Abstract
Background
Mild hypothermia (< 4°C) improves myocardial salvage after infarct reperfusion in animals and in early clinical studies. In this experiment the effect of mild hypothermia during ischemia and early reperfusion on long-term postinfarction left ventricular (LV) remodeling was assessed in an ovine infarct model.
Methods
In the initial phase of the experiment the effect of progressive degrees of hypothermia on infarct size was quantified. Thirty-eight male sheep were subjected to 1 hour of ischemia using a standardized an-teroapical infarct followed by 3 hours of reperfusion. Temperature was maintained at either 39.5°C (n = 11), 38.5°C (n = 7), 37.5°C (n = 7), 36.5°C (n = 7), or 35.5°C (n = 6) for the entire period of ischemia and reperfusion. The area at risk (AR) and infarct size as a percentage of AR (I/AR) were determined with a double staining and planimetry technique. In the second phase of the study, chronic post-infarction remodeling was assessed in animals with nonreperfused infarcts (n = 6), 1 hour of ischemia followed by reperfusion at 39.5°C (n = 6) and 1 hour of ischemia followed by reperfusion at 37.5°C (n = 6). Remodeling was determined at 8 weeks after infarction using echocardiography.
Results
The I/AR in the 39.5°C, 38.5°C, 37.5°C, 36.5°C, and the 35.5°C groups was 71.8 ± 3.0%, 63.1 ± 1.9%, 49.4 ± 1.4%, 38.7 ± 1.4%, and 21.7 ± 2.2%, respectively (p < 0.05 between all groups). In the chronic study LV end systolic volume at 8 weeks after infarction was 81 ± 8 mL in the nonreperfused group, 57 ± 4 mL in the 39.5°C reperfusion group, and 41 ± 3 mL in the 37.5°C reperfusion group (p < 0.05 for between group differences).
Conclusions
Subtle degrees of hypothermia can significantly improve immediate myocardial salvage and long-term LV remodeling after infarct reperfusion.
Prompt opening of the infarct-related artery salvages myocardium, preserves microvascular integrity, and is the most effective treatment for acute myocardial infarction (MI) [1]. Because infarct size is an important predictor of early and late survival after MI, adjunctive therapies that improve myocardial salvage after reperfusion would likely improve clinical outcomes [2]. While numerous pharmacologic interventions have been proposed and tested clinically, few have produced significant improvement over reperfusion alone [3].
Recent experimental studies have demonstrated that small reductions in myocardial temperature during ischemia can a have significant cardioprotective effect after reperfusion [4–8]. This work has resulted in the development of intravascular and topical cooling devices that have produced encouraging results in early clinical trials [9–11]. To date, the effect of adjunctive mild hypothermia on long-term left ventricular (LV) post-MI remodeling has not been studied in animals or patients.
The primary goal of this experiment was to determine the effect of very mild hypothermia (≤2°C), instituted during myocardial ischemia and maintained through the early reperfusion period, on long-term LV remodeling in a well-characterized ovine anteroapical infarct model. Prior to conducting the long-term remodeling experiments, we studied the dose-response relationship between core temperature and myocardial salvage in sheep.
Material and Methods
Experimental Design
The initial acute portion of the experiment was conducted to characterize the dose-response relationship between mild hypothermia (1°C to 4°C) and myocardial salvage in the sheep anteroapical infarct model. All animals were subjected to 1 hour of ischemia followed by 3 hours of reperfusion. Temperature was maintained constant throughout ischemia and reperfusion at the following values: 39.5°C (n = 11), 38.5°C (n = 7), 37.5°C (n = 7), 36.5°C (n = 7), and 35.5°C (n = 6). Ischemia was not induced until the goal temperature was reached. The area at risk (AR) and infarct size were determined with Evans blue dye and triphenyltetrazolium chloride staining, and planimetry.
