Abstract
Background
Cyclosporine A (CsA) limits myocardial reperfusion injury and preserves mitochondrial integrity, but its influence on mitochondrial function has not been described in vivo. Auto-fluorescence of mitochondrial nicotinamide adenine dinucleotide and flavin adenine dinucleotide correlate with mitochondrial dysfunction. We hypothesized that CsA limits mitochondrial dysfunction and that fluorometry can quantify this influence.
Methods
Seventeen rabbits were studied: untreated (UnT, n = 7), CsA preinfarction (CsAp, n = 6), and CsA on reperfusion (CsAr, n = 4). Animals underwent 30 minutes of myocardial ischemia and 3 hours reperfusion. Infarct size was determined by staining. Nicotinamide adenine dinucleotide and flavin adenine dinucleotide fluorescence was continually measured in the risk area. The redox ratio was calculated [flavin adenine dinucleotidef/(flavin adenine dinucleotidef + nicotinamide adenine dinucleotidef)]. Electron microscopy evaluated mitochondria morphology.
Results
The infarct size by group was 39.1% ± 1.7% in CsAp, 39.1% ± 1.7% in CsAr, and 53.4% ± 1.9% in UnT (p < 0.001). During ischemia, the CsAp group demonstrated less hypoxic reduction, with the redox ratio decreasing to 75.6% ± 4.1% of baseline. The UnT and CsAr groups deceased to 67.1% ± 4.0% and 67.2% ± 3.6%, respectively (p < 0.005). During reperfusion the UnT group redox ratio increased to 1.59 ± 0.04 times baseline. This increase was blunted in the CsAp (1.17 ± 0.04, p = 0.026) and CsAr (1.35 ± 0.02, p = 0.056) groups. Electron microscopy revealed reduced mitochondrial disruption in CsAp (19.7% ± 7.6%) and CsAr (18.1% ± 7.1%) rabbits compared with UnT (53.3% ± 12.5%).
Conclusions
Fluorometric spectroscopy can be used in vivo to quantitatively assess the time course of CsA’s influence on the mitochondrial dysfunction associated with myocardial ischemia and reperfusion.
The myocardial protective influence of cyclosporine A (CsA) has been described extensively in isolated hearts [1–3] and in in vivo animal experiments [4, 5] of ischemia-reperfusion injury. Interest in its clinical use has also recently been piqued by a report demonstrating CsA effectiveness in limiting infarct size in patients presenting with myocardial infarction and undergoing percutaneous coronary revascularization [6].
Mitochondria are key regulators of necrotic and apoptotic cell death responsible for reperfusion injury [7, 8]. Evidence suggests that CsA protects mitochondrial function during the ischemic and reperfusion phases. Although the exact mechanism is unclear during ischemia, it has been proposed that CsA, given before ischemia, induces metabolic changes similar to hypoxia that result in a type of “pharmacologic” preconditioning [9]. During the reperfusion phase, CsA has been shown to protect against opening of the mitochondrial permeability transition (MPT) pore, which prevents collapse of membrane potential, uncoupling of the respiratory chain, mitochondrial disruption, and the release of cytochrome c, as well as other proapoptotic factors [4, 5].
To date, the affect of CsA on mitochondrial function has only been assessed in mitochondria isolated from postmortem myocardial specimens. To demonstrate the influence of CsA on an in vivo heart, we have developed an optical catheter device capable of continuously measuring the fluorescence signals of the intrinsic mitochondrial fluorophores nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FAD). The ratio of these fluorescence signals [FADf/(FADf + NADf)], defined as the redox ratio (RR), correlates with different metabolic states and mitochondrial function [10–13]. In addition, the RR has been shown to undergo an oxidative shift in tumor cells with mitochondrial dysfunction associated with apoptosis [14].
