Abstract
Purpose
To assess the risk of developing advanced age-related macular degeneration (AMD) following cataract surgery.
Design
Cohort study
Participants
4577 participants (8050 eyes) from a multi-centered, controlled, randomized clinical trial, the Age-Related Eye Disease Study (AREDS).
Methods
Development of advanced AMD, either neovascular (NV) AMD or geographic atrophy (GA) was evaluated with annual fundus photographs, and history of cataract surgery was assessed every 6 months. Cox proportional hazard models with time-dependent covariates were conducted for NVAMD and GA separately.
Main Outcome Measures
NV AMD, GA, and central GA (involving the center of the macula).
Results
The Cox proportional hazards model of right eyes showed non-significant hazard ratios 1.20, 95% confidence interval (CI ) 0.82–1.75 for NVAMD, 0.80, 95%CI 0.61–1.06 for GA, and 0.87, 95% CI 0.64–1.18 for central GA (CGA). Similar results were obtained for left eyes: 1.07, 95% CI 0.72–1.58 for NV AMD, 0.94, 95%CI 0.71–1.25 for GA, and 0.86, 95%CI 0.63–1.19 for CGA. For participants with advanced AMD in one eye (AREDS Category 4), the hazard ratios for fellow eyes were 1.08 95% CI 0.65–1.72 for NV AMD and 0.98, 95% CI 0.64–1.49 for CGA.
Conclusions
The AREDS results showed no clear effect of cataract surgery on the risk of progression to advanced AMD.
Age-related macular degeneration (AMD) and cataract are two of the leading causes of visual impairment in the United States.1 Cataract surgery is the most commonly performed surgery in this country, with over a million operations performed annually. Although both diseases are associated with increasing age and share some common risk factors, it is not clear whether there is a direct relationship between the two ocular disorders.2–5 The relationship between cataract surgery and the development of advanced AMD has generated interest among ophthalmologists. Concerns have been raised regarding the potential of cataract surgery to accelerate progression to advanced, vision threatening forms of AMD in a number of studies, including a study of autopsy eyes,6 several case series,7–10 and population-based epidemiological studies.11–14
Largely because of the findings from the large epidemiologic studies of an adverse association of cataract surgery with AMD, some investigators have speculated on the risk of cataract surgery in eyes at risk for development of advanced AMD.15 Other investigators have called for more “stringent” indications for cataract surgery in persons with AMD.14 We have the opportunity to further explore the relationship in the Age-Related Eye Disease Study (AREDS), a prospective study in which participants with varying degrees of AMD were enrolled and followed every 6 months by retinal specialists for up to 11 years. In this report, we present the results of these analyses regarding the association of cataract surgery with the incidence of advanced AMD.
Methods and Materials
Study Population
Details of the study design and methods, presented elsewhere,16 are briefly summarized here. Eleven retinal specialty clinics enrolled 4,757 participants in AREDS from 1992 through 1998. Participants were 55 to 80 years of age at enrollment and had best-corrected visual acuity (BCVA) of 20/32 or better in at least one eye (the study eye[s]). Media had to be sufficiently clear to obtain adequate quality stereoscopic fundus photographs of the macula in all study eyes. Visual acuity (VA) was assessed by certified examiners using the Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity chart and a standardized refraction and VA protocol (AREDS Manual of Operations; The EMMES Corporation, Rockville, MD). Persons were enrolled in 1 of 4 AMD categories determined by the presence, size, and extent of drusen and retinal pigment epithelial abnormalities in each eye, the presence of advanced AMD (determined by evaluation of stereoscopic color photographs at a reading center), and VA. IRB approval was obtained by the clinics and informed consent was obtained from all participants.
Briefly, persons in Category 1 were essentially free of AMD, with a total drusen area of less than 5 small drusen (< 63 μm in diameter), and VA of 20/32 or better in both eyes. Category 2 participants had mild age-related macular lesions (multiple small drusen, nonextensive (<20) intermediate drusen (63–124 μm in diameter), pigment abnormalities, or any combination of these) in their most advanced eye, and visual acuity of 20/32 or better in both eyes. Category 3 required absence of advanced AMD in both eyes and at least 1 eye with VA of 20/32 or better with at least 1 large druse (≥125 μm in diameter), extensive (as measured by drusen area) intermediate drusen, or geographic atrophy (GA) that did not involve the center of the macula, or any combination of these. In Category 3a both eyes met these criteria, while in Category 3b one eye had either reduced VA not due to AMD or a disqualifying ocular condition. Category 4 participants had VA of 20/32 or better and no advanced AMD (GA involving the center of the macula or features of choroidal neovascularization) in the study eye, and the fellow eye had either lesions of advanced AMD (Category 4a) or VA less than 20/32 and AMD abnormalities sufficient to explain reduced VA (Category 4b) as determined by examination of photographs at the reading center. Persons aged 55 to 59 years were eligible for the study only if they were in Category 3 or 4.
