Abstract
Janus kinase 3 (JAK3) is a non-receptor tyrosine kinase vital to the regulation of T-cells. We report that JAK3 is a mediator of IL-8 stimulation of a different class of hematopoietic relevant cells: human neutrophils. IL-8 induced a time- and concentration-dependent activation of JAK3 activity in neutrophils and differentiated HL-60 leukemic cells. JAK3 was more robustly activated by IL-8 than other kinases: p70S6K, mTOR, MAPK or PKC. JAK3 silencing severely inhibited IL-8-mediated chemotaxis. Thus, IL-8 stimulates chemotaxis through a mechanism mediated by JAK3. Further, JAK3 activity and chemotaxis were inhibited by the flavonoid apigenin (4,5,7-trihydroxyflavone) at ~5 nM IC50. These new findings lay the basis for understanding the molecular mechanism of cell migration as it relates to neutrophil-mediated chronic inflammatory processes.
1. Introduction
The non-receptor tyrosine kinase Janus kinase 3 (JAK3) is vital to the regulation of T-cell signaling, lymphoid development and severe combined immunodeficiency (SCID) [1,2]. JAK3 is exclusively expressed in myeloid and lymphoid cell lines and in hematopoietic tissues like the thymus, spleen, bone marrow and fetal liver [3,4]. Mice lacking a catalytically intact JAK3 exhibit defects in B lymphocyte maturation and T lymphocyte activation [5,6]. Thymocytes and bone marrow progenitor cells from Jak 3−/− mice have decreased chemotactic responses to the chemokines CXCL12 and CCL25 [7]. Additionally, leukemic cells require an active JAK3 enzyme in order to be killed by small molecule tyrosine kinase inhibitors [8]. Similar to other JAK family kinases, JAK3 contains 7 JAK homology domains (JH) [9]. The JH1 domain is the putative kinase domain whose activity is regulated by tyrosine phosphorylation at Y980 and Y981. The pseudokinase JH2 domain is catalytically inactive and is rumored to interact with signal transducers and STAT proteins and negatively regulate the JH1 domain. The N-terminal JH6 and JH7 domains are implicated in binding to the gamma chain (γc) receptor and mutation at Y100 eliminates this interaction, which ultimately inhibits JAK3 activation. Additionally, JAK3 is implicated in signaling pathways of several cytokines that are involved in allergic airway disease/pulmonary inflammation (IL-2, -4, -7, -9 and -15) via phosphorylation of downstream STAT proteins [10–12], which directly links growth factor receptors to gene transcription.
Interleukin-8 (IL-8) is involved in several human diseases including inflammation, wound repair, angiogenesis, chronic obstructive pulmonary disease (COPD), atherosclerosis and cancer metastasis, and its primary target is induction of chemotaxis in granulocytic neutrophils and lymphocytes [13–18]. Besides inducing chemotaxis, IL-8 also induces changes in cytosolic calcium, neutrophil lipid metabolism, exocytosis and recruits neutrophils by binding and activating specific receptors, termed Cys-X-Cys-R (CXCR) -1 and -2 [19–23]. IL-8 mediated cell migration begins with polarization of neutrophils in the direction of the inflammation site followed by chemotaxis towards host-or pathogen-derived chemoattractants [24]. Neutrophils from stage I COPD patients have normal responses to IL-8, but in the more advanced stages of disease (II-IV), neutrophils showed markedly reduced spontaneous migration and chemotaxis in response to IL-8 [25].
To date, there has been no analysis of JAK3 kinase activity of stimulated human polymorphonuclear neutrophils (PMN). Here, we have determined the effect of IL-8-mediated activation of JAK3 in human PMN and in the neutrophil-like differentiated HL-60 cells (dHL-60) and found that JAK3 is robustly involved in IL-8-iduced chemotaxis. Additionally, we are also demonstrating a potent effect of the flavonoid apigenin in neutrophil and HL-60 cell motility.