In this study we hypothesized that our continuous fluorescent spectroscopy technique could be used to quantify the time course of CsA on limiting myocardial mitochondrial dysfunction during ischemia-reperfusion in a rabbit model. A method to assess mitochondrial dysfunction at the time of reperfusion therapy would provide a potentially valuable clinical tool given its important role in reperfusion injury. Such a device would allow for an immediate quantitative measure of therapeutic success and could potentially be used to guide acute and long-term management decisions.
Material and Methods
The animals in this study were treated under experimental protocols approved by the University of Pennsylvania’s Institutional Animal Care and Use Committee and in compliance with National Institutes of Health Publication No. 85-23, revised 1996.
Surgical Protocol
Seventeen male New Zealand white rabbits (weight, 3.2 to 4.0 kg) were sedated with intramuscular ketamine (50 mg/kg), glycopyrrolate (0.01 mg/kg), and buprenorphine (0.05 mg/kg). They were intubated and then ventilated with a mechanical respirator (Hallowell EMC Model AWS; Pittsfield, Massachusetts) using room air enriched with oxygen at 0.6 L/min. Anesthesia was maintained with an intravenous infusion of ketamine (20 mg/kg/h) and supplemental pentothal (2.5 to 5 mg/kg) as needed.
A left thoracotomy was performed. A coronary snare was placed around a large branch of the circumflex coronary artery at approximately 50% of the distance from base to apex of the heart and threaded through a small piece of polyethylene tubing.
A hyper/hypothermia unit was used to maintain core temperature between 39.0° and 40.0°C. Arterial blood gases were measured and pH was maintained between 7.35 and 7.45 throughout the protocol.
Experimental Protocol
The animals were divided into three groups: the untreated group (UnT, n = 7), the preischemia CsA group (CsAp, n = 6), and the CsA-on-reperfusion group (CsAr, n = 4). The UnT and the CsAp animals received a 1-hour, continuous 20-mL infusion of phosphate buffered saline vehicle (UnT) or 25 mg/kg of CsA. The CsAr animals received a 25-mg/kg intravenous bolus of CsA immediately at the onset of reperfusion.
The coronary snare was tightened to produce an ischemic region of the left ventricle (LV). Ischemia was confirmed by a visible color change in the ischemic myocardial region, ST elevations on the electrocardiogram, and regional wall motion abnormalities on the echocardiogram. At the end of the 30-minute ischemic period, the coronary snares were loosened and the previously ischemic myocardium was reperfused for 3 hours.
Myocardial Fluorescence Spectroscopy
Fluorescence spectroscopy of rabbit myocardium was conducted with a fluorometer (Fig 1). This fluorometer is a mobile optical-electrical apparatus that collects fluorescence signals of any type of tissue through a 3-mm-tip light guide catheter. The incident light is a broadband mercury arc lamp that can be filtered at 2 pairs of excitation/emission wavelengths by an air turbine filter wheel rotating at 50 Hz. Consequently, up to 4 signals can be multiplexed to a photodetector to make 4-wavelength channel optical measurements of tissue metabolism. In this experiment, 2 channels were used for excitation and 2 for emission signals. The light intensity that is incident on tissue at the fiber tip is 3 µW/mm2.
Fig 1.