Participants included in the analyses were in the randomized, placebo-controlled clinical trial of the effect of antioxidant and mineral supplements on progression to advanced AMD and development of cataract (1992–2001). Data was also included for those who continued in the follow-up phase of the trial, until file closure November 2004. Eyes with cataract surgery or advanced AMD at baseline were excluded, leaving 8050 eyes from 4577 participants for consideration for analysis. These numbers were further reduced by exclusions specific to each analysis.
Procedures
Detailed questionnaires were administered to obtain demographic information, history of smoking and sunlight exposure, medical history, history of specific prescription drug and nonprescription medication use, and history of vitamin and mineral use. General physical and ophthalmic examinations included height, weight, blood pressure, manifest refraction, best corrected visual acuity, intraocular pressure, slit-lamp biomicroscopy, and ophthalmoscopy. Date of cataract surgery was obtained by history at 6-month intervals. Stereoscopic film-based color fundus photographs of the macula and lens photographs (red reflex, slit lamp and Neitz) were taken at baseline and annually beginning at the 2 year annual study visit. Photographs were graded at a reading center, where the various lesions associated with AMD and the severity of lens opacities by type were assessed with standardized grading procedures.17,18
Outcomes
Progression to neovascular AMD for a study eye was based on clinical center reports of photocoagulation for choroidal neovascularization, or photographic documentation at the reading center of at least 1 of the following: subretinal fibrosis, non-drusenoid retinal pigment epithelial detachment, serous or hemorrhagic retinal detachment, and hemorrhage under the retina or the retinal pigment epithelium.
Progression to geographic atrophy: 1) GA (>175 um in diameter within the grid to be comparable with previous studies) or 2) central GA (involving the the center of the macula regardless of size), was based on photographic documentation at the reading center.
Risk Factor Definitions
Risk factors for incident advanced AMD (neovascular AMD or central GA) were assessed in an earlier report.19 Factors included in covariate adjusted analyses were age at baseline (either continuous or in categories 55 to 64, 65 to 69, and 70 to 80 years), gender, race (white, other), history of smoking (ever smoked, never smoked), AREDS treatment group (placebo, active treatment [antioxidants, zinc, or both]; or antioxidants vs no antioxidants), and eye-specific AMD severity status based on a 9-point scale.20
Statistical Modeling and Analyses
Cox proportional hazards regression
This model was used to estimate the risk of advanced AMD associated with cataract surgery. Separate analyses were conducted for NV AMD and GA or CGA. In analyses of GA and CGA, eyes with NV AMD occurring before GA or CGA were excluded. Since some covariates (cataract surgery, AMD status, age) change their values over the follow-up and contain information of disease progression, the Cox proportional hazards regression model was fitted to understand the interrelationships between advanced AMD outcome and time-dependent covariates and potentially causal mechanisms.
This model (Procedure PHREG, SAS version 8.2, SAS Institute, Cary, NC) estimates the effects of time-dependent covariates cataract surgery (0 until surgery, then 1), age, and AMD severity (based on the AREDS nine point severity scale) on the outcome variable, “time to developing advanced AMD.” Analysis is done separately for right eyes and left eyes because software for time-dependent covariates is not available to jointly model the two eyes. A hazard ratio > 1 for cataract surgery would suggest an increased risk of advanced AMD in eyes following surgery compared with non-surgery eyes, accounting for time-dependent covariates age and AMD status as well as the independent covariates gender and smoking history. Eyes were excluded from this analysis if they were pseudophakic/aphakic or had advanced AMD at baseline.
Matched pair analysis
This analysis uses matching to adjust for potential confounding variables. It addresses the question of whether cataract surgery accelerates the development of advanced AMD by observing if advanced AMD occurs sooner after cataract surgery compared with matched pair eyes that have not had cataract surgery. Eyes were matched so that they had similar risk factors for the development of advanced AMD. Persons with cataract surgery in either eye at baseline were excluded. A cataract surgery eye (case) was included if surgery was performed after baseline and occurred before any evidence advanced AMD. If surgery was performed in both eyes during follow-up, the “earlier” eye was selected for analysis. A non-surgery eye (control) was eligible for matching if it has a natural lens during all AREDS follow-up.
Characteristics of surgery eyes for matching were age at time of surgery, AMD severity status at time of surgery (based on the AREDS 9-point severity scale from the annual fundus photographs obtained prior to surgery), advanced AMD status of the fellow eye at time of surgery, assigned AREDS treatment (active--antioxidants, zinc, or both; vs. placebo), baseline participant AMD category (Category 2 or 3, or those with 2 study eyes; or Category 4 or those with 1 study eye), length of follow-up after surgery (time to advanced AMD event, or to end of follow-up if no subsequent advanced AMD event).
