecular mass markers; lane 2, wild-type F. johnsoniae; lane 3, sprD HimarEm2 insertion mutant FJ162; lane 4, FJ162 complemented with pSN80; lane 5, FJ162 complemented with pMM339; lane 6, sprD insertion mutant CJ1695; lane 7, fjoh_0983 insertion mutant CJ1708; lane 8, sprC Tn4351 insertion mutant CJ996; lane 9, sprC point mutant UW102-91; lane 10, sprC HimarEm2 insertion mutant FJ105; lane 11, sprB HimarEm2 insertion mutant FJ156; lane 12, sprB HimarEm2 insertion mutant FJ114; lane 13, sprCDB deletion mutant CJ1584; lane 14, sprF insertion mutant CJ1814; lane 15, secDF mutant CJ974. (C) Detection of SprB. Lane 1, molecular mass markers; lane 2, wild-type F. johnsoniae; lane 3, sprB HimarEm2 insertion mutant FJ156; lane 4, FJ156 with pSN60; lane 5, FJ156 complemented with pSN60 and pRR48; lane 6, sprB HimarEm2 insertion mutant FJ114; lane 7, FJ114 with pSN60; lane 8, fjoh_0983 insertion mutant CJ1708; lane 9, sprC Tn4351 insertion mutant CJ996; lane 10, sprC point mutant UW102-91; lane 11, sprC HimarEm2 insertion mutant FJ105; lane 12, sprD insertion mutant CJ1695; lane 13, sprD HimarEm2 insertion mutant FJ162; lane 14, sprCDB deletion mutant CJ1584; lane 15, sprF insertion mutant CJ1814; lane 16, secDF mutant CJ974. Forty micrograms of protein was loaded in each lane. Some strains carried empty control vector pCP23 (panel A, lanes 2, 3, 6, 7, 8, 9, 10, 11, 13, and 14; panel B, lanes 2, 3, 6, 7, 8, 9, 10, 11, 13, and 14; panel C, lanes 2, 8, 9, 10, 11, 12, 13, 14, and 15) or pCP29 (panel A, lane 12; panel B, lanes 8, 10, and 12; panel C, lanes 3 and 6), which had no effect on expression of SprB, SprC, or SprD.
Analysis of mutants by Western blotting provided support for the operon structure suggested above. Cells of CJ1708, which had pLYL03 inserted in fjoh_0983, displayed a dramatic reduction of SprC, SprD, and SprB (Fig. 4A, lane 8, B, lane 7, and C, lane 8). The transposon insertions in the sprC mutants CJ996 and FJ105 also appeared to be polar on sprD and sprB, since little SprD and SprB were present in these cells (Fig. 4B, lanes 8 and 10, and C, lanes 9 and 11). In contrast, cells of UW102-91, which had a point mutation in SprC, produced SprD and SprB (Fig. 4B, lane 9, and C, lane 10). Cells of CJ1695, which had pLYL03 inserted within sprD, produced little SprB, confirming the polarity of this mutation (Fig. 4C, lane 12). In contrast, cells of the sprD mutant FJ162 made normal levels of SprB protein, confirming that the HimarEm2 insertion in sprD was not polar on sprB (Fig. 4C, lane 13) and suggesting that a HimarEm2 promoter reading toward IR2 resulted in expression of sprB. Insertions in sprB resulted in elimination of full-length SprB protein, as previously reported (23). CJ1814, which had a mutation in sprF, produced wild-type levels of SprC, SprD, and SprB, as expected (Fig. 4A, lane 14, B, lane 14, and C, lane 15).
Strains with mutations in other motility genes were also examined for the presence of SprC and SprD proteins. SprC and SprD were present in cells of each of the gld mutants (data not shown), but cells with a mutation in secDF had little if any SprC or SprD (Fig. 4A, lane 15, and B, lane 15). SprB and GldJ are also absent or nearly absent in secDF mutant cells (23, 25). SecDF may play a role in export across the cytoplasmic membrane, and the mislocalized proteins in the secDF mutant may be subject to degradation.
