Abstract
Clinical trials often aggregate daily alcohol consumption data across long-term follow-up intervals (e.g., 6 or 12 months). Although important in understanding general treatment outcomes, these analyses tell us little about when treatment effects emerge or decline. We previously demonstrated that motivational interviewing (MI) reduced heavy drinking (vs. personalized feedback only; FO) among young adult drinkers (N = 198; ages 18-24) recruited in a hospital Emergency Room (ER) using aggregated drinking data from 6-month follow up. In the current study, we used daily alcohol consumption data from a calendar-assisted interview (Timeline Followback) to examine the timing and course of these treatment effects. Participants in both conditions received brief telephone booster sessions at 1 and 3 months. There were no treatment effects in the time between the initial intervention session and the 3-month booster session. Significant effects emerged after the 3-month booster and were driven by an increase in heavy drinking within the FO group. This suggests that the effects of brief interventions may not emerge immediately following an initial session. Aggregated data would be unable to detect this time trend. This research underscores the potential value added by examining the day-to-day timing of effects following treatments for alcohol use.
Keywords: Daily Drinking, Timeline Followback, Motivational Interviewing, Screening and Brief Intervention, Young Adult
1.0. Introduction
Alcohol and drug use data are frequently collected in clinical trials via calendar-based methods, in which participants are asked to report their consumption each day over a specified interval (e.g., Timeline Followback [TLFB], Sobell & Sobell, 1995; Form-90, Miller, 1996). Daily quantity and frequency data typically are aggregated over a period of time (e.g., over the last 30 days at 6-month follow-up) to calculate an efficacy endpoint that can be used to compare treatment groups. Although this strategy provides important information about the relative efficacy of the treatments, it provides little detail about the dynamics of treatment effects over time. For example, if treatments increase motivation to stop or reduce alcohol consumption, treatment effects may emerge immediately following the intervention. Alternatively, if interventions initiate a change process that unfolds over time, effects may gradually appear. These patterns of alcohol use may be better assessed in terms of fine-grained temporal fluctuations rather than summary measures at 3- or 6-month intervals (Neal et al., 2006; Rice, 2007; Wang, Winchell, McCormick, Nevius, & O’Neill, 2002). This information may increase our understanding of intervention effects.
The value of examining the temporal patterning of multiple drinking episodes, rather than just time to a single event or a single summary of drinking frequency, has been discussed elsewhere (see multiple failure time approach; Wang et al., 2002). Examining a single outcome, such as occurrence of relapse or average number of drinks per day, may mask meaningful temporal trends, such as clustering of drinking episodes over time or when treatment effects emerge. However, we are unaware of any study that has used daily drinking data to examine temporal trends in the efficacy of an alcohol intervention. The purpose of the present research was to assess the utility of examining daily drinking behavior in the context of a brief alcohol intervention trial. The goal of this paper is not to assess the efficacy of the intervention (discussed elsewhere, Monti et al., 2007), but rather to show how the examination of daily data can enhance the description of treatment effects for addictive behaviors, in general, by showing when the treatment effects emerge in the course of a multi-session intervention. This approach could provide a model for analyzing psychosocial intervention and pharmacotherapy data that goes beyond examining aggregated data at long-term follow-up points.
To demonstrate this approach, we used data from a recent controlled trial contrasting a single motivational interview (MI) session including personalized feedback on alcohol use with a feedback only (FO) condition including minimal clinician contact. The study was designed to test these interventions in a sample of young adult problem drinkers who were recruited in an emergency room (ER), and therefore, considered at high risk for alcohol abuse and related injury (Office of Applied Studies, 2003). We previously showed that the MI condition significantly reduced alcohol use, when drinking data were aggregated across 6- and 12-month periods (Monti et al., 2007). In this study, we examined daily heavy drinking in the month prior to hospital entry and in the 6 months following the initial intervention session. The main goal of the analysis was to understand when MI vs. FO treatment group differences emerged by examining time trends in daily heavy drinking. A secondary goal was to describe time trends in the days leading up to the ER visit, in order to better understand the drinking patterns that may lead to crisis events requiring medical assistance.
