Abstract
Myogenic differentiation involves myoblast fusion and induction of muscle-specific gene expression, which are both stimulated by pharmacological (LiCl), genetic, or IGF-I-mediated GSK-3β inactivation. To assess whether stimulation of myogenic differentiation is common to ligand-mediated GSK-3β inactivation, myoblast fusion and muscle-specific gene expression were investigated in response to Wnt-3a. Moreover, crosstalk between IGF-I/GSK-3β/NFATc3 and Wnt/GSK-3β/β-catenin signaling was assessed. While both Wnt-3a and LiCl promoted myoblast fusion, muscle-specific gene expression was increased by LiCl, but not by Wnt-3a or β-catenin over-expression. Furthermore, LiCl and IGF-I, but not Wnt-3a, increased NFATc3 transcriptional activity. In contrast, β-catenin-dependent transcriptional activity was increased by Wnt-3a and LiCl, but not IGF-I. These results for the first time reveal a segregated regulation of myoblast fusion and muscle-specific gene expression following stimulation of myogenic differentiation in response to distinct ligand-specific signaling routes of GSK-3β inactivation.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-010-0467-7) contains supplementary material, which is available to authorized users.
Keywords: Wnt-3a, GSK-3, β-catenin, Myogenic differentiation, NFAT, Lithium, IGF-I
Introduction
Satellite cells are muscle precursor cells located between the basement and sarcolemmal membrane in skeletal muscles [1]. These mononuclear cells are activated in response to injury or recovery from atrophy, which is required for efficient regeneration and restoration of muscle mass [2, 3]. Proliferating satellite cells are referred to as myoblasts, which subsequently differentiate to fuse with existing myofibers or form new myofibers [4]. In addition to myoblast fusion, myogenic differentiation is characterized by increased transcriptional activity of the muscle regulatory factors (MRFs), which promote expression of muscle-specific genes. Examples of muscle-specific genes are structural proteins of the contractile apparatus such as troponin-I (TnI) and myosin heavy chain (MyHC), and enzymes involved in muscle energy metabolism such as muscle creatine kinase (MCK) [5].
Post-natal muscle growth is stimulated by insulin-like growth factor-I (IGF-I), and IGF-I is known to promote myogenic differentiation [6, 7]. IGF-I induces an increase in muscle-specific gene expression during myogenic differentiation, which involves activation of Akt/PKB, hereafter referred to as Akt, and inactivation of glycogen synthase kinase-3β (GSK-3β) [8]. Genetic or pharmacological inactivation of GSK-3β also promotes muscle-specific gene expression and myotube fusion in differentiating myoblasts, suggesting a central role for GSK-3β in myogenic differentiation [9]. This notion is further supported by in vivo evidence revealing that markers of muscle differentiation and regeneration are inversely related to GSK-3β activity during skeletal muscle regrowth [10]. Of note is that pharmacological inhibition of GSK-3β activity by lithium (LiCl) has a more striking effect on myoblast fusion and myotube formation than IGF-I [8], which could be related to the ability of LiCl to mimic Wnt/β-catenin signaling [11, 12].
Wnt/β-catenin signaling in part depends on the inactivation of GSK-3, which leads to an accumulation of the transcriptional co-activator β-catenin [13, 14]. Under basal conditions, β-catenin levels are regulated by a protein complex containing Axin, adenomatous polyposis coli (APC), GSK-3β, and Casein kinase 1α (CK1α), hereafter termed the ‘degradation complex’. This degradation complex N-terminally phosphorylates β-catenin [15] and thereby labeling it for degradation by the ubiquitin–proteasome pathway [16]. When Wnt ligands form a ternary complex with membrane receptors Frizzled (Fz), a seven-transmembrane protein, and the low-density lipoprotein receptor-related protein 5/6 (LRP5/6) [17], Dishevelled (Dvl) is activated. Activated Dvl recruits Axin from the degradation complex to the membrane [18] preventing GSK-3β from phosphorylating β-catenin, resulting in its stabilization [19]. Cytoplasmic accumulated β-catenin is involved in cell–cell interactions in complex with cadherins [20, 21]. Increased nuclear concentrations of β-catenin result in induction of growth-associated genes such as c-myc and cyclin D1 by interacting with transcriptional co-activators such as members of the T-cell factor/Lymphocyte-enhancement factor-1 (Tcf/Lef-1) family [22].
Wnt/β-catenin signaling is essential in embryogenesis [23, 24] including muscle development, as Wnt ligands regulate the specification of skeletal myoblasts in the paraxial mesoderm [25–27], and induce location-specific expression of MRFs [28]. However, Wnt/β-catenin signaling not only contributes to embryonic but also to post-natal muscle formation, as it has been implicated in muscle regeneration [29] and hypertrophy [30]. Recently, it has been shown that Wnt’s 1, 3A, and 5A are expressed in activated satellite cells. Moreover, ectopic exposure of satellite cells to these Wnts enhanced proliferation [31], while Wnt’s 4 and 6 reduced this process [31]. Conversely, inhibition of Wnt signaling abrogated differentiation [32]. In this study, we evaluated the effects of Wnt/β-catenin signaling induced by Wnt-3a on differentiating myoblasts. We hypothesized that Wnt-3a would induce β-catenin signaling, and promote myogenic differentiation in a similar manner as observed following pharmacological inhibition of GSK-3β. Improved understanding of the processes regulating myogenic differentiation can be applied to therapeutic approaches to stimulate effective skeletal muscle regeneration following muscle trauma or atrophy.
Materials and methods
Cell culture
The murine skeletal muscle cell line C2C12 (ATCC # CRL1772) and the murine fibroblast control L-cells (ATCC # CRL2648) and Wnt-3a secreting L-cells (ATCC # CRL2647) (LGC Promochem, Teddington, UK) were cultured in growth medium (GM). This was composed of low (for C2C12 cells) or high (for L-cells) glucose Dulbecco’s modified Eagle's medium (DMEM) containing antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin) and 9% (v/v) fetal bovine serum (FBS) (all from Gibco, Rockville, MD). Conditioned medium (CM) containing Wnt-3a CM was obtained from Wnt-3a secreting L-cells (or control-CM from control L-cells) by culturing them on high-glucose DMEM containing 5% FBS without supplementation of antibiotics for 7 days until approximately reaching confluency. Medium was collected after 4 and 7 days, pooled, spun down at 1,500×g for 3 min, filter sterilized (20 μm) and frozen at −20°C in aliquotes. C2C12 cells were plated at 104 cm2 and cultured in GM for 24 h. To induce spontaneous differentiation by growth factor withdrawal [33], GM was replaced with differentiation medium (DM), which contained low-glucose DMEM with 1.0% heat-inactivated FBS and antibiotics. Alternatively, differentiation was induced with conditioned medium (CM) of control (control-CM) or Wnt-3a secreting L-cells, diluted in DM. C2C12 cells were grown on Matrigel (BD Biosciences, Bedford, MA)-coated (1:50 in DMEM low glucose) dishes as described previously [34]. Murine IGF-I (Calbiochem, La Jolla, CA) or LiCl (Sigma, St. Louis, MO) was added directly after induction of differentiation and again 24 h later when the cells were provided with fresh DM, and then every 48 h thereafter. The anti-mouse Wnt-3a antibody (MAB1324, R&D systems, Abingdon, UK) was used to neutralize Wnt-3a-conditioned medium effects by incubated Wnt-3a-conditioned medium with anti-Wnt-3a at 37°C for 1 h prior to dilution with DM and addition to the C2C12 cells.