Analysis of the products of genes in the sprB operon.
sprC encodes a predicted product of 483 amino acids that exhibits sequence similarity to SprB (28% identity over 239 amino acids). The similarity is limited to a region near the carboxy terminus of SprC but occurs five times in the amino-terminal half of the large repetitive SprB protein. sprD encodes a 1,588-amino-acid product that is highly repetitive and is predicted to have a coiled-coil structure. sprF encodes a 324-amino-acid product that exhibits similarity (E value of 2E−10; 22% identity and 40% similarity over 289 amino acids) to the Porphyromonas gingivalis outer membrane protein PorP (29). P. gingivalis PorP is required for secretion of gingipain protease virulence factors and appears to be a component of the PorSS. P. gingivalis porP is located just upstream of porK, porL, porM, and porN, which also encode proteins involved in gingipain secretion. The F. johnsoniae homologs of PorK, PorL, PorM, and PorN (GldK, GldL, GldM, and GldN, respectively) are required for gliding motility and for secretion of SprB and chitinase (28, 29). The predicted product of fjoh_0982 is not similar in sequence to any proteins in the databases, whereas the products of fjoh_0983 and fjoh_0984 exhibit similarities to bacteroidete proteins of unknown functions. Each of the proteins encoded by the seven genes of the operon are predicted to have N-terminal signal peptides targeting them for export across the cytoplasmic membrane by the sec system. PSORTb and CELLO analyses predict that SprC and SprF are outer membrane proteins and that Fjoh_0984 is a periplasmic protein. Predictions for the other proteins are more complex, with SprB and Fjoh_0983 predicted to be extracellular or outer membrane and SprD and Fjoh_0982 predicted to be extracellular, outer membrane, or periplasmic. Antibodies against SprC and SprD were used in an attempt to determine whether these proteins are exposed on the cell surface. Protein G-coated polystyrene spheres carrying antibodies against SprC or SprD failed to bind to wild-type cells, indicating that the epitopes recognized by these antibodies are not fully exposed on the surface of the cell.
Microscopic observations of cell attachment and movement.
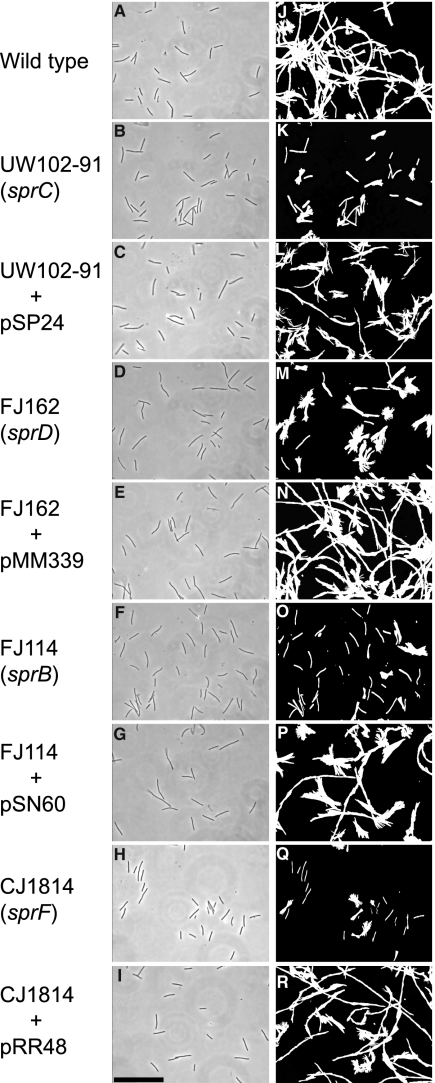
Wild-type and mutant cells were examined for movement on glass as previously described (23). Wild-type cells readily attached to glass and glided at speeds of 2 μm/s. Under these conditions, greater than 95% of the cells that attached to the glass exhibited gliding movements over a 2-min period. Cells with mutations in sprC, sprD, sprB, or sprF attached to glass as well as the wild-type cells did (Fig. 5 and data not shown). Cells of these mutants moved on glass, but less well than did wild-type cells (Fig. 5; see also Movies S1 and S2 in the supplemental material). Wild-type cells moved in long runs, occasionally reversing direction using two different mechanisms. Some cells reversed direction when the leading pole became the lagging pole, whereas other cells flipped or pivoted, thus altering the direction of movement while maintaining the same leading pole. Pivots were easily detected in the cell tracks as starburst patterns, because images of the cells were captured at several stages of each pivot (Fig. 5M and P, for example). Some cells of the mutants moved as rapidly as those of the wild type, but they made little net progress (Fig. 5K, M, O, and Q; see also Movies S1 and S2 in the supplemental material). These mutant cells reversed frequently, either by changing the leading pole or by pivoting. Complementation of the mutants restored normal motility (Fig. 5L, N, P, and R; see also Movies S1 and S2).