2.0. Method
2.1. Participants
Level 1 trauma center patients in a large northeastern hospital (N = 198; ages 18-24) were enrolled. Patients were eligible to participate if they were treated in the ER and: (a) had a blood alcohol concentration greater than .01%, or (b) reported drinking alcohol in the 6 hours prior to the event that precipitated their ER visit, or (c) scored 8 or higher on the Alcohol Use Disorders Identification Test (AUDIT; Saunders at al., 1993). Study staff were always available, through a combination of on-site coverage and on-call availability, eliminating sampling bias. Of the eligible participants, 31.5% were enrolled. The study sample was primarily male (67.7%), Caucasian (74%; followed by African American, 11.5%), and their average age was approximately 20 years old (20.6 ± 1.9). The average AUDIT score was 11.4 (± 6.4). There were no significant group differences in consumption or alcohol problems at baseline. See Monti et al. (2007) for more details.
2.2. Procedure
Patients were screened upon admission to the ER. After enrollment, participants completed a baseline assessment battery. Participants were randomly assigned to treatment condition (MI or FO) and completed follow-up visits 6 and 12 months following treatment. The 6-month follow-up data are presented here (N = 164; 83.3% retention rate, with no difference between treatment groups).
2.3. Treatment conditions
Details of the treatment conditions are described in Monti et al., 2007. Patients in the FO condition (n=100) received the same baseline assessment and computer-generated personalized feedback report as those in MI (n=98). However, counselor contact was minimal (1-3 minutes vs. 30-45 minutes). Telephone booster sessions were conducted one and three months after baseline. MI boosters included assessment of drinking, a review of patient goals, and feedback comparing current drinking behavior to baseline behavior. FO boosters included an assessment of drinking. MI and FO groups did not differ on any drinking variables, including frequency of heavy drinking, at baseline (Monti et al., 2007). Booster compliance was significantly higher in FO (92%) than in the MI condition (82%) at 1-month booster, χ2(1, N = 198) = 4.66, p < .05, and at 3-month booster (FO: 90%; MI: 74%), χ2(1; N = 198) = 9.09, p < .01).
2.4. Measures
The Timeline Followback (TLFB; Sobell & Sobell, 1995) was used to assess the number of drinks per day and then days were categorized as heavy drinking days or not (≥ 4 drinks for women, ≥ 5 drinks for men). At baseline, the TLFB covered the 30 days prior to the ER visit. At the 1-month booster, patients completed a 30-day TLFB. At the 3-month booster, patients completed a 60-day TLFB. At 6-month follow up, the TLFB covered the 30 days prior to the follow-up date.
2.5. Data analysis
Generalized estimating equations (GEE; Liang & Zeger, 1986) were used to examine trends in daily heavy drinking over time in the two treatment groups. Analyses addressed linear and quadratic trends in two baseline time intervals: the entire 30 days and the 7 days prior to the drinking episode. An autoregressive(1) correlation structure type and empirical variance matrix were used. Because the outcome (whether or not a day is a heavy drinking day) is binary, the parameter estimate from the GEE can be interpreted as an odds ratio (OR). The OR indicates the change in odds of heavy drinking, with each passing day. Analysis of treatment group differences included all days and the separate 1-90 and 150-180 intervals. Percent of heavy drinking days at baseline was included as a covariate in the analysis, group assignment was included as a categorical predictor variable (1 = MI, 2 = FO), and the interaction between treatment group and time was examined.