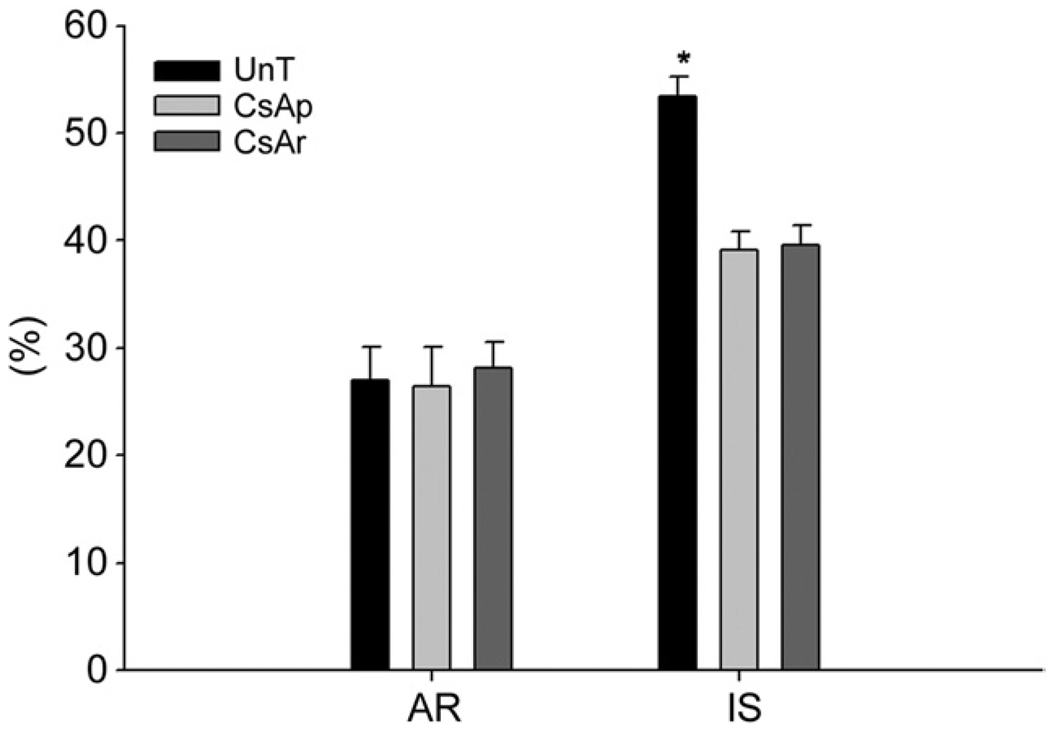
Comparison of area at risk (AR) and infarct sizes (IS) among the untreated (UnT), cyclosporine A (CsA) preinfarction (CsAp), and CsA reperfusion (CsAr) groups. Mean values are shown with the standard error of the mean (error bars). The area at risk is expressed as a percentage of the left ventricle, and the infarct size is expressed as a percentage of the area at risk. The area at risk was not significantly different between groups. Infarct size was significantly greater in the untreated group than in either of the CsA groups (*p < 0.01). Infarct size was the same in both CsA groups.
In cardiac fluorometry experiments, the excitation wavelengths of FAD and NAD are obtained by filtering the resonance lines of the mercury arc lamp at 436 nm and 366 nm by band-pass filters 440DF20 and 365HT25, respectively. The fluorescence intensities are then detected by a photomultiplier tube, converted to an electric voltage, digitized, and displayed. Specific instrument specifications were kept the same for all the experiments.
The fluorometer catheter was placed on the epicardial surface in the center of the anticipated region of ischemia, and continuous recording of the fluorescence signals for FAD and NAD signals was performed during 10 minutes of baseline, 60 minutes of infusion of saline or CsA, 30 minutes of ischemia, and 180 minutes of reperfusion. The RR was calculated as [FADf/(FADf + NADf)] every 5 minutes from the continuously recorded FAD and NAD. The RR in each group was averaged and expressed as mean ± standard error at 5-minute time points for statistical analysis and at 10-minute intervals for spectroscopic graphs.
Analysis of Area at Risk and Infarct Size
At the end of the protocol, the coronary snare was reapplied, vascular clamps were used to occlude the aorta, pulmonary artery, and inferior vena cava, and the right atrium was incised. To delineate the myocardial area at risk (AR), 5 mL of 1% Evans Blue Dye (Sigma, St. Louis, MO) was injected into the left atrium. This was followed by a 20-mEq intraatrial bolus of potassium chloride to arrest the heart. The heart was explanted and LV was isolated and fixed in a 20% gelatin solution for 20 minutes.