In the chronic remodeling phase of the experiment three conditions were studied in the same infarct model used in the acute experiment. A control group had permanent coronary occlusion without reperfusion. A reperfusion group, which was subjected to 1 hour of ischemia followed by reperfusion at normothermia (39.5°C), and a reperfusion + hypothermia group (37.5°C) that had hypothermia induced prior to ischemia and the first 3 hours of reperfusion.
Temperature Management
After the induction of anesthesia sheep were sheared of all wool from neck to rump; cooling-warming pads from the topical hyperthermia-hypothermia unit (Medi-Therm III; Gaymar Industries Inc, Orchard Park, NY) were set up on both sides of the animal and the temperature of the device was set to the goal temperature. Ice bags were put on the neck, axillary, and inguinal regions. If the temperature went down under the maintenance range a halogen warming light was used to increase temperature and the temperature setting on the hyperthermia-hypothermia unit was adjusted. For each experiment the same dedicated technician was assigned to manage the animal’s temperature.
Rectal and left atrial (LA) temperatures were monitored continually throughout the experiment and recorded at 15-minute intervals. Rectal and LA temperature measurements were always within 0.3°C. The LA temperature was used to determine when ischemia would be induced.
Acute Protocol
Thirty-eight male sheep weighing 35 to 40 kg were used in the initial part of the experiment. Animals were treated under experimental protocols approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee (IACUC) and in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23, revised 1996).
Anesthesia was induced with thiopental sodium (10 to 15 mg/kg intravenously), and sheep were intubated, anesthetized with isoflurane (1.5% to 2%), and ventilated with oxygen. Catheters were placed in a femoral artery and internal jugular vein for the continuous measurement of blood pressure and the administration of intravenous medications. Animals underwent a left thoracotomy and silicone vascular loops (Quest Medical Inc, Allen, TX) were placed around the left anterior descending artery (LAD) and its second diagonal branch 40% of the distance from the apex to the base of the heart to allow atraumatic occlusion of these arteries. Occlusion of these arteries at these locations has produced a well-characterized, reproducible model of anteroapical myocardial infarction in our laboratory [12]. After tightening the snares, ischemia was confirmed by a visible color change in the ischemic myocardial region and ST segment elevations on the electrocardiogram (ECG). At the end of the 1-hour ischemic period, coronary snares were loosened and the previously ischemic myocardium was reperfused for 3 hours in all animals. The LA temperature was maintained at the following values prior to the induction of ischemia and throughout the remainder of the experiment: 39.5°C (n = 11), 38.5°C (n = 7), 37.5°C (n = 7), 36.5°C (n = 7), and 35.5°C (n = 6). The normal body temperature range for nonfasting, nonpregnant sheep is 39°C to 40.5°C [13].
After 3 hours of reperfusion, the coronary snares were retightened, vascular clamps were used to occlude the aorta, pulmonary artery, and inferior vena cava, and the right atrium was incised. Evans blue dye (1 mL/kg; Sigma, St. Louis, MO) was injected through the left atrium to delineate the ischemic myocardial risk area. All animals were euthanized by an injection of potassium chloride into the left atrium. The heart was excised and the LV was sectioned perpendicular to its long axis into 6 slices. The thickness of each slice was measured with a digital micrometer and all slices were photographed after 20 minutes of incubation in 2% triphenyltetrazolium chloride at 37°C. All photographs were imported into an image analysis program (Image-Pro Plus; MediaCybernetics, Silver Spring, MD), and computerized planimetry was performed to determine the overall size of the AR and infarct. The AR is expressed as a percentage of the LV, and the infarct size (I) is expressed as a percentage of the AR (I/AR).
Chronic Protocol
Eighteen additional animals were subjected to the same infarction as described above. In six animals reperfusion was not performed (control group). Six animals had 1 hour of coronary occlusion at normothermia followed by reperfusion (reperfusion group). The final six animals (reperfusion + hypothermia group) were cooled to a LA temperature of 37.5°C prior to one hour of ischemia. This temperature was actively maintained for 3 hours after reperfusion and then allowed to passively normalize. Rectal and LA temperature were monitored continually throughout the experiment and recorded at 15-minute intervals.