3.0 . Results
3.1. Pre-intervention interval
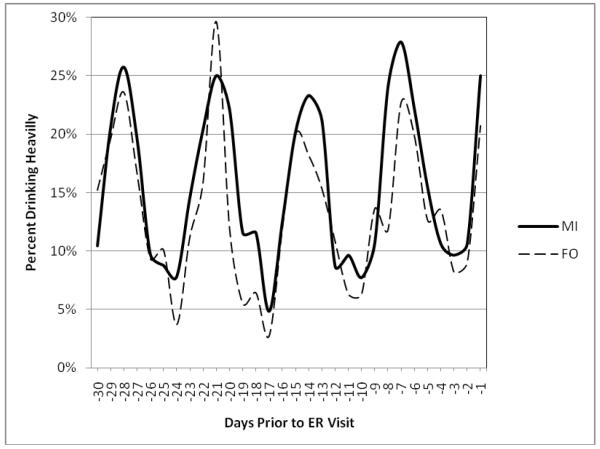
There were no linear (OR = 1.00, 95% CI = 0.99 - 1.01, p=.94) or quadratic (OR = 1.00, 95% CI = 0.99 - 1.01, p=.56) time trends in the 30-day baseline period and treatment groups did not differ (OR = 0.82, 95% CI = 0.57 - 1.17, p=.29) in this interval. However, a significant quadratic trend emerged (OR = 1.11, 95% CI = 1.06 - 1.15, p < .0001) in the week prior to the event leading to the ER visit. This pattern was replicated in the second, third, and fourth weeks prior to the ED visit. Since the vast majority of ER visits occurred on the weekend, the weekly pattern appears to reflect a high frequency of heavy drinking on the weekends and low frequency during the week (Figure 1).
Figure 1.
Occurrence of heavy drinking days during the baseline interval. The data suggest a weekly periodicity, in which heavy drinking peaks on the weekends. MI = Motivational Intervention group, FO = Feedback Only group.
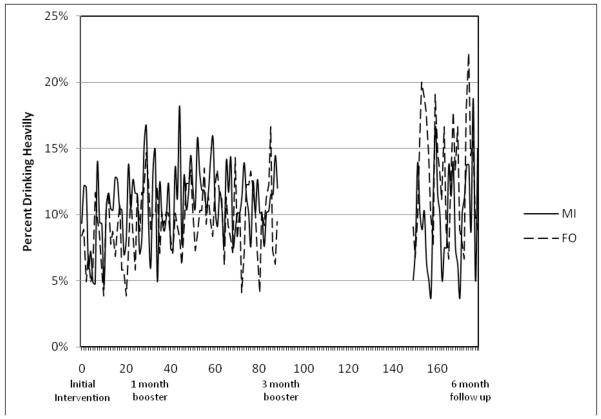
3.2. Post-intervention interval
When all days in the post-intervention period were included in analysis, there was no significant effect of treatment, OR = 1.26, 95% CI = 0.88 - 1.80, p=.22. However, when the follow-up intervals were considered separately, differences in the relationship between treatment group and heavy drinking emerged. In the first 3 months post-intervention, there was no association between treatment and heavy drinking, OR = 1.13, 95% CI = 0.73 - 1.73, p=.58. In the sixth month, FO was significantly associated with an increased likelihood of heavy drinking, OR = 1.72, 95% CI = 1.15 - 2.56, p < .0001. In other words, there were no treatment differences evident in the 3 months after the initial intervention session. Group differences only emerged following the 3-month booster session, such that the odds of heavy drinking on a day were significantly higher in the FO group.
Analysis of changes over time in the post-intervention interval suggested a nonsignificant trend towards increased heavy drinking, OR = 1.01, 95% CI = 0.99 - 1.02, p = .08, and further analyses suggested that this change differed across the treatment groups (treatment x time interaction OR = 1.01, 95% CI = 1.00 - 1.02, p = .03). In the MI group, no linear change was observed, OR = 1.00, 95% CI = 0.99 - 1.01, p=.68, but in the FO group, a positive trend emerged, OR = 1.003, 95% CI = 1.00 - 1.01, p = .006. No treatment x time interactions were observed when the 1-90 or 150-180 day intervals were analyzed separately.
4.0. Discussion
Our previous analyses demonstrated the efficacy of MI vs. FO, when past-month drinking data were evaluated at a 6-month follow up (Monti et al., 2007). Day-to-day analysis of heavy drinking data from the period between intervention and follow up revealed a more nuanced picture of these significant treatment effects. Treatment group differences only emerged after the 3-month booster session and prior to the 6-month follow-up assessment. Without analyzing the drinking data from smaller intervals of time, it would be impossible to detect this important temporal trend.
We also examined the 30-day period prior to ER treatment. Patients generally reported heavy drinking on the weekends. Although a pattern of periodic alcohol consumption may seem typical in a sample of 18 to 24 year olds (e.g., Del Boca et al., 2004), it is undetectable using descriptive measures that are typically included in clinical trials, such as the AUDIT (Saunders et al., 1993) or 30-day summary indices from the TLFB (Sobell & Sobell, 1995).