Stable cell lines
For the assessment of β-catenin-assisted T-cell factor/lymphocyte enhancer factor (TCF/LEF)-dependent transcriptional activation, or the troponin I (TnI) promoter activity during differentiation, stable C2C12 cell lines were created carrying a genomic TCF/LEF promoter-luciferase reporter gene [35] or a TnI promoter-luciferase reporter gene [36]. To determine luciferase activity, the cells were lysed in luciferase lysis buffer (Promega, Madison, WI) and stored at −80°C. Prior to analysis, the lysates were spun at 16,000×g and 4°C, and the soluble fraction was used. Luciferase activity was measured according to the manufacturer’s instructions and expressed after correcting for total protein in the soluble fraction. Total protein concentration was assessed by a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions, R 2 values were >0.98 for the standard curve.
Transfections and plasmids
Transient transfections were performed using Nanofectin (PAA, Pasching, Austria) and in all cases included co-transfection with pSV-β-gal to correct for differences in transfection efficiency (Promega, Madison, WI). According to the manufacturer’s instructions, 1.0 μg plasmid per 3.2 μl nanofectin was used. Per transfection, 1.0–2.5 μg DNA of expression plasmids or empty vector controls was used per 35-mm dish. The transfection mix was added 6 h prior to differentiation induction. TCF/LEF luciferase reporter plasmid was used to measure β-catenin-dependent transcriptional activity, the troponin I (TnI)-luciferase plasmid was used as a reporter for the activity of muscle-specific transcription factors and the 4RTK [37, 38] MRF-sensitive luciferase plasmid was used to determine MRF transcriptional activity. Additionally, an NFAT-sensitive luciferase plasmid [39] was co-transfected with a plasmid encoding NFATc3 [40] to evaluate effects on NFATc3 transcriptional activity. Plasmids encoding WT or K85R (kinase dead) GSK-3β [41], β-catenin [35], and MyoD [9] were transfected in combination with the TCF/LEF, TnI, or 4RTK- reporter as indicated. To determine luciferase and β-galactosidase activity, cells were lysed in luciferase lysis buffer and stored at −80°C. Luciferase (Promega, Madison, WI) and β-galactosidase (Tropix, Bedford, MA) were measured according to the manufacturer’s instructions.
Muscle creatine kinase activity
Myogenic differentiation was assessed biochemically via determination of muscle creatine kinase (MCK) activity. Cells were grown on Matrigel-coated dishes. After induction of differentiation with DM alone or in combination with LiCl, control-CM, or Wnt-3a for 72 h, cells were washed twice in cold PBS, lysed in 0.5% Triton X-100, and scraped from the dish with a rubber policeman (a hand-held flexible natural-rubber scraper). Lysates were centrifuged for 2 min at 16,000×g and 4°C, and the supernatant was stored in two aliquots at −80°C for determination of protein content or MCK activity in presence of 1.25% BSA. MCK activity was measured by using a spectophotometric-based [42] kit from Stanbio (Stanbio, Boerne, TX). Specific MCK activity was calculated after correction for total protein concentration [43], R 2 values were >0.98 for the standard curve.
May-Grunwald Giemsa staining
C2C12 cells were grown on Matrigel-coated 60-mm dishes and after induction of differentiation with DM, LiCl, control-CM or Wnt-3a for 24 or 72 h, cells were washed twice in PBS (RT), fixed in methanol and stained in May-Grunwald Giemsa (Sigma, St. Louis, MO) according to the manufacturer’s instructions. Pictures were taken at 40× and 100× magnifications using a microscope connected to a digital camera (DXM 1200F), both from Nikon (Nikon, Kanagawa, Japan). The 100× magnified images were taken in series of four with fixed overlap.
Nuclei count and myogenic index
The total number of nuclei of four or more fields (100× magnification) were counted to determine the number of nuclei present after 24 and 72 h of differentiation. The myonuclear distribution was assessed by counting all nuclei within four 100× magnified linked images. Counted nuclei were assigned to one of three classes: single nucleated myoblasts, dividing or fusing bi-nucleated myoblasts, or multi-nucleated (>2) myotubes. Per condition, 800–1,800 nuclei were counted and assigned. Of the nuclei contained in myotubes, a subdivision was made in four groups with 3–9, 10–19, 20–29, or >30 nuclei per myotube.
Western blotting
The C2C12 cells were washed twice with ice-cold 1× PBS after which they were scraped and lysed in a whole-cell lysate (WCL) buffer (20 mM Tris, pH 7.4; 150 mM NaCl; 1% Nonidet P-40; 1 mM DTT; 1 mM Na3VO4; 1 mM PMSF; 10 μg/ml Leupeptin and 1% aprotenin) using a rubber policemen. Next, crude lysates were incubated on ice for 30 min, followed by a 30-min centrifugation step at 16,000×g and 4°C. A portion of the supernatant was saved for protein determination, prior to the addition with 4× Laemmli sample buffer (0.25 M Tris–HCl pH 6.8; 8% (w/v) SDS; 40% (v/v) glycerol; 0.4 M DTT, and 0.04% (w/v) Bromophenol Blue). The samples were boiled for 5 min at 95°C and stored at −20°C. Total protein concentration was assessed with the Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. For SDS-PAGE, 0.5–20 μg of protein was loaded per lane and separated on a Criterion™ XT Precast 4–12% Bis–Tris gel (Bio-Rad, #3450124), followed by transfer to a 0.45-μm Whatman® Protran® Nitrocellulose Transfer membrane (Whatman GmbH, #7324007) by electroblotting (Bio-Rad Criterion Blotter) (Bio-Rad, Hercules, CA, USA). The membrane was blocked for 1 h at room temperature in 5% (w/v) NFDM (non-fat dried milk) (ELK, Campina, the Netherlands) diluted in TBS-Tween-20 (0.05%). Nitrocellulose blots were washed in TBS-Tween-20 (0.05%) on a rotating platform, followed by overnight (o/n) incubation at 4°C with specific antibodies directed against: p-GSK-3β (Ser9) (#9336), GSK-3β (#9332), p-Akt (Ser473) (#9271), Akt (#9272), GAPDH (#2118) (all from Cell Signaling Technology, Inc., Danvers, MA,) all were diluted 1/1000 in TBS-Tween-20 (0.05%). After three washing steps of 10 min each, the blots were probed with a peroxidase conjugated secondary antibody (Vector Laboratories, #PI-1000), and visualized by chemiluminescence using Supersignal® WestPico Chemiluminescent Substrate (Pierce Biotechnology, Inc.) according to the manufacturer’s instructions and exposed to film (Biomax light film, Kodak). Western-blot films were imaged and quantified using the Quantity One analysis software from Bio-Rad.
RNA isolation and assessment of mRNA abundance by RT-qPCR
C2C12 cells were washed twice with ice-cold 1xPBS after which Total RNA was isolated using the Totally RNA™ kit (Ambion, Austin, TX) according to the manufacturer’s instructions. After isolation, RNA was dissolved in 1 mM Na-citrate (pH 6.4) and stored at −80°C. The RNA concentrations were measured spectrophotometrically using a Nanodrop® ND-1000 UV–Vis spectrophotometer. RNA was diluted >5x in ddH2O and 400 ng of RNA was reverse transcribed to cDNA using the Transcriptor first strand cDNA synthesis kit (Roche Diagnostics GmbH, Mannheim, Germany) with anchored oligo-dT primers according to the manufacturer’s instructions for generating cDNA fragment of 4 kb with a final reaction volume of 20 μl. RNA of genes of interest (Table 1) were determined by reverse transcription quantitative PCR (qPCR). qPCR primers were designed using Primer Express 2.0 software (Applied Biosystems, Foster City, CA), checked for both primer and amplicon secondary structures, and than obtained from Sigma Genosys (Haverhill, UK). qPCR reactions (20-μl final volume) contained absolute qPCR SyBr Green Fluorescein Mix (Abgene, Leusden, NL) and primers (600 nM). Relative cDNA starting quantities for the samples were derived by the standard curve method. Standard curve samples were generated by serial dilution of pooled cDNA samples and had at least a R 2 > 0.98 and an efficiency between 90 and 110%. The expression of the genes of interest were normalized with a correction factor derived by geNorm, which is based on a combination of the expression levels of β-actin, cyclophillin A, GAPDH, and RPL13A. RT-qPCR reactions were performed on a MyiQ single-color real-time thermal cycler (Bio-Rad, Hercules, CA).