Quantitative two-dimensional echocardiography was performed prior to infarction and at 8 weeks after infarction to assess LV remodeling in all groups. Left ventricular end diastolic (LVEDV) and end systolic volume (LVESV) were determined using a modified Simpson’s rule technique. Ejection fraction (EF) was calculated from the LV volume data.
Heart rate, mean arterial pressure, cardiac output, and pulmonary capillary wedge pressure were also assessed before infarction and after 8 weeks of post-MI remodeling in each group.
In the chronic experiment, after the 8-week echocardiographic and hemodynamic data were recorded, the heart was arrested with potassium chloride and excised. A long-axis tissue section encompassing the infarct and border zone (BZ) regions was fixed in 5% buffered formalin, paraffin embedded, sectioned at 3 to 4 μm, and stained with hematoxylin and eosin as well as Masson’s trichrome stain.
Statistical Analysis
Measurements are reported as means ± standard error of the mean. A one-way analysis of variance (ANOVA) was used for all comparisons between groups; repeated measures ANOVA was used for all comparisons within groups. Individual post hoc comparisons were performed using the Tukey “Honestly Significantly Different” test. All analyses were completed using SPSS Version 11.0 (SPSS Inc, Chicago, IL). Statistically significant differences were established at a p value less than 0.05.
Results
Acute Experiment
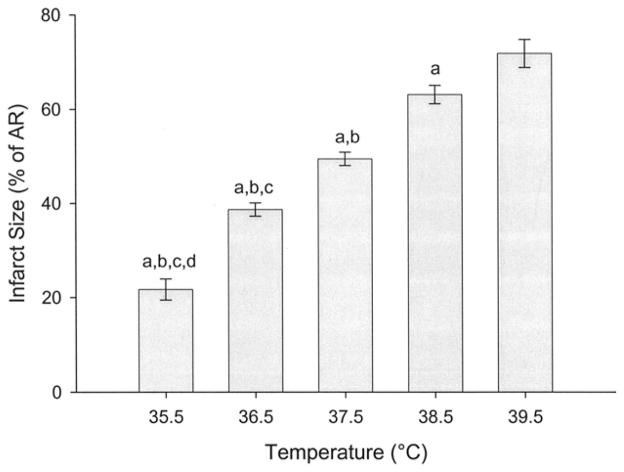
The AR did not vary significantly between temperature groups and averaged 23.5 ± 2.4%. The LA temperature was effectively maintained at the target value in all animals during both the ischemic and reperfusion time periods (Fig 1). Infarct size as a percentage of the AR (I/AR) was very sensitive to small changes in body temperature. The I/AR in the 39.5°C, 38.5°C, 37.5°C, 36.5°C, and 35.5°C groups was 71.8 ± 3.0%, 63.1 ± 1.9%, 49.4 ± 1.4%, 38.7 ± 1.4%, and 21.7 ± 2.2%, respectively. The differences in infarct size among each of the groups reached statistical significance (p < 0.05; Fig 2). Temperature strongly correlated with the ultimate infarct size (r = 0.920, p < 0.001, Fig 3).
Fig 1.