A non-surgery eye was matched if, at some point in time during the study, all of the following were met: (1) the person was the same age ± 2 years as the surgery person at time of surgery; (2) the non-surgery eye AMD severity status was the same as for the surgery eye at time of surgery; (3) available length of follow-up for the non-surgery eye was within ± 3 months of the length of follow-up for the surgery eye post cataract surgery; (4) the fellow eye of the non-surgery eye had the same advanced AMD status as the fellow eye of the surgery eye. The eyes were also matched on baseline AMD category and on assigned AREDS treatment. When n>1 cases had the same matching characteristics, n controls were matched to them.
R is defined as the number of eyes with cataract surgery [cases] with subsequent advanced AMD that developed before their matched controls, divided by the number of eyes without cataract surgery [controls] that developed advanced AMD before their matched cases. An R greater than 1 suggests an increased likelihood of advanced AMD occurring sooner among case eyes than among matched controls, i.e., an acceleration of occurrence of advanced AMD.
Logistic regression
This model follows to some extent the analysis of the combined data from the Beaver Dam Eye Study and the Blue Mountains Eye Study13, using a logistic regression model of 5-year incidence. Eyes with cataract surgery after baseline are included if they had 5-years of follow-up from the time of surgery and were free of advanced AMD at the time of surgery. Non-surgery eyes were included if they had 5 years of follow-up from baseline. In all eyes, the outcome variable is defined as the presence or absence of advanced AMD, assessed at the 5-year follow-up visit without regard to intermediate years. An odds ratio (OR) > 1 would suggest increased risk of advanced AMD in eyes following surgery compared with non-surgery eyes at exactly 5 years of follow-up. Unadjusted and adjusted analyses were done using SAS Procedure GENMOD, accounting for correlation between bilateral outcomes of two eyes in a person using the generalized estimating equations methodology. Eyes were excluded if they had less than 5 years of follow-up, were pseudophakic at baseline, or had advanced AMD at baseline or developed it before cataract surgery. Similar to the BDES/BMES analyses, AMD status was categorized as the presence or absence of late-stage AMD or eye-specific AMD Category 3 at baseline or within 1 year before surgery.
Results
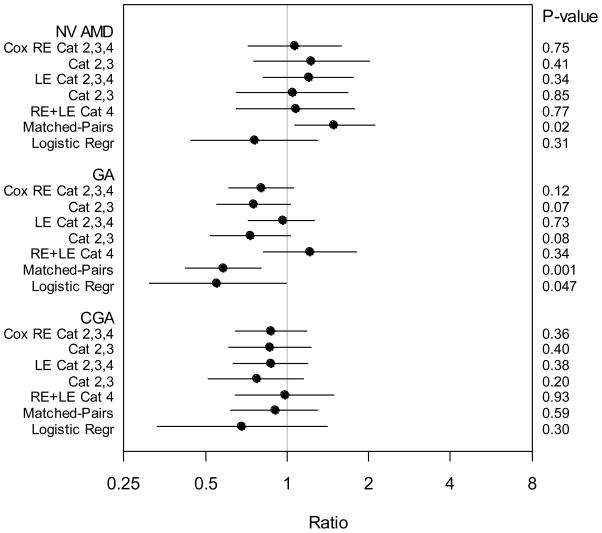
Table 1 gives the distribution of baseline characteristics for participants with and without cataract surgery. Risk of advanced AMD associated with cataract surgery is summarized in Table 2 and Figure 1, and is given in detail in the following.
Table 1.