Table 1.
Genes of interest real-time quantitative PCR primers
| Gene name | Accession number (ensemble) | Primers |
|---|---|---|
| β-actin | ENSMUST00000052678 | FW: 5′-CTGAATGGCCCAGGTCTGA-3′ |
| RV: 5′-CCCTCCCAGGGAGACCAA-3′ | ||
| Cyclophilin A | ENSMUST00000090749 | FW: 5′-TTCCTCCTTTCACAGAATTATTCCA-3′ |
| RV: 5′-CCGCCAGTGCCATTATGG-3′ | ||
| GAPDH | ENSMUST00000118875 | FW: 5′-CAACTCACTCAAGATTGTCAGCAA-3′ |
| RV: 5′-TGGCAGTGATGGCATGGA-3′ | ||
| RPL13A | ENSMUST00000102669 | FW: 5′-CACTCTGGAGGAGAAACGGAAGG-3′ |
| RV: 5′-GCAGGCATGAGGCAAACAGTC-3′ | ||
| Cyclin D1 | ENSMUST00000093962 | FW: 5′-CATTCCCTTGACTGCCGAGAAGTT-3′ |
| RV: 5′-TTGTTCACCAGAAGCAGTT CCATTT-3′ | ||
| PCNA | ENSMUST00000028817 | FW: 5′-CCAAATCAAGAGAAAGTTTCAGACTATGA-3′ |
| RV: 5′-TCACCCGACGGCATCTTTATT-3′ | ||
| c-Myc | ENSMUST00000022971 | FW: 5′-ACCACCAGCAGCGACTCTGA -3′ |
| RV: 5′-GCCCGACTCCGACCTCTTG-3′ | ||
| MCK | ENSMUST00000003643 | FW: 5′-AGGTTTTCCGCCGCTTCT-3 |
| RV: 5′-CGGTGCCCAGGTTGGA-3 | ||
| MyHC 2B | ENSMUST00000018632 | FW: 5′-ACAAGCTGCGGGTGAAGAGC-3 |
| RV: 5′-CAGGACAGTGACAAAGAACG-3 | ||
| MyHC peri(natal) | ENSMUST00000019625 | FW: 5′-ACACATCTTGCAGAGGAAGG-3 |
| RV:5′- TAAACCCAGAGAGGCAAGTG-3 | ||
| Axin 2 | ENSMUST00000052915 | FW: 5′-CTCAGCAAAAAGGGAAATTACAGGTAT-3′ |
| RV: 5′-ACTGTCTCGTCGTCCCAGATCTC-3′ |
DNA content determination
After treatment, cells were washed twice with cold PBS. Then Tris–EDTA buffer was added followed by a freeze–thaw cycle after which dishes were scraped and the lysate was collected. DNA content was determined in the cell lysates using the Quant-iT™ PicoGreen® dsDNA assay kit (Molecular Probes) according to the manufacturer’s protocol.
Immunohistochemical staining
C2C12 cells were grown on glass coverslips coated with Matrigel (BD Biosciences, Bedford, MA) (1:50 in DMEM low glucose) as described previously [34]. After 72 h of differentiation, the cells were washed twice with cold PBS fixed with 4% PFA, permeabilized with 0.1% Triton x-100 in PBS and non-specific binding was blocked with 1% BSA in 0.1% Triton in PBS. MyHC-fast obtained from Sigma-Aldrich (St. Louis, MO USA) was used as a primary antibody 1:250 in 1% BSA/0.1% Triton/PBS and Alexa fluor 488 goat anti IgG from Invitrogen was used as a secondary antibody 1:1000 in 1% BSA/0.1% Triton/PBS washed counter stained with DAPI (20 μg/ml) and mounted with Dako mounting medium. Pictures were taken at 200× magnification using a fluorescence microscope connected to a digital camera (DXM 1200F), both from Nikon (Nikon, Kanagawa, Japan).
Statistical analysis
All values are means ± SEM and raw data was statistically analyzed for one-way t test with unequal variance. Results are considered significantly different with p < 0.05.
Results
Wnt-3a or pharmacological inactivation of GSK-3β promote myoblast fusion during differentiation
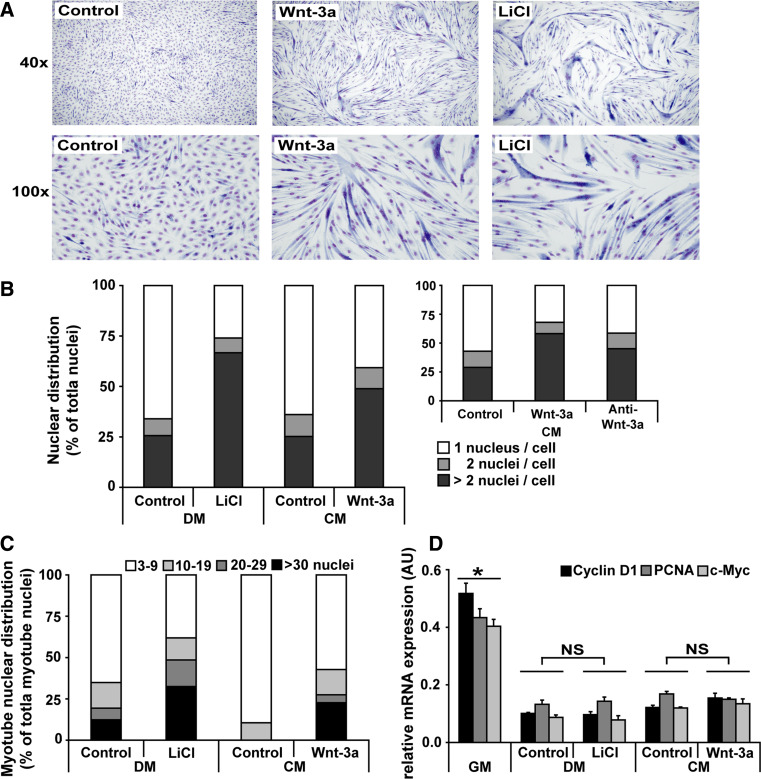
C2C12 myoblasts were differentiated in DM with or without LiCl, or in DM containing diluted medium conditioned (CM) by Wnt-3a-secreting or control L-cells (Control-CM). During the first 48 h of differentiation, alignment (Supplemental Fig. 1), which precedes fusion, appeared to be enhanced by the presence of Wnt-3a compared to control, whereas no effects on myoblast survival were observed (Supplemental Fig. 2). Similar to differentiation with DM, in control-CM treated myoblasts, myotubes were forming at 72 h. Differentiation in the presence of Wnt-3a markedly promoted myoblast fusion, resulting in larger myotubes (Fig. 1a). Pharmacological inhibition of GSK-3β using LiCl also enhanced myotube formation as reported previously [8]. Staining for MyHC-fast confirmed that the multinucleated fused cells were genuine myotubes in all conditions (Supplemental Fig. 3). Myoblast fusion and myotube formation was quantified by determining the number of nuclei in each cell. A greater percentage of nuclei residing in multinucleated cells was observed when myoblasts were differentiated in presence of LiCl or Wnt-3a compared to their respective controls (Fig. 1b). In addition, LiCl and Wnt-3a promoted the formation of larger myotubes containing more nuclei compared to their respective controls (Fig. 1c). The stimulatory effect of Wnt-3a conditioned medium on myoblast fusion and myotube formation was reduced by co-incubation with a Wnt-3a-specific antibody (Fig. 1b inset). Transcript levels of genes associated with proliferation significantly decreased compared to GM, irrespective of treatment conditions (Fig. 1d), indicating that the observed increase in number of nuclei per myotube following LiCl or Wnt-3a were not likely the result of prolonged myoblast proliferation, which was further supported by the absence in changes in total nuclei number or total DNA of adherent cells (Supplemental Fig. 2).