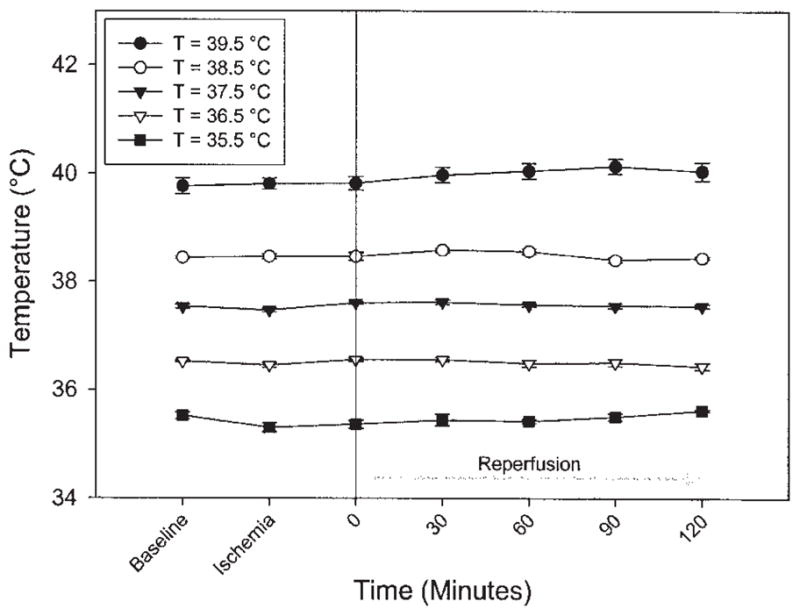
Core body temperature over time as measured in the left atrium in each of the temperature groups in the acute phase of the experiment. Temperature was maintained constant in all groups throughout both ischemia and reperfusion. Baseline is just prior to coronary occlusion; Ischemia is 15 minutes after coronary occlusion.
Fig 2.
Infarct size as a percentage of the area at risk in each of the temperature groups in the acute phase of the experiment. Infarct size was determined by Evans blue/triphenyltetrazolium chloride staining and planimetry. (a p < 0.05 vs 39.5°C, b p < 0.05 vs 38.5°C, c p < 0.05 vs 37.5°C, d p < 0.05 vs 36.5°C.)
Fig 3.
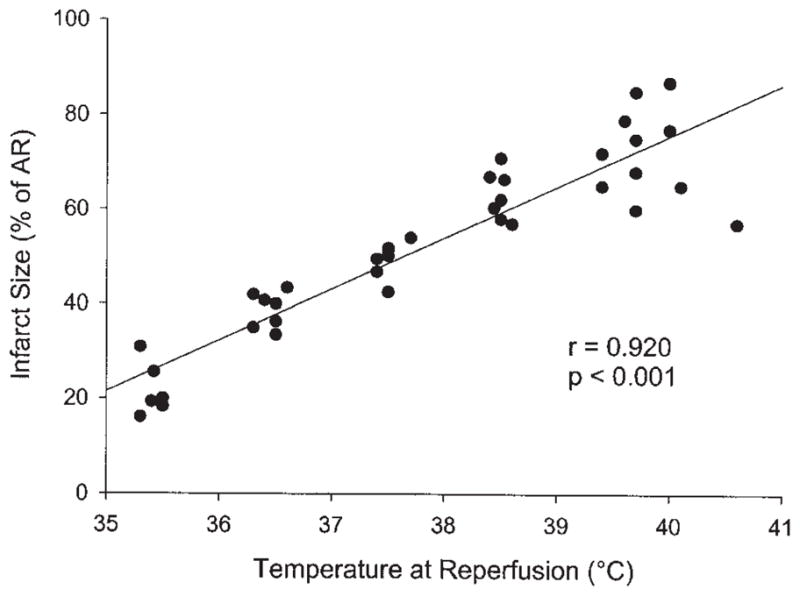
Infarct size plotted as a function of the core body temperature at the time of reperfusion. (AR = area at risk.)