After fixation, the LV was sectioned into eight 2- to 3-mm transverse slices. The thickness of each slice was measured with a digital micrometer and all slices were photographed. The infarct area was delineated by photographing and measuring the slices after 20 minutes of incubation in 2% triphenyltetrazolium chloride at 37° C. All photographs were imported into the Image Pro Plus image analysis program (MediaCybernetics, Silver Spring, MD) and computerized planimetry was performed. The AR and infarct area are expressed as a percentage of the LV, and the infarct size (IS) is expressed as the ratio of infarct area/AR.
Transmission Electron Microscopy
Specimens from myocardial punch biopsies were obtained from the AR from 2 rabbits from each group. Tissue was also obtained from 2 normal rabbits that did not undergo the ischemia-reperfusion protocol. Specimens were preserved in fixative (2.5% glutaraldehyde, 2.0% paraformaldehyde, and 0.1M sodium cacodylate) for 24 hours at 4°C. After several washes in 0.1M sodium cacodylate, samples were post-fixed with buffered 2% osmium tetroxide for 1 hour at 4°C. Subsequent washes in 0.1M sodium cacodylate, water, and 2% aqueous uranyl acetate were used to destain the samples.
Tissue samples were dehydrated in serial washes of ethanol and propylene oxide before a slow infiltration with EPON 812 (54% Polybed 812, 29% Nadic Methyl Anhydride, 16% Dodecenylsuccinic anhydride, 1% DMP-30; Polysciences, Warrington, PA). Samples were cured at 70°C for 48 hours and cut, stained, and imaged on a Jeol-10-10 transmission electron microscope (Jeol, Akishima, Japan). Random images were captured from each sample for comparative analysis. To assess the degree of mitochondrial disruption, 5 random images of mitochondria per rabbit at × 12,000 magnification were captured from each specimen. Morphologic differences in mitochondria were assessed in the nuclear cap, a region surrounding the cell nucleus. The total number of mitochondria and the number of disrupted mitochondria were counted and averaged. The mean percentage of disrupted mitochondria was calculated and reported for each group.
Statistical Analysis
All group results are expressed as the mean ± standard error of the mean. The RR values for the CsAp, CsAr and UnT animals were plotted over time. The resulting curves were compared using random effects models with nonlinear functions over time [15]. Random effects models are similar to standard regression models but are able to adjust for the inherent correlation within measurements from the same animal. Sequential t tests, with adjustment for multiple comparisons, were used to determine the time at which the curves differed within the ischemia and reperfusion periods. Postmortem values for AR and IS were compared using the t test. Analyses were performed using SAS 9.1 software (SAS Institute Inc, Cary, North Carolina).
Results
Infarct Size Measurements
The AR was similar in all groups: 27.0% ± 3.1% in the UnT group, 26.5% ± 3.6% in the CsAp group, and 28.2% ± 2.3% in the CsAr group. The IS was significantly smaller in the CsAp (39.1% ± 1.7%) and CsAr (39.6% ± 1.8%) groups than in the UnT group (53.4% ± 1.9%, p < 0.001; Fig 1). There was no difference in IS between the CsAp and CsAr groups.
Redox Fluorometry
Figure 2 presents the RR at 10-minute intervals during the entire protocol. The RR baseline readings did not differ significantly within groups or between groups and remained constant during the preischemic CsA/saline infusion period, demonstrating that CsA had no effect on the mitochondrial metabolic state of normally perfused myocardium.
Fig 2.