FIG. 5.
Effects of mutations in sprC, sprD, sprB, and sprF on gliding of cells on glass. Cells attached to a glass coverslip on a Palmer cell were observed by phase-contrast microscopy, and digital images of cells of the wild-type strain with control vector pCP23 (A), sprC mutant UW102-91 with pCP23 (B), UW102-91 complemented with pSP24 (C), sprD mutant FJ162 with pCP23 (D), FJ162 complemented with pMM339 (E), sprB mutant FJ114 with control vector pCP29 (F), FJ114 complemented with pSN60 (G), sprF mutant CJ1814 with pCP23 (H), and CJ1814 complemented with pRR48 (I) were recorded at time zero. Tracks illustrating the movements of the cells shown in panels A to I over a 60-s period were obtained by superimposing individual digital video frames of the wild-type strain with pCP23 (J), sprC mutant UW102-91 with pCP23 (K), UW102-91 complemented with pSP24 (L), sprD mutant FJ162 with pCP23 (M), FJ162 complemented with pMM339 (N), sprB mutant FJ114 with pCP29 (O), FJ114 complemented with pSN60 (P), sprF mutant CJ1814 with pCP23 (Q), and CJ1814 complemented with pRR48 (R). Images were recorded using a Photometrics CoolSNAPcf2 camera mounted on an Olympus BH-2 phase-contrast microscope. Bar (shown in panel I; applies to all panels), 40 μm.
SprF is required for secretion of SprB to the cell surface.
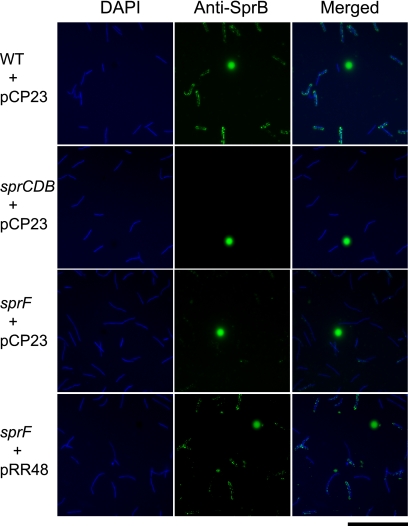
The similarity of SprF to the P. gingivalis PorSS protein PorP suggested that SprF might have a role in protein secretion. The F. johnsoniae PorSS is involved in secretion of SprB and of an extracellular chitinase (28, 29). Wild-type and sprF mutant cells were examined for localization of SprB by immunofluorescence microscopy. SprB was found on the surface of wild-type cells, as previously reported (Fig. 6). In contrast, sprF mutant cells produced SprB (Fig. 4C, lane 15), but little of the SprB was present on the cell surface (Fig. 6). The presence of SprB on the cell surface was also monitored by adding protein G-coated polystyrene spheres and anti-SprB antibodies to live cells. As previously reported (23), antibody-coated spheres attached specifically to wild-type cells expressing SprB (Table 2). Such spheres failed to attach to cells of sprB mutants FJ156 and FJ114, and protein G-coated spheres without antibodies failed to bind to wild-type cells. Cells of strains with polar mutations in fjoh_0983 (CJ1708), sprC (FJ105), or sprD (CJ1695) produced only small amounts of SprB (Fig. 4C) and bound few anti-SprB-coated spheres. In contrast, cells with nonpolar mutations in sprC (UW102-91) or sprD (FJ162) produced approximately wild-type levels of SprB (Fig. 4C) and bound to anti-SprB-coated spheres similar to wild-type cells. Antibody-coated spheres failed to bind to cells of the sprF mutant, indicating that SprB was not exposed on the surface of these cells. Complementation with pRR48 restored surface localization of SprB (Fig. 6; Table 2).
FIG. 6.
Detection of surface-localized SprB protein by immunofluorescence microscopy. Cells of wild-type and mutant F. johnsoniae were exposed to DAPI and to anti-SprB antibodies followed by secondary antibodies conjugated to Alexa-488. The fluorescent spheres are InSpeck relative intensity control fluorescent beads. WT, wild-type F. johnsoniae UW101; sprCDB, sprCDB deletion mutant CJ1584; sprF, sprF insertion mutant CJ1814. pCP23 is a control vector; pRR48 carries sprF. Bar, 20 μm.
TABLE 2.