Chronic Remodeling Experiment
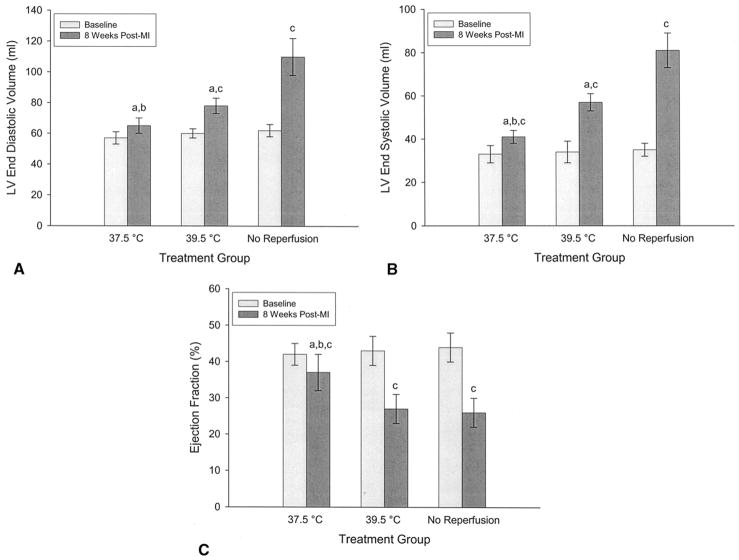
The nonreperfused control group experienced severe LV remodeling as LVEDV increased from 62 ± 4 mL to 110 ± 12 mL, LVESV increased from 35 ± 3 mL to 81 ± 8 mL, and EF decreased from 0.44 ± 0.04 to 0.26 ± 0.04. Reperfusion after 1 hour of normothermic ischemia significantly (p < 0.05) reduced remodeling relative to the control group (LVEDV = 78 ± 5 mL; LVESV =57 ± 4 mL), but did not significantly improve EF (0.27 ± 0.04). Cooling to 37.5°C during ischemia and subsequent reperfusion further improved long-term remodeling (LVEDV = 65 ± 5; LVESV = 41 ± 3 mL) and resulted in improved EF (0.37 ± 0.05). All comparisons among and within groups were significant at the p less than 0.05 level (Fig 4).
Fig 4.
Echocardiographic assessment of global left ventricular (LV) remodeling and function before infarction and 8 weeks after infarction in the reperfusion group (39.5), the reperfusion + hypothermia group (37.5°C), and the control group (no reperfusion). (A) Left ventricular end diastolic volume; (B) left ventricular end systolic volume; (C) ejection fraction a p < 0.05 vs no reperfusion; b p < 0.05 vs 39.5°C (reperfused); c p < 0.05 vs baseline.
Cardiac output decreased significantly from preinfarction values in all groups, but was significantly better in the reperfusion + hypothermia group at 8 weeks after infarction. Pulmonary capillary wedge pressure was increased at 8 weeks in the control group but not in either of the reperfusion groups. Echocardiographic and hemodynamic data are presented in Table 1.
Table 1.
Hemodynamic and Remodeling Data
| 37.5°C (Reperfused) |
39.5°C (Reperfused) |
No Reperfusion |
||||
|---|---|---|---|---|---|---|
| Baseline | 8 Weeks Post-MI | Baseline | 8 Weeks Post-MI | Baseline | 8 Weeks Post-MI | |
| LVEDV (mL) | 57 ± 4 | 65 ± 5a,b | 60 ± 3 | 78 ± 5a,c | 62 ± 4 | 110 ± 12c |
| LVESV (mL) | 33 ± 4 | 41 ± 3a,b,c | 34 ± 5 | 57 ± 4a,c | 35 ± 3 | 81 ± 8c |
| EF | 0.42 ± 0.03 | 0.37 ± 0.03a,b,c | 0.43 ± 0.04 | 0.27 ± 0.04c | 0.44 ± 0.04 | 0.26 ± 0.04c |
| CO (L/m) | 4.3 ± 0.3 | 3.8 ± 0.5a,b,c | 4.3 ± 0.2 | 2.8 ± 0.3c | 4.6 ± 0.6 | 2.4 ± 0.4c |
| PCWP (mm Hg) | 12 ± 2 | 14 ± 2a | 12 ± 3 | 15 ± 2a | 9 ± 2 | 19 ± 2c |
| MAP (mm Hg) | 91 ± 4 | 84 ± 2 | 88 ± 2 | 81 ± 7 | 90 ± 3 | 86 ± 4 |
p < 0.05 vs no reperfusion;
p < 0.05 vs 39.5°C (reperfused);
p < 0.05 vs baseline.