Baseline Characteristics of Participants with Incident Cataract Surgery vs Participants with No Cataract Surgerya
| Baseline Characteristics | Incident Cataract Surgery (n=1167) | No Cataract Surgery (n=3410) | P Valueb | Total (n=4577) |
|---|---|---|---|---|
| Age | <.0001 | |||
| 55–64 | 125 (11) | 944 (28) | 1069 (23) | |
| 65–69 | 385 (33) | 1173 (34) | 1558 (34) | |
| 70–81 | 657 (56) | 1293 (38) | 1950 (43) | |
| Mean Age, yr (SD) | 70 (5) | 68 (5) | <.0001 | 68 (5) |
| Gender | .02 | |||
| Female | 685 (59) | 1870 (55) | 2555 (56) | |
| Male | 482 (41) | 1540 (45) | 2022 (44) | |
| Race | .02 | |||
| White | 1130 (97) | 3244 (95) | 4374 (96) | |
| Other | 37 (3) | 166 (5) | 203 (4) | |
| History of Smoking | .84 | |||
| Ever Smoked | 646 (55) | 1899 (56) | 2545 (56) | |
| Never Smoked | 521 (45) | 1511 (44) | 2032 (44) | |
| AMD Treatment | .89 | |||
| Placebo | 369 (32) | 1071 (31) | 1440 (32) | |
| Treated | 798 (68) | 2339 (69) | 3137 (68) | |
| Participant AMD Category | .46 | |||
| Category 1 | 272 (23) | 841 (25) | 1113 (24) | |
| Category 2 | 259 (22) | 795 (23) | 1054 (23) | |
| Category 3 | 451 (39) | 1089 (32) | 1540 (34) | |
| Category 4 | 185 (16) | 685 (20) | 870 (19) | |
| Nuclear Sclerosisc | <.0001 | |||
| < STD 4 | 900 (77) | 3098 (91) | 3998 (88) | |
| ≥ STD 4 | 262 (23) | 308 (9) | 570 (12) | |
| Length of Follow-up, yr | ||||
| Median | 9.8 | 9.5 | <.0001 | 9.5 |
| Mean (SD) | 9.2 (1.9) | 8.6 (2.5) | <.0001 | 8.8 (2.4) |
Values are expressed as number (%) of participants unless otherwise indicated.
P value derived from t test for means and χ2 test for percentages.
Baseline score based on earliest eye with incident cataract surgery; if no surgery, worse baseline score selected. Data unavailable for 9 participants.
SD = standard deviation; STD 4 = standard photograph 4; AMD = age-related macular degeneration.
Table 2.
Risk of advanced AMD associated with incident cataract surgery
| Method of Analysis | NVAMD |
GA |
CGA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ratioa | 95% CI | P | Ratio | 95% CI | P | Ratio | 95% CI | P | |||
| Cox | RE | Category 2,3,4 | 1.20 | 0.82–1.75 | 0.34 | 0.80 | 0.61–1.06 | 0.12 | 0.87 | 0.64–1.18 | 0.36 |
| No. (%) progressing | 382 (13.4) | 439 (14.9) | 289 (9.8) | ||||||||
| Category 2,3 | 1.05 | 0.65–1.68 | 0.85 | 0.75 | 0.55–1.03 | 0.07 | 0.86 | 0.61–1.22 | 0.40 | ||
| No. (%) progressing | 232 (9.5) | 322 (12.7) | 206 (8.1) | ||||||||
| LE | Category 2,3,4 | 1.07 | 0.72–1.58 | 0.75 | 0.95 | 0.71–1.26 | 0.73 | 0.87 | 0.63–1.19 | 0.38 | |
| No. (%) progressing | 394 (13.5) | 456 (15.1) | 319 (10.5) | ||||||||
| Category 2,3 | 1.23 | 0.75–2.02 | 0.41 | 0.72 | 0.52–1.03 | 0.07 | 0.77 | 0.51–1.15 | 0.20 | ||
| No. (%) progressing | 221 (9.0) | 325 (12.8) | 208 (8.2) | ||||||||
| RE+LE | Category 4 | 1.08 | 0.65–1.77 | 0.77 | 1.21 | 0.82–1.80 | 0.34 | 0.98 | 0.64–1.49 | 0.93 | |
| No. (%) progressing | 323 (37.6) | 460 (17.5) | 194 (21.1) | ||||||||
| Matched-Pairs | 1.49 | 1.07–2.10 | 0.02 | 0.58 | 0.42–0.80 | 0.001 | 0.90 | 0.62–1.29 | 0.59 | ||
| No. (%) progressing | 172 (11.9) | 201 (14.1) | 148 (10.2) | ||||||||
| Logistic Regression | 0.76 | 0.44–1.30 | 0.31 | 0.55 | 0.31–0.99 | 0.047 | 0.68 | 0.33–1.41 | 0.30 | ||
| No. (%) progressing | 282 (5.0) | 219 (3.8) | 122 (2.0) | ||||||||
Ratio is Hazard Ratio (HR) for Cox models, Ratio (R) from matched-pair analysis, Odds Ratio (OR) for Logistic Regressions.
Values of HR, R and OR > 1 suggest increased risk of AMD in eyes following cataract surgery compared with non-surgery eyes.
RE =Right eye, LE =Left eye, NVAMD = Neovascular age-related macular degeneration, GA = Geographic atrophy, CGA = Central geographic atrophy.
Persons in Categories 2, 3, and 4 at baseline are at increasing risk of developing advanced AMD, as described in “Study Population.”
AMD = age-related macular degeneration; CI = confidence interval
Figure 1. Graphical representation of risks and 95% confidence intervals.
Ratio is Hazard Ratio (HR) for Cox models, Ratio (R) from matched-pair analysis, Odds Ratio (OR) for logistic regression.