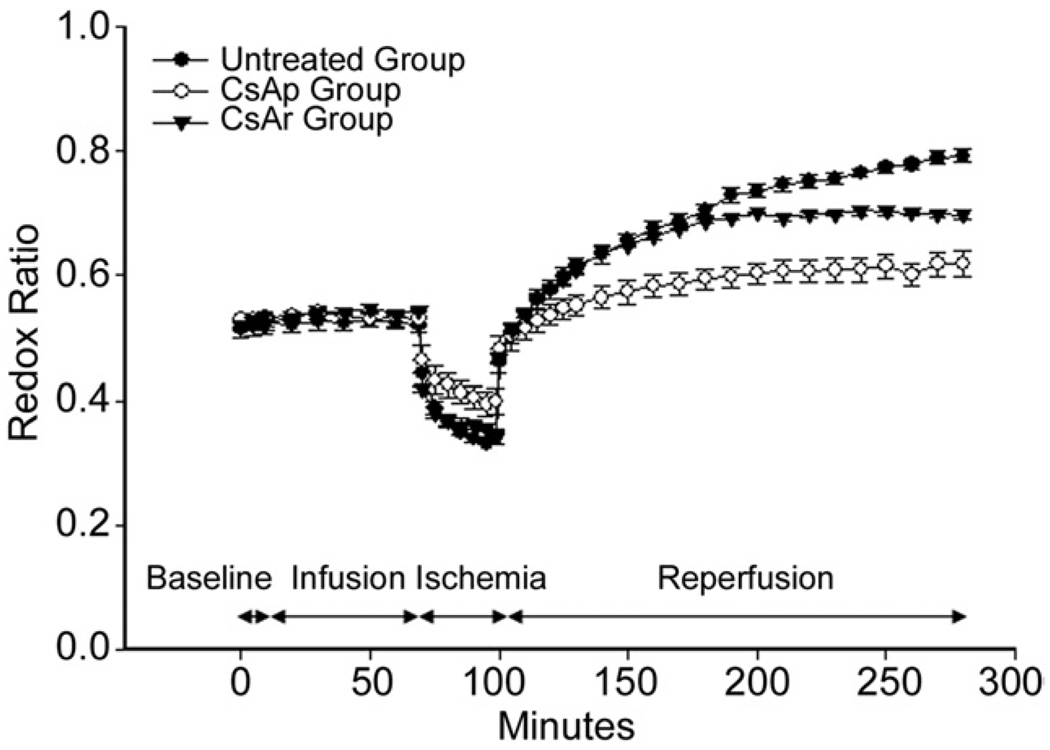
Comparison of mean redox ratio (RR) between the untreated (UnT; black circle), cyclosporine A (CsA) preinfarction (CsAp; white circle) and CsA reperfusion (CsAr; black triangle) groups throughout the time course of the experiment. There were no significant differences among the groups during the baseline or infusion phases. During ischemia, the CsAp curve is significantly different from the UnT and CsAr groups. During reperfusion, all three curves were significantly different. The average rate of increase of the redox ratio during the first 70 minutes of reperfusion, as assessed by a best-fit line through all the data points, was significantly greater in the UnT and CsAr groups than it was in the CsAp group (0.005 min−1 vs 0.003 min−1; p < 0.001). After 90 minutes of reperfusion, the CsAr curve began to deviate from the UnT curve. Error bars represent standard error of the mean.
The RR dropped immediately in all groups with the onset of ischemia. This is consistent with a reduction in the oxidative state of the fluorophores due to decreased oxygen availability. That is, NAD and FAD remain in their reduced form due to back-up of the electron transports chain created by the absence of oxygen, which is the chain’s terminal electron acceptor. The rate at which the RR decreased during the ischemic period was significantly less in the CsAp group (p < 0.001) than in the UnT or CsAr groups. Preischemic treatment with CsA resulted in the RR dropping to only 0.398 ± 0.020 (75.6% ± 4.1% of baseline), whereas the RR dropped to 0.339 ± 0.008 (67.1% ± 4.0% of baseline) in the UnT group and to 0.348 ± 0.004 (67.2% ± 3.6% of baseline) in the CsAr group (p = 0.008). As would be expected, there was no difference in the RR during ischemia in the UnT and CsAr groups.
With the reinstitution of blood flow, the RR increased immediately in all groups. In the UnT group, the RR increased persistently to 0.792 ± 0.010 (1.59 ± 0.04 times normal) during the entire 180 minutes of reperfusion. This drastic increase in the RR represents a “hyperoxidation” of fluorophores and is indicative of dysfunctional mitochondria that are unable to use the tricarboxylic acid cycle to reduce NAD+ and FAD, which prevents their entry into the electron transport chain and ultimately the production of adenosine triphosphate.