Effects of mutations in sprC, sprD, sprB, and sprF on binding of protein G-coated polystyrene spheres carrying antibodies against SprB
| Strain | Description | Antibody added | Avg (SD) % of cells with spheres attacheda |
|---|---|---|---|
| UW101 with control plasmid pCP23 | Wild type | None | 0 (0) |
| UW101 with pCP23 | Wild type | Anti-SprB | 48.0 (5.3) |
| CJ1708 with pCP23 | fjoh_0983 plasmid insertion mutant; polar | Anti-SprB | 2.3 (1.5) |
| UW102-91 with pCP23 | sprC point mutant; nonpolar | Anti-SprB | 37.3 (3.2) |
| FJ105 with pCP23 | sprC HimarEm2 mutant; polar | Anti-SprB | 6.3 (4.9) |
| FJ162 with pCP23 | sprD HimarEm2 mutant; nonpolar | Anti-SprB | 50.3 (7.6) |
| CJ1695 with pCP23 | sprD plasmid insertion mutant; polar | Anti-SprB | 4.0 (1.7) |
| FJ114 with pCP29 | sprB HimarEm2 mutant; nonpolar | Anti-SprB | 0.3 (0.6) |
| FJ114 with pSN60 | FJ114 complemented with sprB | Anti-SprB | 30.3 (4.7) |
| FJ156 with pCP23 | sprB HimarEm2 mutant; polar | Anti-SprB | 0.7 (0.6) |
| CJ1814 with pCP23 | sprF mutant | Anti-SprB | 0.3 (0.6) |
| CJ1814 with pRR48 | CJ1814 complemented with sprF | Anti-SprB | 42.7 (10.1) |
Purified anti-SprB and 0.5-μm-diameter protein G-coated polystyrene spheres were added to cells as described in Materials and Methods. Samples were spotted on a glass slide, covered with a glass coverslip, incubated for 1 min at 25°C, and examined using a phase-contrast microscope. Images were recorded for 30 s, and 100 randomly selected cells were examined for the presence of attached spheres during this period. Numbers in parentheses are standard deviations calculated from three measurements.
SprF is not required for chitin utilization or for secretion of chitinase.
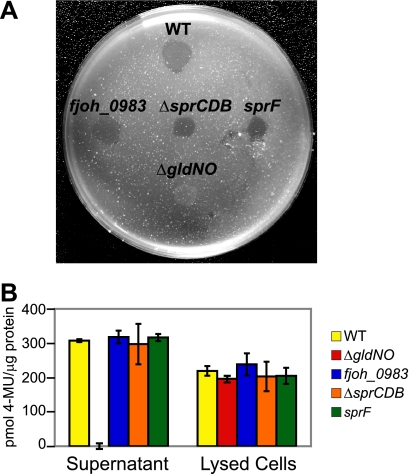
F. johnsoniae produces a suite of enzymes for chitin utilization, including at least one extracellular chitinase, encoded by fjoh_4555 (22, 29). Mutations in F. johnsoniae porSS genes, such as sprT and gldN, result in defects in chitin utilization and defects in secretion of extracellular chitinase, in addition to the defects in SprB secretion (28, 29). Cells of the sprF mutant CJ1814 were examined for extracellular chitinase. The mutant cells secreted extracellular chitinase and digested chitin, indicating that SprF is not involved in secretion of chitinase (Fig. 7). Cells with mutations in the other genes of the sprB operon also secreted extracellular chitinase and digested chitin.
FIG. 7.
Mutations in genes of the sprCDBF operon have no effect on chitin utilization. (A) Digestion of chitin by intact cells. Approximately 106 cells of F. johnsoniae strains were spotted on MYA-chitin medium and incubated at 25°C for 4 days. Strains included wild-type F. johnsoniae UW101 (WT), fjoh_0983 insertion mutant CJ1708 (fjoh_0983), sprCDB deletion strain CJ1584 (ΔsprCDB), sprF insertion mutant CJ1814 (sprF), and nonmotile gldNO deletion mutant CJ1631A (ΔgldNO). Erythromycin was included in the medium to ensure maintenance of the plasmid insertions in fjoh_0983 and sprF. The wild type, CJ1584, and CJ1631A carried control vector pCP29, which conferred erythromycin resistance and allowed these strains to be tested on the same medium as the fjoh_0983 and sprF insertion mutants. Control experiments verified that pCP29 had no effect on chitin utilization. (B) Chitinase activities of cell lysates and culture supernatants of F. johnsoniae strains, determined using the synthetic substrate 4-MU-(GlcNAc)2. Equal amounts of each sample, based on the protein content of the original cell suspension, were incubated with 10 nmol of 4-MU-(GlcNAc)2 for 4 h at 37°C, and the amount of 4-MU released was determined by measuring fluorescence emission at 460 nm following excitation at 360 nm. WT, F. johnsoniae UW101; ΔgldNO, gldNO deletion mutant CJ1631A; fjoh_0983, fjoh_0983 insertion mutant CJ1708; ΔsprCDB, sprCDB deletion mutant CJ1584; sprF, sprF insertion mutant CJ1814. All strains in panel B carried control vector pCP23, which has no effect on chitinase activity.