CO = cardiac output; EF = ejection fraction; LVEDV = left ventricular end diastolic volume; LVESV = left ventricular end systolic volume; MAP = mean arterial pressure; MI = myocardial infarction; PCWP = pulmonary capillary wedge pressure.
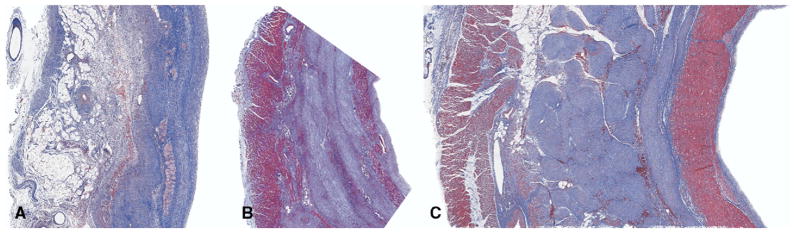
Examination of the pathologic slides in the nonreperfused control group demonstrated occasional islands of myocytes in a well-developed transmural scar. In both reperfusion groups there was an endocardial and epicardial layer of myocytes separated by a dense, mature scar similar to that seen in the control group. In most cases the endocardial and epicardial myocyte layers were thicker in the reperfusion + hypothermia group compared with the normothermic reperfusion group (Fig 5).
Fig 5.
Masson’s trichrome staining of longitudinal section through myocardial infarct specimens 8 weeks after a nonreperfused infarct (A), an infarct reperfused at 39.5°C (B), or an infarct reperfused at 37.5°C (C). All images are 2×. In all images the endocardial surface is to the right and the epicardial surface is to the left. Reperfusion resulted in the salvage myocytes primarily in the endocardial and epicardial regions. These epicardial and endocardial regions appeared thicker in reperfused animals subjected to hypothermia. Nonreperfused infarcts were transmural, with no significant myocyte salvage.
Comment
The results of the acute infarct sizing experiments demonstrate that the ovine myocardial response to an ischemia-reperfusion insult is exquisitely sensitive to temperature. Within the temperature range studied there was a near linear response between myocardial salvage and temperature; for every 1°C reduction in temperature the infarct size within the area at risk was reduced by over 10%. These results are consistent with previous studies conducted in models with both a well-developed system of coronary collateral circulation (dogs [7]), as well as those which are devoid of coronary collaterals (pigs [5, 8] and rabbits [4]).
The mechanism by which mild hypothermia produces its cardioprotective effects is likely multifactorial. Hypothermia has been associated with a wide range of protective mechanisms, which may ameliorate ischemia-reperfusion injury. These include the induction of heat shock proteins [14], reduced apoptosis [15], decreased complement activation, and a reduction in neutrophil degranulation [16, 17]. Mild hypothermia has also been shown by our group and others [18, 19] to limit the microvascular injury associated with myocardial reperfusion injury. Although the broad spectrum of hypothermia-induced effects makes it difficult to pinpoint the exact mechanism of protection, this may be advantageous because pharmacologic therapies targeting a single mechanistic pathway have been generally ineffective adjuncts to reperfusion in clinical trials [3]. The ability to simultaneously suppress multiple pathologic pathways without significant adverse consequences makes mild hypothermia an attractive adjunct to reperfusion therapy for acute MI.
In this experiment, temperature was maintained constant throughout ischemia and subsequent reperfusion. The effect of instituting hypothermia at a more clinically relevant time point either late in the ischemic period or early after reperfusion was not assessed. Other studies in our laboratory have, however, demonstrated that the temperature during late ischemia and (or) early reperfusion is most important for reducing infarct size and preserving microvascular integrity with mild hypothermia [20]. Our work and that of other investigators [21] suggest that it is the temperature of the reperfusate itself that is most critical in determining the degree of myocardial salvage.
The ischemic time used in this experiment was chosen because it has been commonly used in other large animal acute and chronic preclinical studies of myocardial ischemia-reperfusion injury; therefore, it allows the best comparison with the existing literature. The effect of mild hypothermia on myocardial salvage during longer, more clinically relevant, ischemic periods will need to be determined In future studies.