The increase in the RR of the UnT group was significantly blunted by the preischemic (p = 0.026) and on-reperfusion (p = 0.056) administration of CsA. In the CsAp group, the RR ratio only increased to 0.619 ± 0.02 (1.17 ± 0.04 times baseline), whereas in the CsAr group, it increased to 0.697 ± 0.007 (1.35 ± 0.02 times baseline) after 180 minutes of reperfusion.
The RR rose almost twice as fast during the first 70 minutes of reperfusion in both the UnT and CsAr groups than in the CsAp (0.005 min−1 vs 0.003 min−1; p < 0.001). The preischemic administration of CsA blunted reperfusion-induced fluorophore hyperoxidation almost immediately. In the CsAr group, 90 minutes passed before any influence on the RR was observed.
Transmission Electron Microscopy
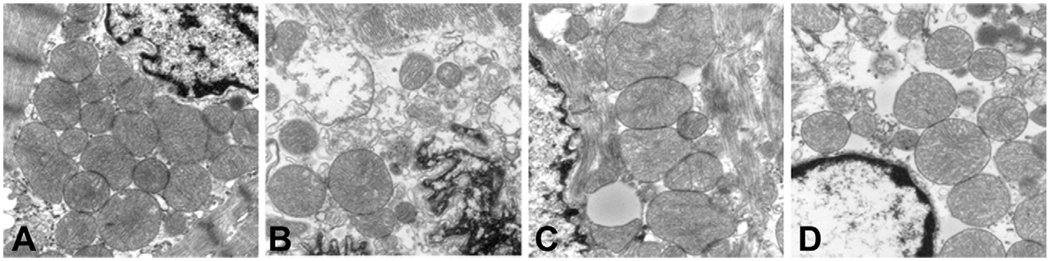
The nuclear cap in the normal animals was densely populated with mitochondria that had well-defined membranes with tightly packed cristae (Fig 3A) The nuclear cap in the UnT group (Fig 3B) contained fewer mitochondria, and the mitochondria that were present were vacuolated, swollen, or possessed poorly defined or had completely absent outer membranes with unraveling cristae. Compared with the normal group, the nuclear cap in the CsAp group (Fig 3C) was less densely populated with mitochondria and contained mitochondria with dilated cristae; however, most of the mitochondria had well-defined outer membranes with normal-appearing cristae.
Fig 3.
Representative transmission electron photomicrographs of rabbit myocardium images (original magnification ×30,000) compare the differences among the mitochondria of the (A) normal, (B) untreated, (C) cyclosporine A (CsA) preinfarction, and (D) CsA reperfusion groups. The mitochondria of the normal myocardium are tightly packed and possess an intact mitochondrial membrane and well-organized cristae, unlike the untreated group, that is characterized by swollen, loosely packed, vacuolated, and completely disrupted mitochondria. Although the CsA preinfarction and reperfusion groups demonstrate mitochondria with dilated cristae, their general morphology is much closer to normal than was the untreated group.
Qualitatively, there were no remarkable differences between the CsAp and CsAr groups. Overall, the CsAp and CsAr groups demonstrated a morphology that was more consistent with that of the normal animals than the nuclear cap morphology observed in the UnT animals. Quantification of these structural differences revealed a higher percentage of disrupted mitochondria in the UnT group (53% ± 16%) compared with the CsAp group (20% ± 9%, p ± 0.01), CsAr (18% ± 7%), or normal rabbits (1.5% ± 1%).
Comment
This study confirms the protective influence of CsA against ischemia-reperfusion injury. Interestingly, there were no significant differences in myocardial salvage or mitochondrial morphology when the drug was given before ischemia or only at reperfusion. Despite the lack of influence on the ultimate infarct size, the timing off CsA administration did have a relatively dramatic influence on mitochondrial function during ischemia and reperfusion, as indicated by the time course curves of the RR (Fig 2).