Movement of anti-SprB-coated protein G-labeled polystyrene spheres on wild-type cells and on cells of nonpolar sprC and sprD mutants.
Protein G-coated polystyrene latex spheres were used to monitor movement of SprB along the cell surface. As previously reported (23), wild-type cells of F. johnsoniae bound and propelled the protein G-coated spheres along the cell surface only when antibodies against SprB were added (see Movie S3 in the supplemental material). Cells of strains with mutations in sprB (FJ114) or sprF (CJ1814) failed to bind or propel anti-SprB-coated spheres, and complementation with the appropriate plasmids (pSN60 for FJ114 and pRR48 for CJ1814) restored the ability to bind and propel the spheres. Cells with nonpolar mutations in sprC (UW102-91) and sprD (FJ162) produced SprB (Fig. 4C) and localized it to the cell surface (Table 2). Anti-SprB-coated spheres attached to cells of these mutants and were rapidly propelled along their surfaces (see Movie S3 in the supplemental material), indicating that SprC and SprD are not required for surface localization or movement of SprB.
Bacteriophage sensitivity of spr mutants.
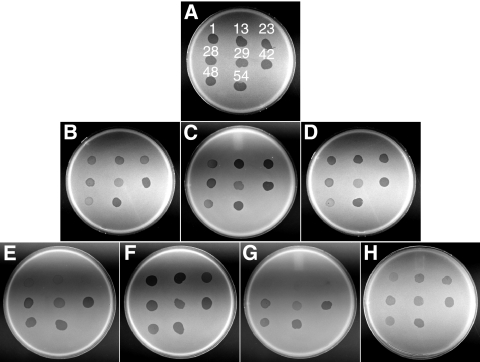
Nonmotile gld mutants of F. johnsoniae are resistant to infection by all known F. johnsoniae bacteriophages, including φCj1, φCj13, φCj23, φCj28, φCj29, φCj42, φCj48, and φCj54 (1, 5, 6, 11-13, 19, 20, 31). Cells with mutations in sprB exhibit increased resistance to φCj1, φCj13, φCj23, and φCj29 (23) (Fig. 8 E). SprB may function as a receptor for these phages. CJ1814, which has a mutation in sprF, exhibited the same resistance pattern as did the sprB mutants (Fig. 8G). CJ1814 produces SprB protein (Fig. 4C), but it is apparently not secreted to the cell surface (Fig. 6; Table 2), which may explain the increased resistance to bacteriophages φCj1, φCj13, φCj23, and φCj29. Unlike all motility mutants previously examined, cells with nonpolar mutations in sprC and sprD were sensitive to infection by all of the phages tested (data not shown). These mutants produce SprB and secrete it to the cell surface, which probably accounts for their phage sensitivity. Surprisingly, even cells with polar mutations in fjoh_0983, sprC and sprD were sensitive to phage infection (Fig. 8B, C, and D). Western blot analyses revealed small amounts of SprB produced by these polar mutants (Fig. 4C). This small amount of SprB may be sufficient to confer phage sensitivity.
FIG. 8.
Effects of mutations on bacteriophage resistance. Bacteriophages (in 5-μl aliquots of lysates containing approximately 109 PFU/ml) were spotted onto lawns of cells in CYE overlay agar. The plates were incubated at 25°C for 24 h to observe lysis. Bacteriophages were spotted in the following order from left to right, as indicated also by the numbers in panel A: top row, φCj1, φCj13, and φCj23; middle row, φCj28, φCj29, and φCj42; bottom row, φCj48 and φCj54. (A) Wild-type F. johnsoniae UW101 with control vector pCP23; (B) fjoh_0983 mutant CJ1708 with pCP23; (C) sprC mutant FJ105 with pCP23; (D) sprD mutant CJ1695 with pCP23; (E) sprB mutant FJ114 with control vector pCP29; (F) sprB mutant FJ114 with pSN60, which carries sprB; (G) sprF mutant CJ1814 with pCP23; (H) sprF mutant CJ1814 with pRR48, which carries sprF.