Values of HR, R and OR > 1 suggest increased risk of age-related macular degeneration in eyes following cataract surgery compared with non-surgery eyes.
RE =Right eye, LE =Left eye, NVAMD = Neovascular age-related macular degeneration, GA = Geographic atrophy, CGA = Central geographic atrophy, Logistic Regr = Logistic Regression.
Persons in Categories 2, 3, and 4 at baseline are at increasing risk of developing advanced AMD, as described in “Study Population.”
Progression to NV AMD
Cox proportional hazard analysis
The risk of NV AMD with cataract surgery among AMD Category 2, 3, and 4 participants (2880 right eyes and 2961 left eyes) expressed as a hazard ratio (HR) was (right eye) 1.20, 95%CI 0.82–1.75, (left eye) 1.07, 95%CI 0.72–1.58. The model included covariates gender and baseline smoking status, as well as time-dependent covariates age, AMD status, and cataract surgery. Among Category 2 and 3 participants HR = (right eye) 1.05, 95%CI 0.65–1.68, (left eye) 1.23, 95%CI 0.75–2.02. In Category 4 participants, RE+LE (all study eyes), HR = 1.08, 95%CI 0.65–1.77.
Matched-pair analysis
Among 722 matched pairs, 569 (79%) had no NV AMD events in matched follow-up. More surgery eyes (91) had NV AMD events before their matched control eyes than non-surgery eyes (61) had before their matched case eyes, R = 91/61 = 1.49, 95%CI 1.07–2.10.
Logistic Regression
There were 6037 eligible eyes with follow-up at the 5-year visit, and at that visit NV AMD had occurred in 22 (6.0%) of 364 eyes known to have had cataract surgery since baseline, and in 260 (4.6%) of 5673 eyes without surgery. The odds of NV AMD at 5 years follow-up, in eyes following cataract surgery compared to non-surgery eyes, adjusted for covariates, was OR = 0.76, 95%CI 0.44–1.30.
Progression to GA and CGA
Cox proportional hazard analysis
The risk of GA with cataract surgery among AMD Category 2, 3, and 4 participants (2964 right eyes and 3035 left eyes) expressed as a hazard ratio (HR) was (right eye) 0.80, 95%CI 0.61–1.06, (left eye) 0.94, 95%CI 0.71–1.25. The model included covariates gender and baseline smoking status, as well as time-dependent covariates age, AMD status, and cataract surgery. Among Category 2 and 3 participants HR = (right eye) 0.75, 95%CI 0.55–1.02, (left eye) 0.72, 95%CI 0.51–1.02. In Category 4 participants, RE+LE (all study eyes), HR = 1.22, 95%CI 0.82–1.80. The corresponding results for CGA were (Category 2, 3, and 4 participants) HR = (right eye) 0.87, 95%CI 0.64–1.18, (left eye) 0.86, 95%CI 0.63–1.19; (Category 2 and 3 eyes) HR = (right eye) 0.86, 95%CI 0.61–1.22, (left eye) 0.77, 95%CI 0.51–1.15; (Category 4 participants, RE+LE) HR = 0.98, 95%CI 0.64–1.49.
Matched-pair analysis
Among 713 matched pairs, 539 (76%) had no GA events in matched follow-up. Fewer surgery eyes (64) had GA events before their matched control eyes than non-surgery eyes (110) had before their matched case eyes, R = 64/110 = 0.58, 95%CI 0.42–0.80. Among 724 matched pairs, 597 (82%) had no CGA events in matched follow-up. Fewer surgery eyes (60) had CGA events before their matched control eyes than non-surgery eyes (67) had before their matched case eyes, R = 60/67 = 0.90, 95%CI 0.62–1.29.
Logistic Regression
There were 6127 eligible eyes with follow-up at the 5-year visit, and at that visit GA occurred in 14 (2.6%) of 539 eyes known to have had cataract surgery since baseline, and in 205 (3.7%) of 5588 eyes without surgery. The odds of GA at 5 years follow-up, in eyes following cataract surgery compared with non-surgery eyes, adjusted for covariates, was OR = 0.55, 95%CI 0.31–0.99. For CGA, OR = 0.68, 95%CI 0.33–1.41.
Discussion
The results from the Cox proportional hazards models used to examine AREDS data show little evidence of a detrimental effect of cataract surgery on progression to advanced AMD. Use of the Cox regression model allows us to take advantage of a unique feature of AREDS, the ability to examine the effect of important covariables, including time dependent covariables, on progression to late AMD. Results from the Cox models are not statistically significant and there are no consistent trends among models, with point estimates for risk of neovascular AMD in the harmful direction for the matched pair analyses and in the protective direction for the logistic regression analyses. For GA results, all models had point estimates in the direction of protection, suggesting that it is unlikely that there is any risk of cataract extraction on the development of GA. The absence of any consistent pattern in the direction of harm across models reinforces our conclusion that AREDS data provide little evidence that cataract surgery increases the risk of progression to late AMD.