Fig. 1.
Wnt-3a or LiCl promote myoblast fusion during differentiation. C2C12 myoblasts were differentiated for a 72 h in control-CM or Wnt-3a-CM (each diluted 1/10 in DM), or DM in presence or absence of LiCl (10 mM), fixed and stained with May-Grunwald Giemsa to determine myoblast fusion and myotube formation or for 24 h to determine mRNA expression. Shown are representative pictures of >10 independent experiments at 40× and 100× magnification. From these pictures (b), myoblast fusion was quantified by determining nuclear distribution of 800–1,800 nuclei for each separate condition, which is expressed as the percent of nuclei residing in cells containing 1, 2, or >2 nuclei, reflecting mononucleated myoblasts (one nucleus), dividing or fusing myoblasts (two nuclei) or myotubes (>2 nuclei), respectively shown is representative data of three independent experiments. b Wnt-3a-CM was pre-incubated with an anti-Wnt-3a antibody, and cells were cultured for 120 h and myonuclear distribution was assessed. c Stratification of myonuclear content in myotubes of LiCl or Wnt-3a-treated cultures. d C2C12 myoblasts were differentiated for 24 h and RNA was extracted for assessment of proliferation-associated mRNA transcripts shown are (c, d) representative data of three independent experiments (n = 3 ± SEM), *p < 0.01, and NS non-significant
Muscle-specific gene expression during differentiation is stimulated by pharmacological inactivation of GSK-3β but not by Wnt-3a
Next we investigated whether enhanced myotube formation by Wnt-3a was associated with increased muscle-specific gene expression. Muscle creatine kinase (MCK) activity was increased by LiCl compared with control after 72 h (Fig. 2a), which is in line with previous results [8]. In contrast, MCK activity was not increased by Wnt-3a when compared to controls (Fig. 2a). Similarly, mRNA expression levels of MCK, but also myosin heavy chain (MyHC)-IIB and MyHC-perinatal at 72 h were only increased by LiCl, but not by Wnt-3a treatment (Fig. 2b). Differentiation-induced transcriptional activation, of the troponin I (TnI) promoter, evaluated in a stable reporter cell line, was increased by LiCl but not Wnt-3a (Fig. 2c) as increasing concentration of Wnt-3a even slightly decreased TnI-promoter transactivation. In line with this, over-expression of β-catenin, did not promote TnI-promoter transactivation (Supplemental Fig. 4A). Next, MRF activity was assessed with a transiently transfected, MyoD-sensitive (supplemental Fig. 4B), 4RTK luciferase reporter. Only LiCl induced an increase in MRF transcriptional activity compared to control, whereas Wnt-3a did not affect MRF activity (Fig. 2d), in line with the absence of a stimulatory effect on muscle-specific gene expression.
Fig. 2.
Muscle-specific gene expression during differentiation is stimulated by LiCl but not by Wnt-3a. C2C12 myoblast cells were cultured in DM with or without LiCl (10 mM), control, or Wnt-3a CM (diluted 1/10, or as indicated, in DM). a After 72 h, lysates were prepared for determination of muscle creatine kinase activity and total protein. Results are expressed as specific enzyme activity (units/mg protein). b After 72 h, lysates were prepared to determine mRNA expression levels of MCK, MyHC IIB, and perinatal. c Myoblasts containing a stable genomically integrated troponin I (TnI) luciferase reporter construct were cultured for 48 h in DM, ±LiCl (10 mM), or Wnt-3a or control-CM (1/10 diluted in DM). Alternatively, (d) C2C12 myoblasts were transfected with a 4RTK luciferase-reporter construct and plasmid encoding β-gal (0.25μg each) and cultured in DM with or without LiCl (10 mM), control, or Wnt-3a CM (diluted 1/10 in DM). Lysates were prepared for luciferase and β-galactosidase enzyme activity (RLU/mg protein) or (RLU luciferase/β-gal activity). Shown are representative graphs of three independent experiments (n = 3 ± SEM), *p < 0.05, # p < 0.01, $ p < 0.001, and NS non-significant
Wnt-3a or pharmacological inhibition of GSK-3β induce β-catenin stabilization and β-catenin-dependent transcriptional activity in differentiating myoblasts
To investigate whether Wnt-3a induces Wnt/β-catenin signaling in C2C12 myoblasts, β-catenin protein abundance was assessed. A clear increase (>2.5 fold) in cellular β-catenin protein content was observed following 24 h differentiation in DM containing 1/10 diluted Wnt-3a-CM compared to control-CM (Fig. 3a). To assess whether β-catenin accumulation was accompanied by increases in its functional activity as a transcriptional co-activator, β-catenin-dependent TCF/LEF transcriptional activity was assessed in C2C12 cells stably transfected with a TCF/LEF-sensitive promoter reporter construct. Wnt-3a induced a concentration- (Fig. 3b) and time-dependent (Fig. 3c) increase in TCF/LEF transcriptional activity. Moreover, Wnt-3a induced TCF/LEF transcriptional activity was abrogated by co-incubation with a Wnt-3a-specific, but not isotype control, antibody (Fig. 3d). In addition to Wnt-3a, pharmacological inhibition of GSK-3β activity by LiCl also induced TCF/LEF transcriptional activity (Fig. 3d). Finally, to assess endogenous β-catenin mediated gene expression, Axin 2 mRNA levels were assessed. Axin 2 mRNA expression was increased by LiCl (>2.5 fold) and Wnt-3a (>14 fold) compared with their respective controls (Fig. 3e).
Fig. 3.
Wnt-3a or LiCl induce β-catenin stabilization and β-catenin-dependent transcriptional activity in differentiating myoblasts. C2C12 myoblasts were differentiated for a 24 h in control or Wnt-3a CM (each diluted 1/10 in DM), lysates were prepared and cellular β-catenin and GAPDH protein content were visualized and quantified. Myoblasts containing a stable genomically integrated TCF/LEF luciferase reporter construct were cultured for b 24 h in DM, Wnt-3a CM diluted as indicated, or c or for the indicated time in control or Wnt-3a CM diluted 1/10 in DM. C2C12 myoblasts were cultured for d 24 h in DM with or without LiCl (10 mM), control or Wnt-3a CM (pre-incubated with a Wnt-3a-specific or isotype control antibody for 1 h at 37°C) diluted 1/10 in DM. After the indicated times, the lysates were prepared for specific enzyme activity (RLU/mg protein). Myoblast were e harvested 24 h after induction of differentiation for determination of endogenous Axin 2 mRNA levels. Shown are representative graphs of three independent experiments (n = 3 ± SEM), *p < 0.05, # p < 0.01, $ p < 0.001, and NS non-significant
GSK-3β activity directly inhibits β-catenin-dependent TCF/LEF transcriptional activity
To address GSK-3β regulation of β-catenin-dependent transcriptional activity in skeletal muscle cells, C2C12 myoblasts were transiently transfected with a TCF/LEF responsive promoter luciferase reporter plasmid. Simultaneous over-expression of β-catenin resulted in increased TCF/LEF transcriptional activity (Fig. 4). In contrast, over-expression of wild-type (WT-)GSK-3β led to suppressed β-catenin-induced TCF/LEF transcriptional activity (Fig. 4), whereas over-expression of a dominant negative (dn-)GSK-3β (K85R) mutant actually enhanced β-catenin-dependent transcriptional activity (Fig. 4). Therefore, inhibition of β-catenin-mediated transcriptional activation requires enzymatically active GSK-3β.
Fig. 4.