DISCUSSION
The large cell surface protein SprB was previously identified as a component of the F. johnsoniae gliding motility machinery (23). SprB appears to be propelled rapidly along the cell surface by the gliding motor and is thought to function as an adhesin that transmits the force of the motor to the surface over which the cell crawls. A novel protein secretion system, the PorSS, is required for translocation of SprB across the outer membrane (28, 29). The results of this study identified SprC, SprD, and SprF as additional motility proteins. SprF appears to be needed for secretion of SprB, whereas the functions of SprC and SprD are not known.
The molecular and genetic analyses presented here suggest that sprC, sprD, sprB, and sprF are part of a large operon spanning three additional genes (fjoh_0982, fjoh_0983, and fjoh_0984). Previous results suggested that sprB was expressed independently of neighboring genes (23). This was based primarily on the fact that sprB HimarEm2-induced mutants, such as FJ114, could be complemented by pSN60, which carries just sprB. The results described in this study present a more complicated picture. Some sprB HimarEm2 insertion mutations (such as FJ114, which has IR2 facing downstream) are complemented by pSN60, whereas others (with HimarEm2 inserted in the opposite orientation, such as FJ156) exhibit polar effects on the downstream gene and thus require introduction of both sprB and sprF for complementation. We previously reported complementation of FJ156 by pSN60 (23). However, further examination revealed that this required integration of pSN60 into the genome by recombination. The complementation data, Western blot assays, and molecular analyses suggest that the sprB operon spans seven genes and 29.3 kbp of DNA. They also suggest that the HimarEm transposons contain a fortuitous promoter(s) reading toward IR2 that functions in F. johnsoniae. These studies also revealed that the promoter driving expression of sprB on pSN60 is probably not the native promoter. Cloning of the 21.5-kbp fragment spanning sprB to generate pSN60 relied on a HimarEm2 transposon insertion within the upstream gene sprD, which provided a selectable marker (23). Expression of sprB on pSN60 appears to be driven by the HimarEm2 promoter(s).
Cells with mutations in sprC, sprD, sprB, or sprF form nonspreading colonies on agar that are indistinguishable from those formed by completely nonmotile gld mutants. However, whereas cells of gld mutants are deficient in attachment to glass (28), cells of the sprC, sprD, sprB, and sprF mutants attach readily to glass. They also glide rapidly on glass surfaces, but they make little net progress because they frequently reverse their direction of movement. Cells of the sprC, sprD, sprB, and sprF mutants differ in phenotype from sprA and sprT mutants. Cells with mutations in sprA or sprT are deficient in attachment to glass and are almost completely nonmotile (24, 29), presumably because they have a general defect in their PorSS.
The genetic organization of sprC, sprD, sprB, and sprF and the similar phenotypes of cells with mutations in these genes suggest that the encoded proteins function together in assembly or operation of the same cell surface motility apparatus. SprC and SprD are required for formation of spreading colonies, but their exact roles in gliding are not known. Neither SprC nor SprD is required for expression or proper localization of SprB, and SprB appears to move rapidly along the surface of sprC or sprD mutant cells, as it does on wild-type cells. SprF is similar in sequence to P. gingivalis PorP, a component of the multiprotein PorSS involved in secretion of gingipain protease virulence factors. F. johnsoniae has a similar PorSS, comprised of GldK, GldL, GldM, GldN, SprA, and SprT, required for secretion of SprB, and of an extracellular chitinase. SprF is required for secretion of SprB to the cell surface but is not required for secretion of extracellular chitinase. SprF may function as an adaptor to the PorSS that is needed specifically to promote secretion of SprB.
The roles of the three genes that lie upstream of sprC are unclear. These genes appear to be cotranscribed with sprC, sprD, sprB, and sprF, and a polar insertion in fjoh_0983 resulted in motility defects. Homologs of fjoh_0984 and fjoh_0983 of unknown function have been found in other bacteroidetes, whereas fjoh_0982 appears to be unique to F. johnsoniae. Since homologs to fjoh_0982 are not present in the genomes of gliding bacteroidetes, such as Flavobacterium psychrophilum, Capnocytophaga ochraceae, Chitinophaga pinensis, and Cytophaga hutchinsonii, they do not appear to be required for gliding. Fjoh_0983 is also not required for gliding or for the formation of spreading colonies. Disruption of fjoh_0983 resulted in motility defects, but polar effects on the downstream genes may explain these, since introduction of plasmids that express sprC, sprD, sprB, and sprF, but that lack fjoh_0983, resulted in partial complementation.