In the current study, reperfusion at normothermia improved post-MI remodeling to a degree that was consistent with previous studies in our laboratory [22, 23]. Reducing temperature by 2°C resulted in a further improvement in remodeling that was statistically significant. While it was not possible to precisely quantify infarct size directly in the chronic study, the acute study and the reproducibility of the model allow us to make a reliable estimate. In the nonreperfused control group the infarct size was approximately 25% of the LV mass. In the normothermia reperfusion group infarct size was approximately 17% (AR × I/AR = 25 × 0.7), and in the reperfusion + hypothermia group infarct size was close to 12% (AR × I/AR = 25 × 0.5) of the total LV mass. This analysis demonstrates that relatively small reductions in the absolute infarct size induced by low levels of hypothermia can have important effects on LV remodeling. These results suggest that future clinical studies of reperfusion therapy with adjunctive hypothermia need to be designed to assess long-term outcomes in addition to the more commonly reported short-term outcomes and quantification of infarct size. Based on the data in the current study, reductions in myocardial loss secondary to mild hypothermia measured in the early post-MI period may seem modest, but, as seen from our results, they may translate into substantial improvements in long-term remodeling.
The current study employed a model of anteroapical MI. We have recently demonstrated that the myocardial response to reperfusion and hypothermia is dependent on location within the ventricle, with the most apical regions having the best responses to hypothermic therapy [19]. Therefore, the results of this study cannot be extrapolated to infarcts located at the base of the heart [19]. Finally, while we have focused exclusively on myocardial salvage it is possible that hypothermia may influence other biologic properties known to affect the remodeling process, such as extracellular matrix biochemistry and the post-MI inflammatory response.
Acknowledgments
This study was supported by the National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda MD (HL63954, HL71137, and HL 76560). Robert Gorman and Joseph Gorman are supported by individual Established Investigator Awards from the American Heart Association.
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomized trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.Bolognese L, Neskovic AN, Parodi G, et al. Left ventricular remodeling after primary coronary angioplasty: patterns of left ventricular dilation and long-term prognostic implications. Circulation. 2002;106:2351–7. doi: 10.1161/01.cir.0000036014.90197.fa. [DOI] [PubMed] [Google Scholar]
- 3.Kloner RA, Rezkalla SH. Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol. 2004;44:276–86. doi: 10.1016/j.jacc.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 4.Chien GL, Wolff RA, Davis RF, van Winkle DM. “Normothermic range” temperature affects myocardial infarct size. Cardiovasc Res. 1994;28:1014–7. doi: 10.1093/cvr/28.7.1014. [DOI] [PubMed] [Google Scholar]
- 5.Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol Heart Circ Physiol. 1996;270:H1189–99. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- 6.Hale SL, Kloner RA. Myocardial temperature in acute myocardial infarction: protection with mild regional hypothermia. Am J Physiol Heart Circ Physiol. 1997;273:H220–7. doi: 10.1152/ajpheart.1997.273.1.H220. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz LM, Verbinski SG, Vander Heide RS, Reimer KA. Epicardial temperature is a major predictor of myocardial infarct size in dogs. J Mol Cell Cardiol. 1997;29:1577–83. doi: 10.1006/jmcc.1997.0391. [DOI] [PubMed] [Google Scholar]
- 8.Dae MW, Gao DW, Sessler DI, Chair K, Stillson CA. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am J Physiol. 2002;282:H1584–91. doi: 10.1152/ajpheart.00980.2001. [DOI] [PubMed] [Google Scholar]