GSK-3β activity directly inhibits β-catenin-dependent TCF/LEF transcriptional activity. C2C12 myoblasts were transiently transfected with a TCF/LEF luciferase reporter plasmid and plasmids encoding β-gal (0.25 μg each), β-catenin (0.5 μg), WT or kinase dead (K85R)-GSK-3β (1.0 μg each). After 24 h incubation in DM with or without LiCl (5 mM), cells were lysed to measure luciferase and β-gal activity. Shown is a representative graph of three independent experiments (n = 3 ± SEM), *p < 0.05, # p < 0.01, $ p < 0.001, and NS non-significant
Wnt-3a and IGF-I regulate distinct GSK-3β substrates in differentiating myoblasts
In contrast to Wnt-3a, inactivation of GSK-3β by IGF-I did not result in β-catenin accumulation after 24 h (Fig. 5a), or affect β-catenin-dependent transcriptional activity after incubation 24 h (Fig. 5b), 48, 96, or 120 h of incubation (data not shown). GSK-3β inactivation following IGF-I signaling results from phosphorylation GSK-3β at Ser-9 [44]. GSK-3β phosphorylation was increased by IGF-I (Fig. 5c), while no effect was observed in response to Wnt-3a. Similar results were seen for Akt phosphorylation (Supplemental Fig. 5). This indicates that Wnt/β-catenin signaling in skeletal muscle cells is not dependent on GSK-3β phosphorylation at Ser-9, which is in line with previous reports [45, 46]. Increased muscle-specific gene expression in response to genetic or pharmacological GSK-3β inhibition was previously shown to be mediated by the GSK-3β phospho-substrate and transcription factor NFATc3 [9]. Evaluation of NFATc3 transcriptional activity using a transiently transfected NFAT luciferase reporter construct revealed increased NFAT-dependent transcription following either IGF-I or LiCl, but not in response to Wnt-3a or over-expression of β-catenin (Fig. 5d).
Fig. 5.
Wnt-3a and IGF-I regulate distinct GSK-3β substrates in differentiating myoblasts. C2C12 myoblasts were differentiated for a 24 h in DM with or without IGF-I (5nM) or LiCl (10 mM), control, or Wnt-3a CM (each diluted 1/10 in DM), lysates were prepared and cellular β-catenin, and GAPDH protein content were visualized and quantified. Alternatively, b myoblasts containing a stable genomically integrated TCF/LEF luciferase reporter construct were cultured for 24 h under the same conditions. c C2C12 myoblasts were treated for 2 h in DM with IGF (5nM) or LiCl (10 mM), or 2 h incubated in control or Wnt-3a CM (each diluted 1/10 in DM). Lysates were prepared and cellular phospho-GSK-3β and total GSK-3β protein content were visualized and quantified. d C2C12 myoblasts were transfected with a NFAT-sensitive promoter-luciferase reporter plasmid and plasmids encoding β-gal (0.25 μg each), NFATc3 (1.0 μg each) β-catenin, or empty vector (1.0 μg each) and treated as indicated. After 48 h incubation, the cells were lysed to measure luciferase and β-galactosidase activity. Shown are representative graphs of three independent experiments (n = 3 ± SEM), *p < 0.05, # p < 0.01, $ p < 0.001, and NS non-significant
Discussion
Recent work identified GSK-3β as a negative regulator of myogenic differentiation controlled by IGF-I signaling [8]. Wnt/β-catenin signaling also involves regulation of GSK-3β, and is reported to participate in the initiation of pre-myogenic cell differentiation [47]. Besides its well-established role in skeletal muscle formation during embryogenesis [23], recent studies propose the involvement of Wnt/β-catenin signaling in postnatal skeletal muscle hypertrophy [48, 49]. However, whether Wnt/β-catenin signaling affects myogenic differentiation during skeletal muscle growth was not assessed in these studies. Our results reveal a potential role for Wnt/β-catenin signaling in myogenic differentiation, as Wnt-3a induced a marked increase in C2C12 myoblast fusion and myotube formation during differentiation. This is in line with findings describing stimulation of myotube formation by co-culture of myoblasts on Wnt1-presenting monolayers [50]. In our study, however, myoblasts were provided with cell-free conditioned medium from Wnt-3a-secreting L-cells to rule out producer-cell-myoblast interaction effects. To confirm that stimulation of fusion (Fig. 1b inset) and TCF/LEF transcriptional activity (Fig. 3e) was attributable to Wnt-3a, the conditioned medium was incubated with a Wnt-3a-specific antibody, which partly neutralized Wnt-3a-mediated effects. Finally, opposed to the study by Rochat et al., we did not supplement our cells with insulin, as one of our aims was to distinguish between effects of GSK-3β inhibition by Wnt-3a and IGF-I/insulin signaling.
Improvement of myoblast fusion by Wnt-3a, reflected by an increased myogenic index, was similar to stimulation of fusion following GSK-3β inhibition by LiCl [8]. Although Wnt-3a can induce expression of genes promoting proliferation like Cyclin-D1 and c-myc [51, 52], the increased myogenic index did not result from sustained cell division, as the decrease in proliferation marker mRNA levels (two-fold or more) following induction of differentiation was not affected by Wnt-3a or LiCl. Alternatively, improved myoblast survival could contribute to an increased myogenic index, as inhibition of GSK-3β reduces caspase activity and cellular apoptosis [53]. Determination of DNA content and total nuclear count of the adherent cells 24–48 h after induction of differentiation revealed no changes in the presence of either LiCl or Wnt-3a. This leads to the observation that the increased myogenic index did not result from either increased proliferation or survival of myoblasts, but is likely attributable to improved cell–cell interactions and subsequent myoblast alignment. Following the improved alignment, larger myotubes after Wnt-3a or LiCl stimulation are observed, which could result from either faster or enhanced myoblast-myoblast, myoblast-myotube, or myotube-myotube fusion [54, 55]. We can, however, not discern at present which of these processes results in those larger myotubes. Although they are already observed 3 days after induction of differentiation.
Wnt-3a as well as LiCl-induced GSK-3 inactivation stabilized and increased cellular levels of β-catenin leading to β-catenin-dependent TCF/LEF transcriptional activity in differentiating myoblasts, which is in agreement with previous reports [11, 56, 57]. In addition, over-expression of WT-GSK-3β in myoblasts confirmed direct inhibition of β-catenin-induced TCF/LEF transcriptional activity by GSK-3β. Conversely, inhibition of endogenous GSK-3 activity or over-expression of dominant-negative (dn)-GSK-3β (K85R) increased TCF/LEF reporter activity, the latter likely resulting from competition with endogenous GSK-3 for participation in the degradation-complex to phosphorylate β-catenin [58, 59].
GSK-3β inactivation by IGF-I [8] or LiCl (shown here) resulted in increased muscle-specific gene expression during differentiation, such as increased MCK and MyHC mRNA expression, and TnI-promoter activity. Wnt-3a-mediated GSK-3 inactivation stimulated myoblast fusion, which coincided with increased β-catenin-dependent transcriptional activity. Wnt/β-catenin signaling is shown to precede MRF expression, which controls myogenic differentiation [5, 26, 60], and some reports suggest Wnt signaling may increase MyoD expression [61, 62]. Therefore, we expected Wnt-3a to increase muscle-specific gene expression. To our surprise however, Wnt-3a-mediated GSK-3 inactivation did not increase muscle-specific protein abundance or mRNA transcripts, muscle-specific promoter transactivation or MRF transcriptional activity. The latter results appear to be in contrast to postulated effects of Wnt signaling on MRF expression. However, very different experimental settings were used to obtain these results, including over-expression of Wnt ligands in combination with other stimulatory factors. Nevertheless, as any biologically significant effects on MRF expression should result in altered MRF activity, we are confident Wnt does not alter MRF functionally in differentiating myoblasts, as MRF-dependent transcriptional activity was not altered in presence of Wnt-3a. Previously, increased muscle-specific gene expression following GSK-3β inhibition was shown to involve increased NFATc3 transcriptional activity [9]. In line with the absence of a stimulatory effect on muscle-specific gene expression and in contrast to IGF-I or LiCl, Wnt-3a did not induce NFATc3 transcriptional activity. Furthermore, β-catenin over-expression did not affect NFATc3 transcriptional or TnI-promoter activity.