The RR is calculated using intrinsic NAD and FAD fluorescence measurements and has been shown by members of our group to be a sensitive index of mitochondrial metabolism. Because the fluorescence of NAD and FAD varies inversely with the mitochondrial redox state, the RR [FADf/(FADf + NADf)] has been found to correlate more strongly with mitochondrial function than either of the individual fluorescent measurements alone [10–13].
When CsA was given before ischemia, there was significantly smaller hypoxic-induced mitochondrial dysfunction indicated by a blunted drop in the RR during ischemia. It has been hypothesized that CsA given before ischemia induces metabolic changes similar to hypoxia, resulting in a type of pharmacologic preconditioning. The findings of the current study do not support this hypothesis. No such “hypoxic” physiology is noted on the CsAp RR curve in Figure 2. If CsA were inducing hypoxic like conditions, we would have expected a decrease in the RR compared with the CsAr and UnT groups.
Multiple studies have demonstrated that MPT pore opening is triggered by reperfusion and that its opening is a pivotal event in necrotic and apoptotic cell death [16, 17]. After myocardial ischemia-reperfusion injury, opening of this nonspecific pore results in inner membrane potential collapse, uncoupling of the respiratory chain, and outer membrane rupture with efflux of small molecules such as cytochrome c and other proapoptotic factors [18–20]. The uncoupling of the respiratory chain prevents the reduction of NAD and FAD, resulting in a reperfusion-induced hyperoxidation of NAD and FAD that causes a rise in the RR.
CsA has been shown to be a powerful inhibitor of MPT pore opening [4, 5], and our study corroborates this. CsA given before ischemia almost completely prevented the hyperoxidation of mitochondrial fluorophores and the associated rise in the RR that was seen in the untreated group. When CsA was given only at the time of reperfusion, 90 minutes elapsed before any sign of RR normalization, and mitochondrial functional improvement was seen. Despite this delay, the myocardial salvage rate was almost identical between the CsAp and the CsAr groups. An explanation for this may be that a window of opportunity exists during which initially dysfunctional mitochondria are still salvageable by the protective influence of CsA. It also indicates that CsA not only prevents MPT pore opening but also promotes pore resealing.
The results of this study demonstrate that mitochondrial function after ischemia and reperfusion, as well as CsA’s influence on it, follows a complex time course. This complexity may be responsible for past difficulties in translating promising animal studies of CsA efficacy into clinically applicable treatment strategies.
We believe the data presented provide convincing evidence that catheter-based fluorometry is a promising tool for assessing other pharmacologic strategies designed to protect mitochondria and limit myocardial profusion injury. With further development of the technology, it may also become an effective clinical tool to assess myocardial injury early after reperfusion without tissue biopsy. Such a device could provide improved early evaluation of the success of reperfusion therapy and potentially identify patients that are at increased risk for postinfarction ventricular remodeling. Early identification of such patients would allow the institution of more aggressive treatment to prevent heart failure than would be justifiable without proper patient identification.
Mitochondrial dysfunction is also associated with established chronic heart failure [21] and myocardial rejection after heart transplantation [22]. These disease states are currently treated with complex pharmacologic strategies that can be hard to optimize. Considering the findings of this study, it is possible that fluorometry may be used to assess and monitor the progression of these diseases without tissue biopsy and allow physicians to make more informed decisions regarding drug selection and dosing.
The proposed clinical applications of fluorometry to the quantitative assessment of cardiovascular disease are highly speculative. However, one can envision a catheter-based fluorometer, introduced through standard percutaneous venous or arterial techniques, that could be used to assess reperfusion injury, ventricular remodeling, and allograft rejection. Such a technology would improve the care of these very complex patients while minimizing their discomfort and treatment risk.
Acknowledgment
This research was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland (HL76560, HL71137, and HL63954) and Individual American Heart Association Established Investigator Awards to R. Gorman and J. Gorman.