Our results are contrary to the results of some previously published epidemiologic studies, including two reports that each pooled data from different population based studies. Combined cross-sectional data from the Salisbury Eye Evaluation, Proyecto VER, and the Baltimore Eye Survey found that “a history of cataract surgery may be associated with an increased prevalence of late AMD” (OR 1.7, 95%CI 1.1–2.6).11 In this study severe cataract was associated with a non-statistically significant increase in the prevalence of late AMD (OR 1.4, 95% CI 0.8–2.4). Exclusion of these persons with severe cataract from the reference (no surgery) group in the analyses may have influenced the finding of a positive association between history of cataract surgery and prevalence of late AMD. The authors noted the difficulties in establishing causal relationships with cross sectional studies and called for additional studies, citing the possible effects of residual confounding or bias on their results.
The second report pooled the 5-year results of the Beaver Dam Eye Study and the Blue Mountains Eye Study.12 They identified persons with and without a history of cataract extraction at a baseline examination and reexamined them for incident AMD at 5 and 10 years. They found an association between cataract surgery and the 5-year incidence of late AMD (NV AMD and GA) with an adjusted OR 5.7 (95%CI 2.4–13.6).12 Analyses from the individual studies after 10 years of follow-up showed that the relationships largely persisted. For the Beaver Dam Eye Study, cataract surgery before baseline was associated with an increased risk of advanced AMD (Relative Risk (RR) 3.81,95%CI 1.89–7.69), NV AMD (RR 4.31, 95%CI 1.71–10.9), and GA (RR 1.95, 95%CI 1.17–3.25).13 In the Blue Mountains Eye Study, there was a 3-fold increased risk of advanced AMD (RR 3.3, 95%CI 1.1–9.9) and NV AMD (RR 3.4, 95%CI 1.1–10.9) in nonphakic eyes compared with phakic eyes.14 No significant association for cataract surgery and the development of geographic atrophy was noted in the Blue Mountains Eye Study. Another population-based study conducted in Australia, the Visual Impairment Project, demonstrated cataract surgery was not associated with progression of AMD.21
We used a logistic regression model of 5-year incidence in an attempt to follow to some extent the analysis of the combined data from the Beaver Dam Eye Study and the Blue Mountains Eye Study. No statistically significant associations were noted for cataract surgery and occurrence of advanced AMD. In secondary adjusted analyses of their 10 year data both the Beaver Dam and the Blue Mountains Eye Studies found no statistically significant differences in the incidence of late AMD in eyes that underwent cataract surgery between the baseline and 5 year follow-up examinations compared with eyes that did not undergo cataract surgery (RR 1.62, 95% CI 0.60–4.40 for Beaver Dam and OR 0.82, 95%CI 0.26–2.59 for Blue Mountain)13,14.
There are many differences between our study and earlier studies that reported an association between cataract surgery and progression of AMD. We can only speculate on which of these differences might have caused the discrepant results. We studied a select population of volunteers that was at much higher risk of developing advanced AMD than was the case in the population based studies. Over 40% of our cohort had high risk characteristics for late AMD—at least 1 large druse, extensive intermediate drusen, or geographic atrophy (GA) that did not involve the center of the macula. The inclusion of such a high proportion of participants who already had demonstrated a propensity to develop AMD may have decreased our ability to detect additional risk factors, such as the impact of cataract surgery. Moreover, AREDS participants were volunteers for a clinical trial of nutritional supplements. Clinical trial volunteers are generally healthier and more health conscious than the overall population, characteristics that might make them less susceptible to a possible insult of cataract surgery on the macula.
Another possible difference in studies was how decisions to operate may have been made. In the communities that participated in the population-based studies, it is more likely that general ophthalmologists made the decision to operate, while the decision to proceed on AREDS participants was likely to be influenced by the retinal specialists who were examining the participants at regular intervals. As a result, participants in AREDS with subtle macular pathology may have been less likely to undergo surgery. In the Beaver Dam Eye Study the age-adjusted 5-year incidence of cataract surgery in right eyes was 3.5% in persons without early AMD and 6.5% in those with early stage AMD at baseline.13 This suggests that the presence of early AMD may influence the likelihood of surgery, perhaps because of a subtle effect on visual acuity. Unpublished ten year data showed no difference in cataract surgery rates in those who did or did not develop early AMD at the 5-year exam. However, the diagnosis of early AMD may have resulted in closer follow-up of macular status that made cataract surgery less likely.