This analysis has some limitations to consider. This was a secondary data analysis; the study was not designed to examine daily drinking patterns over the entire course of the follow-up period. For this reason, drinking on days 90 to 150 are missing. This ‘blind spot’ in the data may obscure meaningful trends, including the exact time when the beneficial effects of the MI treatment emerged. Fewer MI patients attended the booster session and this may have influenced the results. For example, if patients who were drinking less in the MI condition did not attend the booster, this could obscure group differences. However, analyses of 6-month follow up data suggest that there were no differences in heavy drinking between those who attended the 3-month follow up and those who did not. Approximately 17% of participants did not attend the 6-month follow up; it is unknown how this may have affected the data. Finally, it is not clear that these results generalize to other clinical populations; replication in other samples is needed.
5.0. Conclusions
Analyzing endpoints on a daily basis can provide information about how and when treatments begin to exert their effects and when these effects deteriorate. It provides a more detailed description of treatment effects than aggregate indices or single events. This more detailed description may provide information that could be used to improve interventions, such as highlighting time intervals when the treatment is ineffective and supplemental treatment may be needed. The current study may serve as a model of examining daily data in other clinical trials.
Research Highlights.
Beneficial effects of motivational interviewing vs. feedback only appear after 3 months
Examining endpoints on daily basis can enhance understanding of treatment effects
Figure 2.
Occurrence of heavy drinking days during the post-intervention interval. MI = Motivational Intervention group, FO = Feedback Only group.
Acknowledgments
Role of Funding Sources
This investigation was supported by research grant AA09892 from the National Institute on Alcohol Abuse and Alcoholism, and by a Department of Veterans Affairs Senior Career Research Scientist Award to Peter M. Monti. The funding agencies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication.
Footnotes
Conflict of Interest
Chad Gwaltney serves as a consultant to PRO Consulting and invivodata, inc., which provides electronic diary services for clinical research. All other authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Del Boca FK, Darles J, Greenbaum PE, Goldman MS. Up close and personal: Temporal variability in the drinking of individual college students during their first year. J Cons Clin Psychol. 2004;72:155–164. doi: 10.1037/0022-006X.72.2.155. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- Miller WR. Motivational interviewing: Research, practice, and puzzles. Addictive Behaviors. 1996;21(6):835–842. doi: 10.1016/0306-4603(96)00044-5. [DOI] [PubMed] [Google Scholar]
- Monti PM, Barnett NP, Colby SM, Gwaltney CJ, Spirito A, Rohsenow DJ, Woolard R. Motivational interviewing vs. feedback only in emergency care for young adult problem drinking. Addiction. 2007;102:1234–1243. doi: 10.1111/j.1360-0443.2007.01878.x. [DOI] [PubMed] [Google Scholar]
- Neal DJ, Fromme K, Del Boca FK, Parks KA, King LP, Pardi AM, et al. Capturing the moment: Innovative approaches to daily alcohol assessment. Alcohol: Clin Exp Res. 2006;30:282–291. doi: 10.1111/j.1530-0277.2006.00025.x. [DOI] [PubMed] [Google Scholar]
- Rice C. Retest reliability of self-reported daily drinking: Form-90. J Stud Alcohol Drugs. 2007;68:615–618. doi: 10.15288/jsad.2007.68.615. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, De La Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol timeline followback users’ manual. Addiction Research Foundation; Toronto, Canada: 1995. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2003 National Survey on Drug Use and Health: National Findings. Office of Applied Studies; Rockville, MD: 2004. NSDUH Series H–25, DHHS Publication No. SMA 04–3964. [Google Scholar]
- Wang SJ, Winchell CJ, McCormick CG, Nevius SE, O’Neill RT. Short of Complete Abstinence: An Analysis Exploration of Multiple Drinking Episodes in Alcoholism Treatment Trials. Alcoholism: Clinical and Experimental Research. 26:1803–1809. doi: 10.1097/01.ALC.0000042009.07691.12. [DOI] [PubMed] [Google Scholar]