Abbreviations and Acronyms
- AR
area at risk
- CsA
cyclosporine A
- CsAp
cyclosporine A preinfarction
- CsAr
cyclosporine A reperfusion
- FAD
flavin adenine dinucleotide
- FADf
FAD fluorescence
- IS
infarct size
- MPT
mitochondria permeability transition
- NAD
nicotinamide adenine dinucleotide
- NADf
NAD fluorescence
- RR
redox ratio
- UnT
untreated
References
- 1.Weinbrenner C, Liu GS, Downey JM, Cohen MV. Cyclosporine A limits myocardial infarct size even when administered after onset of ischemia. Cardiovasc Res. 1998;38:678–684. doi: 10.1016/s0008-6363(98)00064-9. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths EJ, Halestrap AP. Protection by cyclosporin A of ischemia/reperfusion-induced damage in isolated rat hearts. J Mol Cell Cardiol. 1993;25:1461–1469. doi: 10.1006/jmcc.1993.1162. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 4.Argaud L, Gateau-Roesch O, Muntean D, et al. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol. 2005;38:367–374. doi: 10.1016/j.yjmcc.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Leshnower BG, Kanemoto S, Matsubara M, et al. Cyclosporine preserves mitochondrial morphology after myocardial ischemia/reperfusion independent of calcineurin inhibition. Ann Thor Surg. 2008;86:1286–1292. doi: 10.1016/j.athoracsur.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piot C, Criosille P, Staat P, Thibault H, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bromme HJ, Holtz J. Apoptosis in the heart: when and why? Mol Cell Biochem. 1996;164:261–275. doi: 10.1007/BF00408667. [DOI] [PubMed] [Google Scholar]
- 9.Niemann CU, Saeed M, Akabari H, et al. Close association between the reduction in myocardial energy metabolism and infarct size: dose-response assessment of cyclosporine. J Pharmacol Exp Ther. 2002;302:1123–1128. doi: 10.1124/jpet.102.036848. [DOI] [PubMed] [Google Scholar]
- 10.Hassinen I, Chance B. “Oxidation-reduction properties of the mitochondrial flavoprotein chain,”. Biochem Biophys Res Commun. 1968;31:895–900. doi: 10.1016/0006-291x(68)90536-6. [DOI] [PubMed] [Google Scholar]
- 11.Chance B, Schoener B, Oshino R, Itshak F, Nakase Y. Oxidation-reduction ratio studies of mitochondria in freeze-trapped samples. NADH and flavoprotein fluorescence signals. J Biol Chem. 1979;254:4764–4771. [PubMed] [Google Scholar]
- 12.Chance B, Oshino N, Sugano T, Mayevsky A. Basic principles of tissue oxygen determination from mitochondrial signals. Adv Exp Med Biol. 1973;37:277–282. doi: 10.1007/978-1-4684-3288-6_35. [DOI] [PubMed] [Google Scholar]
- 13.Ranji M, Matsubara M, Leshnower BG, et al. Quantifying acute myocardial injury using ratiometric fluorometry. IEEE Trans Biomed Eng. 2009;56:1556–1563. doi: 10.1109/TBME.2008.2006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranji M, Jaggard DL, Chance B. Observation of mitochondrial morphology and biochemistry changes undergoing apoptosis by angularly resolved light scattering and cryoimaging. Proc SPIE 6087. 2006 60780K1-9. [Google Scholar]
- 15.Laird NM, Ware JH. Random effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 16.Kroemer G, Dallaporta B, Resche-Rignon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 17.Duchen MR, McGuiness O, Brown LA, Crompton M. On the involvement of a cyclosporin: A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc Res. 1993;27:1790–1794. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- 18.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia but open upon reperfusion. Biochem J. 1995;307:93–99. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 21.Narula J, Haider N, Virmani R, et al. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 22.Narula J, Elmo R, Acio, et al. Annexin-V imaging for noninvasive detection of cardiac allograft rejection. Nat Med. 2001;7:1347–1352. doi: 10.1038/nm1201-1347. [DOI] [PubMed] [Google Scholar]