Overall, these data indicate that stimulation of muscle-specific gene expression corresponds with increased transcriptional activity of IGF-I but not Wnt-3a signaling associated GSK-3β phospho-substrates.
In contrast to our findings, increased MyHC-IIB mRNA expression by IGF-I was reported to in part depend on nuclear β-catenin accumulation [63]. However, the ~seven-fold lower IGF-I concentrations used in our studies did not affect β-catenin protein content or β-catenin transcriptional activity (Fig. 5a, b), despite a robust increase in GSK-3β phosphorylation (Fig. 5c) or muscle-specific gene expression [8] in differentiating myoblasts. Based on this, we postulate that β-catenin presence may aid IGF-I stimulated MyHC-IIB promoter activity [63], but that stabilization of β-catenin is not sufficient to induce muscle-specific gene expression.
Nevertheless, most literature suggests that GSK-3β inactivation by IGF-I/Akt signaling is not sufficient to stabilize β-catenin or induce β-catenin-dependent transcription [18, 45, 46, 64]. The inability of IGF-I to affect the GSK-3β substrate β-catenin transcriptional activity likely relates to the unique signaling route by which Wnt inactivates GSK-3, i.e., via its sequestration by Axin [46, 57, 65]. Moreover, Wnt-mediated GSK-3 inactivation is not dependent on the Akt phosphorylation sites of GSK-3 [45, 66]. In agreement with this, Wnt-3a did not induce GSK-3β phosphorylation in differentiating myoblasts, nor did it affect NFATc3 transcriptional activity, as opposed to IGF-I mediated GSK-3β inactivation. These data support the notion that the differential inactivation of GSK-3β by IGF-I and Wnt/β-catenin signaling constitutes the molecular basis to differentiate between GSK-3β substrates [46, 64].
During myogenic differentiation, β-catenin, and NFATc3 may represent the GSK-3 substrates subject to independent regulation by the distinct GSK-3 signaling pools, and may be responsible for the separate stimulation of myoblast fusion and muscle-specific gene expression by Wnt-3a and IGF-I, respectively. In line with this, LiCl, which inhibits GSK-3 enzymatic activity towards both β-catenin and NFAT-c3, promoted both myoblast fusion and myogenic gene expression. Although the segregation of these aspects of myogenic differentiation has been reported previously [67, 68], this is the first report to imply the dissociation of myoblast fusion and gene expression may be controlled by these distinct modes of GSK-3β inactivation.
The observed effect of Wnt-3a on increased myoblast fusion may relate to the function of β-catenin as an essential binding partner for the cytoplasmic tail of various cadherins, including M-cadherin [69]. β-catenin co-localizes with M-cadherin at the cell–cell contact sites in membranes and is essential for proper fusing of myoblasts [70–72]. Therefore, increased myoblast fusion observed in response to Wnt-3a or LiCl inactivation of GSK-3 may rather relate to the function of β-catenin in cell–cell contact than its activity as a transcriptional co-regulator. This idea is supported by the observation that LiCl caused a less potent induction of TCF/LEF transcriptional activation or Axin-2 expression compared to 1/10 diluted Wnt-3a, despite similar effects on myoblast fusion, thereby demonstrating that the effects of β-catenin activity modulation do not linearly correlate with β-catenin-dependent transcriptional activity.
In conclusion, our results demonstrate that Wnt-3a induces β-catenin signaling in differentiating myoblasts, which can be mimicked by pharmacological, but not IGF-I-dependent GSK-3 inhibition. Moreover, Wnt-3a strongly promotes myoblast fusion and myotube formation without enhancing muscle-specific gene expression. These data demonstrate that two distinct signaling routes controlling GSK-3 activity independently regulate myoblast fusion and muscle-specific gene expression during myogenic differentiation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 Wnt-3a and LiCl enhance alignment of differentiating myoblasts prior to myotube formation. C2C12 myoblasts were differentiated for 48 h in DM with or without LiCl (10 mM), control, or Wnt-3a CM (diluted 1/10 in DM), fixed and stained with May-Grunwald Giemsa. Shown are representative pictures of >10 independent experiments at 100× magnification (TIFF 10061 kb)
Supplementary Fig. 2 LiCl or Wnt-3a does not affect cell survival in differentiating myoblasts. C2C12 myoblasts were differentiated for (A) 24h (B) 72h in DM with or without LiCl (10mM), control, or Wnt-3a CM (diluted 1/10 in DM), fixed and stained with May-Grunwald Giemsa to determine number of nuclei per 100x magnified microscopic field and >4 microscopic field per condition were quantified. (C) C2C12 myoblasts were differentiated for 0, 24, and 48h in DM with or without LiCl (10 mM), control, or Wnt-3a CM (diluted 1/10 in DM). Adherent cells were lysed and total DNA content was determined as a measure for total cell number. Shown are representative graphs of three independent experiments (n = 3 ±SEM), *: p < 0.05, #: p < 0.01, $: p < 0.001 and NS non-significant (TIFF 254 kb)
Supplementary Fig. 3 Enhanced formation of MyHC expressing myotubes by Wnt-3a. C2C12 myoblasts were differentiated for 72 h in DM with or without LiCl (10 mM), control, or Wnt-3a CM (diluted 1/10 in DM), fixed and immunohistochemically stained for MyHC-fast and DAPI, and visualized by fluorescence microscopy to determine bona fide myotube formation. Shown are representative pictures of >3 independent experiments at 200x magnification (TIFF 5967 kb)
Supplementary Fig. 4 Muscle-specific gene expression during differentiation is stimulated by LiCl and MyoD but not by β-catenin. (A) C2C12 myoblasts were transfected with a TnI-promoter luciferase-reporter construct and plasmids encoding β-gal (0.25μg each), β-catenin or empty vector (0.5 μg each) and cultured +/- LiCl (5 mM). (B) C2C12 myoblasts were transfected with a 4RTK luciferase-reporter construct and plasmids encoding β-gal (0.25 μg) and pEMSV-MyoD or empty pcDNA3.1 (1.0 µg) and cultured in DM with or without LiCl (10mM), control, or Wnt-3a CM (diluted 1/10 in DM). Lysates were prepared for luciferase and β-galactosidase enzyme activity. Shown are representative graphs of three independent experiments (n = 3 ±SEM), *: p < 0.05, #: p < 0.01, $: p < 0.001 and NS non-significant (TIFF 109 kb)
Supplementary Fig. 5 Wnt-3a does not affect Akt phosphorylation in differentiating myoblasts. C2C12 myoblasts were treated for 2h in DM with IGF (5 nM) or LiCl (10 mM), or 2 h incubated in control or Wnt-3a CM (each diluted 1/10 in DM). Lysates were prepared and cellular phospho-Akt and total Akt protein content were visualized and quantified. Shown are representative graphs of three independent experiments (n = 3 ±SEM), *: p < 0.05 and NS non-significant (TIFF 167 kb)
Acknowledgments
We would like to kindly thank Dr. Albert Baldwin (University of North Carolina, Chapel Hill, NC) for providing us with the troponin I (TnI)-luciferase plasmid, Dr. S Sokol (Harvard Medical School, Boston, MA) for providing plasmids encoding wild-type GSK-3β and kinase dead GSK-3β K85R, Dr. Leon de Windt (Department of Cardiology, UM) for providing the NFAT luciferase reporter and NFATc3, Dr. D. Guttridge for providing us with the 4RTK-luciferase reporter plasmid, Dr. B. Winter for providing us with the pEMSV-MyoD expression vector, and Dr. W. M. Blankesteijn for providing us with plasmid encoding β-catenin and a TCF/LEF promoter-luciferase reporter plasmid. This work was supported by the trans University Limburg and the Netherlands Astma Foundation (grant NAF 3.2.07.017).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