Secretion of SprB is thought to require the sec system for export across the cytoplasmic membrane and the PorSS for secretion across the outer membrane. Involvement of the sec system in export of SprB across the cytoplasmic membrane is not surprising, since SprB and other bacteroidete proteins secreted by PorSSs have signal peptides expected to be recognized by the sec system. Mutations in the sec and porSS genes result in different outcomes for SprB protein. Cells with mutations in sprF or in genes encoding components of the PorSS, such as GldN or SprT, result in accumulation of SprB intracellularly rather than its being exposed on the cell surface (28, 29), whereas cells with mutations in secDF result in the absence of SprB protein (23) (Fig. 4C). Since SecDF is likely involved in translocation across the cytoplasmic membrane, SprB may be trapped in the cytoplasm or cytoplasmic membrane and targeted for degradation in cells of secDF mutants. In contrast, in cells of sprF and porSS mutants, SprB may accumulate in the periplasm, where it is apparently stable.
SprB is thought to function as a mobile cell surface adhesin that is propelled by the gliding motor. SprF appears to be needed for secretion of SprB, whereas SprC and SprD may support SprB function. Homologs of sprC, sprD, sprB, and sprF are found in all of the motile bacteroidetes for which genome sequences are currently available. In F. johnsoniae and in most of the other motile bacteroidetes, multiple paralogs of sprC, sprD, sprB, and sprF are present (22). These paralogs may explain the residual motility exhibited by cells with mutations in sprC, sprD, sprB, and sprF. The presence of SprB-like cell surface adhesins may also explain the ability of F. johnsoniae to crawl efficiently over such diverse surfaces as agar, glass, and Teflon. Five of the seven F. johnsoniae sprF paralogs are located immediately downstream of sprB paralogs. These SprF-like proteins may serve as adaptors for the PorSS, allowing efficient secretion of their cognate SprB-like proteins. SprB and the other cell surface motility proteins provide convenient handles for continued exploration of the functioning of the entire motility apparatus.
Supplementary Material
Acknowledgments
This research was supported by grants MCB-0641366 and MCB-1021721 from the National Science Foundation.
Footnotes
Published ahead of print on 3 December 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Agarwal, S., D. W. Hunnicutt, and M. J. McBride. 1997. Cloning and characterization of the Flavobacterium johnsoniae (Cytophaga johnsonae) gliding motility gene, gldA. Proc. Natl. Acad. Sci. U. S. A. 94:12139-12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez, B., P. Secades, M. J. McBride, and J. A. Guijarro. 2004. Development of genetic techniques for the psychrotrophic fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 70:581-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340:783-795. [DOI] [PubMed] [Google Scholar]
- 5.Braun, T. F., M. K. Khubbar, D. A. Saffarini, and M. J. McBride. 2005. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J. Bacteriol. 187:6943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, T. F., and M. J. McBride. 2005. Flavobacterium johnsoniae GldJ is a lipoprotein that is required for gliding motility. J. Bacteriol. 187:2628-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, L. Y. E., J. L. Pate, and R. J. Betzig. 1984. Isolation and characterization of nonspreading mutants of the gliding bacterium Cytophaga johnsonae. J. Bacteriol. 159:26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, S., M. Bagdasarian, M. G. Kaufman, A. K. Bates, and E. D. Walker. 2007. Mutational analysis of the ompA promoter from Flavobacterium johnsoniae. J. Bacteriol. 189:5108-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, A. J., A. P. Kalinowski, N. B. Shoemaker, and A. A. Salyers. 1997. Construction and characterization of a Bacteroides thetaiotaomicron recA mutant: transfer of Bacteroides integrated conjugative elements is RecA independent. J. Bacteriol. 179:6221-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland, I. B. 2010. The extraordinary diversity of bacterial protein secretion mechanisms. Methods Mol. Biol. 619:1-20. [DOI] [PubMed] [Google Scholar]