Unadjusted confounding is another concern in epidemiological studies and is of particular concern in a study of the effect of cataract surgery on the occurrence of AMD. Cataract and AMD share some common risk factors, in particular increasing age, which is by far the most important factor for each condition. Some but not all studies have reported associations between the presence of cataract and AMD.2–5 In the 10 year data from the Beaver Dam Eye Study, cataract at baseline was associated with incidence of early AMD, soft indistinct drusen, increased retinal pigment and progression of AMD, all risk factors for late AMD, but not with late AMD itself.13 Lack of association with late AMD may have been due to low power to detect differences because of the small number of incident late AMD cases (41 right and 48 left eyes). A link between cataract and AMD or incomplete adjustment for important known or unknown covariates in our analyses or the analyses of others could have affected the findings or their interpretation. In our study, lens opacity status before cataract surgery showed no pattern of independent association with subsequent advanced AMD.
Another factor that might have resulted in differences between our results and the results from the pooled population based studies is the period that the cataract surgery had been conducted. Techniques in cataract surgery and types of lens replacement may have changed over time. Beaver Dam, and Blue Mountains Eye Study participants had cataract surgery prior to recruitment, before 1988–1990 for Beaver Dam and before 1992–1994 for the Blue Mountains Eye Study. Similarly, the Baltimore Eye Study and the Salisbury Eye Evaluation Studies participants had cataract surgery during an earlier period of time than AREDS participants.11 AREDS participants had their surgery sometime after the recruitment interval of 1992–1998. About a quarter of Beaver Dam Eye Study participants had aphakic cataract surgery.13 Ten year follow-up results from the Beaver Dam Study showed that eyes that were aphakic at baseline had considerably higher relative risks for most of the AMD end points (except for neovascular AMD) compared with eyes that had intraocular lenses (IOLs) at baseline, though rates for late AMD were still significantly higher for participants with IOL’s than participants without cataract surgery. Moreover AREDS participants, with their later occurring surgery, were probably more likely to have had UV-B blocking lenses inserted than persons who had lenses implanted earlier. Some have hypothesized that insertion of such lenses may decrease the risk of AMD by blocking UV-B exposure of the macula. Thus, the greater likelihood of extracapsular cataract extractions with insertion of UV blocking lenses in AREDS participants may explain some of the differences in findings. Some support for this possibility is provided from both the Beaver Dam Eye Study and the Blue Mountains Eye Studies that found, in secondary analyses, no significant difference in the incidence of late AMD in eyes that did and did not have surgery between the 5 and 10 year examinations.13,14 Such eyes had surgery at a later time than eyes with a history of cataract surgery at the baseline examination.
Our study had some major strengths. Stereo retinal photographs were taken in a standardized fashion at baseline and then annually after the first yearly visit. They were graded at a reading center which had a rigorous quality control program. Unlike some of the other studies, we were able to document macular status prior to cataract surgery. This is of importance in establishing the temporal relationship between development of macular lesions and occurrence of cataract surgery.
The large number of participants in AREDS, the inclusion of participants at increased risk of advanced AMD, and the long period of follow-up increased the study’s power to detect associations by providing a large number of cases with cataract surgery (about 1700) and incident advanced AMD (over 750). The population based studies had far fewer cases of each.
With the low losses to follow-up (2% during the entire clinical trial portion and 4% during the later nonintervention portion of AREDS, not including deaths) and the frequent participant contacts, information on both cataract surgery and progression to advanced AMD was captured for almost all of participants. The population-based studies had rates of loss to follow-up that ranged from almost 20 to 33%.
In the AREDS population that was carefully followed at frequent, regular intervals for a long period of time, it appears that cataract surgery was not associated with a clinically important increase in the rates of development of advanced AMD. However, it remains important that individuals with large drusen and pigmentary changes have an understanding of their risk for progression to advanced AMD with or without cataract surgery and that this risk is discussed with the patient prior to cataract surgery. Persons with intermediate AMD (bilateral large drusen) or with unilateral advanced AMD should be aware of the fact that the risk of developing advanced AMD is as high as 50% in 5 years 22, regardless of cataract surgery. Although the results of AREDS analyses suggest that this risk does not appear to be accelerated by cataract surgery, the patient should still be counseled as to the risk of development of advanced AMD because of the natural course of the disease.
In summary, the AREDS data suggest that there is no clinically important increased risk of progression to advanced AMD following cataract surgery. This is the only prospective study in which the severity of AMD was documented prior to and following cataract surgery in a large number of cases with more than 5 years of regular follow-up. These data, that are contrary to previously reported results, may provide some reassurance to patients with AMD who are considering cataract surgery.