N. A. M. Pansters and J. L. J. van der Velden contributed equally to this work.
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan JE, Partridge TA. Muscle satellite cells. Int J Biochem Cell Biol. 2003;35:1151–1156. doi: 10.1016/S1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- 3.Rosenblatt JD, Yong D, Parry DJ. Satellite cell activity is required for hypertrophy of overloaded adult rat muscle. Muscle Nerve. 1994;17:608–613. doi: 10.1002/mus.880170607. [DOI] [PubMed] [Google Scholar]
- 4.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 5.Olson EN. Signal transduction pathways that regulate skeletal muscle gene expression. Mol Endocrinol. 1993;7:1369–1378. doi: 10.1210/me.7.11.1369. [DOI] [PubMed] [Google Scholar]
- 6.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 7.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 8.van der Velden JL, Langen RC, Kelders MC, Wouters EF, Janssen-Heininger YM, Schols AM. Inhibition of glycogen synthase kinase-3beta activity is sufficient to stimulate myogenic differentiation. Am J Physiol Cell Physiol. 2006;290:C453–C462. doi: 10.1152/ajpcell.00068.2005. [DOI] [PubMed] [Google Scholar]
- 9.van der Velden JL, Schols AM, Willems J, Kelders MC, Langen RC. Glycogen synthase kinase 3 suppresses myogenic differentiation through negative regulation of NFATc3. J Biol Chem. 2008;283:358–366. doi: 10.1074/jbc.M707812200. [DOI] [PubMed] [Google Scholar]
- 10.van der Velden JL, Langen RC, Kelders MC, Willems J, Wouters EF, Janssen-Heininger YM, Schols AM. Myogenic differentiation during regrowth of atrophied skeletal muscle is associated with inactivation of GSK-3beta. Am J Physiol Cell Physiol. 2007;292:C1636–C1644. doi: 10.1152/ajpcell.00504.2006. [DOI] [PubMed] [Google Scholar]
- 11.Du WJ, Li JK, Wang QY, Hou JB, Yu B. Lithium chloride regulates connexin43 in skeletal myoblasts in vitro: possible involvement in Wnt/beta-catenin signaling. Cell Commun Adhes. 2008;15:261–271. doi: 10.1080/15419060802198587. [DOI] [PubMed] [Google Scholar]
- 12.Du WJ, Li JK, Wang QY, Hou JB, Yu B. Lithium chloride preconditioning optimizes skeletal myoblast functions for cellular cardiomyoplasty in vitro via glycogen synthase kinase-3beta/beta-catenin signaling. Cells Tissues Organs. 2009;190:11–19. doi: 10.1159/000167699. [DOI] [PubMed] [Google Scholar]
- 13.Novak A, Dedhar S. Signaling through beta-catenin and Lef/Tcf. Cell Mol Life Sci. 1999;56:523–537. doi: 10.1007/s000180050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 15.Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/S0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 17.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Yuan H, Weaver CD, Mao J, Farr GH, 3rd, Sussman DJ, Jonkers J, Kimelman D, Wu D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999;18:4233–4240. doi: 10.1093/emboj/18.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X, Rubin JS, Kimmel AR. Rapid, Wnt-induced changes in GSK3beta associations that regulate beta-catenin stabilization are mediated by Galpha proteins. Curr Biol. 2005;15:1989–1997. doi: 10.1016/j.cub.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 20.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 21.Nusse R. Cell biology: relays at the membrane. Nature. 2005;438:747–749. doi: 10.1038/438747a. [DOI] [PubMed] [Google Scholar]
- 22.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 24.Geetha-Loganathan P, Nimmagadda S, Scaal M. Wnt signaling in limb organogenesis. Organogenesis. 2008;4:109–115. doi: 10.4161/org.4.2.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cossu G, Borello U. Wnt signaling and the activation of myogenesis in mammals. EMBO J. 1999;18:6867–6872. doi: 10.1093/emboj/18.24.6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petropoulos H, Skerjanc IS. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem. 2002;277:15393–15399. doi: 10.1074/jbc.M112141200. [DOI] [PubMed] [Google Scholar]
- 27.Galli LM, Willert K, Nusse R, Yablonka-Reuveni Z, Nohno T, Denetclaw W, Burrus LW. A proliferative role for Wnt-3a in chick somites. Dev Biol. 2004;269:489–504. doi: 10.1016/j.ydbio.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Tajbakhsh S, Borello U, Vivarelli E, Kelly R, Papkoff J, Duprez D, Buckingham M, Cossu G. Differential activation of Myf5 and MyoD by different Wnts in explants of mouse paraxial mesoderm and the later activation of myogenesis in the absence of Myf5. Development. 1998;125:4155–4162. doi: 10.1242/dev.125.21.4155. [DOI] [PubMed] [Google Scholar]
- 29.Polesskaya A, Seale P, Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45 + adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/S0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong DD, Wong VL, Esser KA. Expression of beta-catenin is necessary for physiological growth of adult skeletal muscle. Am J Physiol Cell Physiol. 2006;291:C185–C188. doi: 10.1152/ajpcell.00644.2005. [DOI] [PubMed] [Google Scholar]
- 31.Otto A, Schmidt C, Luke G, Allen S, Valasek P, Muntoni F, Lawrence-Watt D, Patel K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]
- 32.Descamps S, Arzouk H, Bacou F, Bernardi H, Fedon Y, Gay S, Reyne Y, Rossano B, Levin J. Inhibition of myoblast differentiation by Sfrp1 and Sfrp2. Cell Tissue Res. 2008;332:299–306. doi: 10.1007/s00441-008-0574-z. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7:159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 34.Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. Faseb J. 2001;15:1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- 35.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/S0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 36.Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Enhanced myogenic differentiation by extracellular matrix is regulated at the early stages of myogenesis. In Vitro Cell Dev Biol Anim. 2003;39:163–169. doi: 10.1007/s11626-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 37.Johnson SE, Wang X, Hardy S, Taparowsky EJ, Konieczny SF. Casein kinase II increases the transcriptional activities of MRF4 and MyoD independently of their direct phosphorylation. Mol Cell Biol. 1996;16:1604–1613. doi: 10.1128/mcb.16.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res. 2000;87:E61–E68. doi: 10.1161/01.res.87.12.e61. [DOI] [PubMed] [Google Scholar]
- 40.van Rooij E, Doevendans PA, de Theije CC, Babiker FA, Molkentin JD, de Windt LJ. Requirement of nuclear factor of activated T-cells in calcineurin-mediated cardiomyocyte hypertrophy. J Biol Chem. 2002;277:48617–48626. doi: 10.1074/jbc.M206532200. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez I, Itoh K, Sokol SY. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szasz G, Gruber W, Bernt E. Creatine kinase in serum: 1. Determination of optimum reaction conditions. Clin Chem. 1976;22:650–656. [PubMed] [Google Scholar]
- 43.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 44.Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296(Pt 1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aschenbach WG, Ho RC, Sakamoto K, Fujii N, Li Y, Kim YB, Hirshman MF, Goodyear LJ. Regulation of dishevelled and beta-catenin in rat skeletal muscle: an alternative exercise-induced GSK-3beta signaling pathway. Am J Physiol Endocrinol Metab. 2006;291:E152–E158. doi: 10.1152/ajpendo.00180.2005. [DOI] [PubMed] [Google Scholar]
- 46.Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, Schutte M, Clevers H. Phosphatidylinositol 3-kinase (PI3 K) signaling does not activate the Wnt cascade. J Biol Chem. 2009;284:35308–35313. doi: 10.1074/jbc.M109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong DD, Esser KA. Wnt/beta-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2005;289:C853–C859. doi: 10.1152/ajpcell.00093.2005. [DOI] [PubMed] [Google Scholar]