- 11.Hunnicutt, D. W., M. J. Kempf, and M. J. McBride. 2002. Mutations in Flavobacterium johnsoniae gldF and gldG disrupt gliding motility and interfere with membrane localization of GldA. J. Bacteriol. 184:2370-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunnicutt, D. W., and M. J. McBride. 2001. Cloning and characterization of the Flavobacterium johnsoniae gliding motility genes gldD and gldE. J. Bacteriol. 183:4167-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunnicutt, D. W., and M. J. McBride. 2000. Cloning and characterization of the Flavobacterium johnsoniae gliding motility genes, gldB and gldC. J. Bacteriol. 182:911-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kempf, M. J., and M. J. McBride. 2000. Transposon insertions in the Flavobacterium johnsoniae ftsX gene disrupt gliding motility and cell division. J. Bacteriol. 182:1671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, L.-Y., N. B. Shoemaker, and A. A. Salyers. 1995. Location and characterization of the transfer region of a Bacteroides conjugative transposon and regulation of the transfer genes. J. Bacteriol. 177:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, J., M. J. McBride, and S. Subramaniam. 2007. Cell-surface filaments of the gliding bacterium Flavobacterium johnsoniae revealed by cryo-electron tomography. J. Bacteriol. 189:7503-7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lupas, A. 1996. Prediction and analysis of coiled-coil structures. Methods Enzymol. 266:513-525. [DOI] [PubMed] [Google Scholar]
- 18.McBride, M. J. 2001. Bacterial gliding motility: multiple mechanisms for cell movement over surfaces. Annu. Rev. Microbiol. 55:49-75. [DOI] [PubMed] [Google Scholar]
- 19.McBride, M. J., and T. F. Braun. 2004. GldI is a lipoprotein that is required for Flavobacterium johnsoniae gliding motility and chitin utilization. J. Bacteriol. 186:2295-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McBride, M. J., T. F. Braun, and J. L. Brust. 2003. Flavobacterium johnsoniae GldH is a lipoprotein that is required for gliding motility and chitin utilization. J. Bacteriol. 185:6648-6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride, M. J., and M. J. Kempf. 1996. Development of techniques for the genetic manipulation of the gliding bacterium Cytophaga johnsonae. J. Bacteriol. 178:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McBride, M. J., et al. 2009. Novel features of the polysaccharide-digesting gliding bacterium Flavobacterium johnsoniae as revealed by genome sequence analysis. Appl. Environ. Microbiol. 75:6864-6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson, S. S., S. Bollampalli, and M. J. McBride. 2008. SprB is a cell surface component of the Flavobacterium johnsoniae gliding motility machinery. J. Bacteriol. 190:2851-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson, S. S., P. P. Glocka, S. Agarwal, D. P. Grimm, and M. J. McBride. 2007. Flavobacterium johnsoniae SprA is a cell surface protein involved in gliding motility. J. Bacteriol. 189:7145-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson, S. S., and M. J. McBride. 2006. Mutations in Flavobacterium johnsoniae secDF result in defects in gliding motility and chitin utilization. J. Bacteriol. 188:348-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pate, J. L., and D. M. De Jong. 1990. Use of nonmotile mutants to identify a set of membrane proteins related to gliding motility in Cytophaga johnsonae. J. Bacteriol. 172:3117-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pate, J. L., S. J. Petzold, and L.-Y. E. Chang. 1979. Phages for the gliding bacterium Cytophaga johnsonae that infect only motile cells. Curr. Microbiol. 2:257-262. [Google Scholar]
- 28.Rhodes, R. G., et al. 2010. Flavobacterium johnsoniae gldN and gldO are partially redundant genes required for gliding motility and surface localization of SprB. J. Bacteriol. 192:1201-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato, K., et al. 2010. A protein secretion system linked to bacteroidete gliding motility and pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 107:276-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf, E., P. S. Kim, and B. Berger. 1997. MultiCoil: a program for predicting two- and three-stranded coiled coils. Protein Sci. 6:1179-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolkin, R. H., and J. L. Pate. 1986. Phage adsorption and cell adherence are motility-dependent characteristics of the gliding bacterium Cytophaga johnsonae. J. Gen. Microbiol. 132:355-367. [Google Scholar]
- 32.Wolkin, R. H., and J. L. Pate. 1985. Selection for nonadherent or nonhydrophobic mutants co-selects for nonspreading mutants of Cytophaga johnsonae and other gliding bacteria. J. Gen. Microbiol. 131:737-750. [Google Scholar]
- 33.Yu, C. S., Y. C. Chen, C. H. Lu, and J. K. Hwang. 2006. Prediction of protein subcellular localization. Proteins 64:643-651. [DOI] [PubMed] [Google Scholar]
- 34.Yu, N. Y., et al. 2010. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.