Acknowledgments
The authors gratefully acknowledge the extensive statistical assistance of Molly Harrington, MA, Jonghyeon Kim, PhD, and Martin Ho, MS of the EMMES Corporation.
This report was supported by contracts from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD
The funding organization had no role in the design or conduct of this research.
Footnotes
No authors have any financial/conflicting interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Jensen SC, Cruickshanks KJ. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol. 1998;116:506–13. doi: 10.1001/archopht.116.4.506. [DOI] [PubMed] [Google Scholar]
- 3.Wang JJ, Mitchell PG, Cummings RG, Lim R. Cataract and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 1999;6:317–26. doi: 10.1076/opep.6.4.317.4182. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Wang Q, Moss SE. Is age-related maculopathy associated with cataracts? Arch Ophthalmol. 1994;112:191–6. doi: 10.1001/archopht.1994.01090140067025. [DOI] [PubMed] [Google Scholar]
- 5.Liu IY, White L, LaCroix AZ. The association of age-related macular degeneration and lens opacities in the aged. Am J Public Health. 1989;79:765–9. doi: 10.2105/ajph.79.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Schaft TL, Mooy CM, de Bruijin WC, et al. Increased prevalence of disciform macular degeneration after cataract extraction with implantation of an intraocular lens. Br J Ophthalmol. 1994;78:441–5. doi: 10.1136/bjo.78.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair CJ, Ferguson J., Jr Exacerbation of senile macular degeneration following cataract extraction. Am J Ophthalmol. 1979;87:77–83. doi: 10.1016/0002-9394(79)90195-8. [DOI] [PubMed] [Google Scholar]
- 8.Pollack A, Marcovich A, Bukelman A, Oliver M. Age-related macular degeneration after extracapsular cataract extraction with intraocular lens implantation. Ophthalmology. 1996;103:1546–54. doi: 10.1016/s0161-6420(96)30464-8. [DOI] [PubMed] [Google Scholar]
- 9.Sutter FK, Menghini M, Barthelmes D, et al. Is pseudophakia a risk factor for neovascular age-related macular degeneration? Invest Ophthalmol Vis Sci. 2007;48:1472–5. doi: 10.1167/iovs.06-0766. [DOI] [PubMed] [Google Scholar]
- 10.Armbrecht AM, Findlay C, Aspinall PA, et al. Cataract surgery in patients with age-related macular degeneration: one-year outcomes. J Cataract Refract Surg. 2003;29:686–93. doi: 10.1016/s0886-3350(02)01650-4. [DOI] [PubMed] [Google Scholar]
- 11.Freeman EE, Munoz B, West SK, et al. Is there an association between cataract surgery and age-related macular degeneration? Data from three population based studies. Am J Ophthalmol. 2003;135:849–56. doi: 10.1016/s0002-9394(02)02253-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang JJ, Klein R, Smith W, et al. Cataract surgery and the 5-year incidence of late-stage age-related maculopathy: pooled findings from the Beaver Dam and Blue Mountains eye studies. Ophthalmology. 2003;110:1960–7. doi: 10.1016/s0161-6420(03)00816-9. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Wong TY, et al. The association of cataract surgery with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2002;120:1551–8. doi: 10.1001/archopht.120.11.1551. [DOI] [PubMed] [Google Scholar]
- 14.Cugati S, Mitchell P, Rochtchina E, et al. Cataract surgery and the 10-year incidence of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2006;113:2020–5. doi: 10.1016/j.ophtha.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 15.de Jong PT, Lubsen J. The standard gamble between cataract extraction and AMD. Graefes Arch Clin Exp Ophthalmol. 2004;24:103–5. doi: 10.1007/s00417-003-0833-3. [DOI] [PubMed] [Google Scholar]
- 16.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS): design implications. AREDS report no. 1. Control Clin Trials. 1999;20:573–600. doi: 10.1016/s0197-2456(99)00031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study report no. 6. Am J Ophthalmol. 2001;132:668–81. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 18.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study (AREDS) system for classifying cataracts from photographs. AREDS report no. 4. Am J Ophthalmol. 2001;131:167–75. doi: 10.1016/s0002-9394(00)00732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Age-Related Eye Disease Study Research Group. Risk factors associated with age-related macular degeneration: a case-control study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study report no. 3. Ophthalmology. 2000;107:2224–32. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123:1484–98. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarty CA, Mukesh BN, Fu CL, et al. Risk factors for age-related maculopathy: the Visual Impairment Project. Arch Ophthalmol. 2001;119:1455–62. doi: 10.1001/archopht.119.10.1455. [DOI] [PubMed] [Google Scholar]
- 22.Age-Related Eye Disease Study Research Group. A simplified severity scale for age-related macular degeneration: AREDS report no. 18. Arch Ophthalmol. 2005;123:1570–4. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]