- 9.Dixon SR, Whitbourn RJ, Dae MW, et al. Induction of mild systemic hypothermia with endovascular cooling during primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2002;40:1928–34. doi: 10.1016/s0735-1097(02)02567-6. [DOI] [PubMed] [Google Scholar]
- 10.O’Neill WW on behalf of the COOL-MI Investigators. A prospective, randomized trial of mild systemic hypothermia during PCI treatment of ST elevation MI. Presented at the 15th Annual Transcatheter Cardiovascular Therapeutics Symposium; Washington, DC. Sept 15–19, 2003. [Google Scholar]
- 11.Ly HQ, Denault A, Dupuis J, et al. A pilot study: the noninvasive surface cooling thermoregulatory system for mild hypothermia induction in acute myocardial infarction (the NICAMI Study) Am Heart J. 2005;150:933. doi: 10.1016/j.ahj.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Markovitz LJ, Savage EB, Ratcliffe MB, et al. Large animal model of left ventricular aneurysm. Ann Thorac Surg. 1989;48:838–45. doi: 10.1016/0003-4975(89)90682-6. [DOI] [PubMed] [Google Scholar]
- 13.Mohr EG, Krzywanek H. Endogenous oscillator and regulatory mechanisms of body yemperature in sheep. Physiol Behav. 1995;57:339–47. doi: 10.1016/0031-9384(94)00273-8. [DOI] [PubMed] [Google Scholar]
- 14.Qing M, Vasquez-Jimenez JF, Schumacher K, et al. Moderate hypothermia during cardiopulmonary bypass increases intramyocardial synthesis of heat shock protein 72. J Thorac Cardiovasc Surg. 2002;124:724–31. doi: 10.1067/mtc.2002.124498. [DOI] [PubMed] [Google Scholar]
- 15.Ning XH, Chen SH, Xu CS, et al. Hypothermia protection of the ischemic heart via alterations in apoptotic pathways as assessed by gene arrays analysis. J Appl Physiol. 2002;92:2200–7. doi: 10.1152/japplphysiol.01035.2001. [DOI] [PubMed] [Google Scholar]
- 16.Anttila V, Hagino I, Zurakowski D, Lidov HG, Jonas RA. Higher bypass temperature correlates with increased white cell activation in the cerebral microcirculation. J Thorac Cardiovasc Surg. 2004;127:1781–8. doi: 10.1016/j.jtcvs.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 17.Chello M, Mastroroberto P, Romano R, Ascione R, Pantaleo D, De Amicis V. Complement and neutrophil activation during cardiopulmonary bypass: a randomized comparison of hypothermic and normothermic circulation. Eur J Cardiothorac Surg. 1997;11:162–8. doi: 10.1016/s1010-7940(96)01102-5. [DOI] [PubMed] [Google Scholar]
- 18.Hale SL, Dae MW, Kloner RA. Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res. 2003;59:715–22. doi: 10.1016/s0008-6363(03)00456-5. [DOI] [PubMed] [Google Scholar]
- 19.Hamamoto H, Leshnower BG, Parish LM, et al. Regional heterogeneity of myocardial reperfusion injury: effect of mild hypothermia. Ann Thorac Surg. 2009;87:164–71. doi: 10.1016/j.athoracsur.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanemoto S, Matsubara M, Noma M, et al. Mild hypothermia to limit myocardial ischemia-reperfusion injury: importance of timing. Ann Thorac Surg. 2009;87:157–63. doi: 10.1016/j.athoracsur.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otake H, Shite J, Paredes OL, et al. Catheter-based transcoronary myocardial hypothermia attenuates arrhythmia and myocardial necrosis in pigs with acute myocardial infarction. J Am Coll Cardiol. 2007;49:250–60. doi: 10.1016/j.jacc.2006.06.080. [DOI] [PubMed] [Google Scholar]
- 22.Bowen FW, Hattori T, Narula N, et al. Reappearance of myocytes in ovine infarcts produced by six hours of complete ischemia followed by reperfusion. Ann Thorac Surg. 2001;71:1845–55. doi: 10.1016/s0003-4975(01)02642-x. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto H, Parish LS, Hamamoto H, et al. The effect of reperfusion on left ventricular regional remodeling strains after myocardial infarction. Ann Thorac Surg. 2007;84:1528–36. doi: 10.1016/j.athoracsur.2007.05.060. [DOI] [PubMed] [Google Scholar]