- 49.Zhang CG, Jia ZQ, Li BH, Zhang H, Liu YN, Chen P, Ma KT, Zhou CY. beta-Catenin/TCF/LEF1 can directly regulate phenylephrine-induced cell hypertrophy and Anf transcription in cardiomyocytes. Biochem Biophys Res Commun. 2009;390:258–262. doi: 10.1016/j.bbrc.2009.09.101. [DOI] [PubMed] [Google Scholar]
- 50.Rochat A, Fernandez A, Vandromme M, Moles JP, Bouschet T, Carnac G, Lamb NJ. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol Biol Cell. 2004;15:4544–4555. doi: 10.1091/mbc.E03-11-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samarzija I, Sini P, Schlange T, Macdonald G, Hynes NE. Wnt3a regulates proliferation and migration of HUVEC via canonical and non-canonical Wnt signaling pathways. Biochem Biophys Res Commun. 2009;386:449–454. doi: 10.1016/j.bbrc.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 52.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 53.Takadera T, Ohtsuka M, Aoki H. Chelation of extracellular calcium-induced cell death was prevented by glycogen synthase kinase-3 inhibitors in PC12 Cells. Cell Mol Neurobiol. 2010;30:193–198. doi: 10.1007/s10571-009-9442-y. [DOI] [PubMed] [Google Scholar]
- 54.Shimada Y. Electron microscope observations on the fusion of chick myoblasts in vitro. J Cell Biol. 1971;48:128–142. doi: 10.1083/jcb.48.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robertson TA, Grounds MD, Mitchell CA, Papadimitriou JM. Fusion between myogenic cells in vivo: an ultrastructural study in regenerating murine skeletal muscle. J Struct Biol. 1990;105:170–182. doi: 10.1016/1047-8477(90)90111-O. [DOI] [PubMed] [Google Scholar]
- 56.Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD., Jr Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008;42:669–680. doi: 10.1016/j.bone.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 57.Kishida M, Koyama S, Kishida S, Matsubara K, Nakashima S, Higano K, Takada R, Takada S, Kikuchi A. Axin prevents Wnt-3a-induced accumulation of beta-catenin. Oncogene. 1999;18:979–985. doi: 10.1038/sj.onc.1202388. [DOI] [PubMed] [Google Scholar]
- 58.Farr GH, 3rd, Ferkey DM, Yost C, Pierce SB, Weaver C, Kimelman D. Interaction among GSK-3, GBP, axin, and APC in Xenopus axis specification. J Cell Biol. 2000;148:691–702. doi: 10.1083/jcb.148.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277:30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- 60.Ridgeway AG, Petropoulos H, Wilton S, Skerjanc IS. Wnt signaling regulates the function of MyoD and myogenin. J Biol Chem. 2000;275:32398–32405. doi: 10.1074/jbc.M004349200. [DOI] [PubMed] [Google Scholar]
- 61.Takata H, Terada K, Oka H, Sunada Y, Moriguchi T, Nohno T. Involvement of Wnt4 signaling during myogenic proliferation and differentiation of skeletal muscle. Dev Dyn. 2007;236:2800–2807. doi: 10.1002/dvdy.21327. [DOI] [PubMed] [Google Scholar]
- 62.Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 2009;150:1259–1268. doi: 10.1210/en.2008-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shanely RA, Zwetsloot KA, Childs TE, Lees SJ, Tsika RW, Booth FW. IGF-I activates the mouse type IIb myosin heavy chain gene. Am J Physiol Cell Physiol. 2009;297:C1019–C1027. doi: 10.1152/ajpcell.00169.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ding VW, Chen RH, McCormick F. Differential regulation of glycogen synthase kinase 3beta by insulin and Wnt signaling. J Biol Chem. 2000;275:32475–32481. doi: 10.1074/jbc.M005342200. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of axin, a Wnt signal negative regulator, by glycogen synthase kinase-3beta regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- 66.McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett AM, Tonks NK. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- 68.Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion. Mol Biol Cell. 2009;20:3422–3435. doi: 10.1091/mbc.E09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM. The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol. 1992;118:681–691. doi: 10.1083/jcb.118.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ishido M, Uda M, Masuhara M, Kami K. Alterations of M-cadherin, neural cell adhesion molecule and beta-catenin expression in satellite cells during overload-induced skeletal muscle hypertrophy. Acta Physiol (Oxf) 2006;187:407–418. doi: 10.1111/j.1748-1716.2006.01577.x. [DOI] [PubMed] [Google Scholar]
- 71.Kramerova I, Kudryashova E, Wu B, Spencer MJ. Regulation of the M-cadherin-beta-catenin complex by calpain 3 during terminal stages of myogenic differentiation. Mol Cell Biol. 2006;26:8437–8447. doi: 10.1128/MCB.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mukai A, Kurisaki T, Sato SB, Kobayashi T, Kondoh G, Hashimoto N. Dynamic clustering and dispersion of lipid rafts contribute to fusion competence of myogenic cells. Exp Cell Res. 2009;315:3052–3063. doi: 10.1016/j.yexcr.2009.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Wnt-3a and LiCl enhance alignment of differentiating myoblasts prior to myotube formation. C2C12 myoblasts were differentiated for 48 h in DM with or without LiCl (10 mM), control, or Wnt-3a CM (diluted 1/10 in DM), fixed and stained with May-Grunwald Giemsa. Shown are representative pictures of >10 independent experiments at 100× magnification (TIFF 10061 kb)
Supplementary Fig. 2 LiCl or Wnt-3a does not affect cell survival in differentiating myoblasts. C2C12 myoblasts were differentiated for (A) 24h (B) 72h in DM with or without LiCl (10mM), control, or Wnt-3a CM (diluted 1/10 in DM), fixed and stained with May-Grunwald Giemsa to determine number of nuclei per 100x magnified microscopic field and >4 microscopic field per condition were quantified. (C) C2C12 myoblasts were differentiated for 0, 24, and 48h in DM with or without LiCl (10 mM), control, or Wnt-3a CM (diluted 1/10 in DM). Adherent cells were lysed and total DNA content was determined as a measure for total cell number. Shown are representative graphs of three independent experiments (n = 3 ±SEM), *: p < 0.05, #: p < 0.01, $: p < 0.001 and NS non-significant (TIFF 254 kb)
Supplementary Fig. 3 Enhanced formation of MyHC expressing myotubes by Wnt-3a. C2C12 myoblasts were differentiated for 72 h in DM with or without LiCl (10 mM), control, or Wnt-3a CM (diluted 1/10 in DM), fixed and immunohistochemically stained for MyHC-fast and DAPI, and visualized by fluorescence microscopy to determine bona fide myotube formation. Shown are representative pictures of >3 independent experiments at 200x magnification (TIFF 5967 kb)
Supplementary Fig. 4 Muscle-specific gene expression during differentiation is stimulated by LiCl and MyoD but not by β-catenin. (A) C2C12 myoblasts were transfected with a TnI-promoter luciferase-reporter construct and plasmids encoding β-gal (0.25μg each), β-catenin or empty vector (0.5 μg each) and cultured +/- LiCl (5 mM). (B) C2C12 myoblasts were transfected with a 4RTK luciferase-reporter construct and plasmids encoding β-gal (0.25 μg) and pEMSV-MyoD or empty pcDNA3.1 (1.0 µg) and cultured in DM with or without LiCl (10mM), control, or Wnt-3a CM (diluted 1/10 in DM). Lysates were prepared for luciferase and β-galactosidase enzyme activity. Shown are representative graphs of three independent experiments (n = 3 ±SEM), *: p < 0.05, #: p < 0.01, $: p < 0.001 and NS non-significant (TIFF 109 kb)
Supplementary Fig. 5 Wnt-3a does not affect Akt phosphorylation in differentiating myoblasts. C2C12 myoblasts were treated for 2h in DM with IGF (5 nM) or LiCl (10 mM), or 2 h incubated in control or Wnt-3a CM (each diluted 1/10 in DM). Lysates were prepared and cellular phospho-Akt and total Akt protein content were visualized and quantified. Shown are representative graphs of three independent experiments (n = 3 ±SEM), *: p < 0.05 and NS non-significant (TIFF 167 kb)