2. Materials and Methods
2.1. Chemicals
Human IL-8 was from R & D Systems (Minneapolis, MN). Myelin basic protein (MBP) to be used as the MAPK substrate and S6 Kinase (RsK2) substrate peptide 2 (KKRNRTLTV) were from Millipore (Temecula, CA). The PKC substrate (QKRPSQRSKYL) and JAK3tide substrate (GGEEEEYFELVKKKK) were from Upstate (Lake Placid, NY). Apigenin was from Sigma (St. Louis, MO).
2.2. Isolation of Peripheral Blood Neutrophils and HL-60 Differentiation (dHL-60)
Neutrophils were isolated from peripheral blood of human donors who had signed an IRB-approved consent form similar to [26] and were estimated to be >95% pure. HL-60 cells were maintained in Iscove’s DMEM containing 40% fetal calf serum, 2 mM L-glutamine and penicillin/streptomycin. Cell density was maintained between 1–2 × 106 cells/ml. HL-60s were differentiated (dHL-60) for 4 days using 1.75% (v/v) DMSO in the complete growth media in order to achieve the expression of the neutrophilic phenotype. Both neutrophils and dHL-60 cells were ultimately each resuspended in HBSS at a concentration 1.5 × 106 cells/ml for use in chemotaxis assays or 1 × 107 cells/ml for use in both PLD and kinase assays.
2.3. dsRNA Transfection of dHL-60 Cells
Twenty-four hr after induction of differentiation, HL-60 cells were transfected with 300 NM dsRNA’s using nucleofection per the manufacturer’s protocol (Amaxa, Gaithersburg, MD). Fresh DMSO to 1.75% (v/v) was added to the media post-nucleofection, and cells were cultured for an additional 72 hr period. For JAK silencing, we used a “Selected validated” dsRNA from Applied Biosystems (Foster City, CA) that targeted exon 19; sense sequence: 5′-GUAUCGUGGUGUCAGCUAUtt-3′. For PKC silencing, we used a dsRNA from Santa Cruz Biotechnology (Santa Cruz, CA) that targeted 5 different exons specific for the PKC isoforms α, β, δ, μ and ι. The sequences target the following 5 regions: ACCAAGCAGAAGACCAACA; CACUGCACCGACUUCAUCU; UCAGUCCAUCAACAAGCAA; GGGAUGUGCAAAGAGAACA and CAGAGAAGCACGUGUUUGA. A negative control for all silencing was 100 nM siRNA “Neg-siRNA#2”, purchased from Applied Biosystems. This control siRNA is a 19 bp scrambled sequence with 3′ dT overhangs (sequence not disclosed by Applied Biosystems) certified not to have significant homology to any known gene sequences from mouse, rat or human and causes no significant changes in gene expression of transfected cells after 48 hrs at the same concentration as the dsRNA in test.
2.4 Chemotaxis Assays
Neutrophils or dHL-60s were resuspended at a concentration 1.5 × 106 cells/ml in chemotaxis buffer (HBSS + 0.5 % bovine serum albumin). 200 μl cells were applied to the upper chamber of 5 μm Transwells (24-well format) with a 6.5 mm diameter membrane. Twenty nM IL-8 in 500 μl of chemotaxis buffer was placed in the lower wells. Reactions were incubated for 1 hr at 37 °C in a 5 % CO2 cell culture incubator. Results were quantified in triplicate as number of cells migrated per insert.
2.5 Kinase Assays
Approximately 5 × 106 human neutrophils or dHL-60 cells were incubated with 20 nM IL-8 for 14 min at 37 °C. After stimulation, cells were sedimented, washed and finally lysed via sonication in 20 μl SLB containing protease inhibitors. Each lysate was incubated in the presence of the following final concentration of each: 8.25 mM HEPES, pH 7.5, 18.75 mM MgCl2, 1.25 mM EGTA, 18.75 μM Na Orthovanadate, 3.125 μM p-nitrophenylphosphate (PNPP), 0.625 μCi [32Pγ]-ATP, 40 μM cold ATP and the relevant kinase substrate to yield a 40 μl total kinase reaction volume. Cell lysates were immunoprecipitated with an antibody at 1 μg/μl intended to be used for the kinase assay (i.e., with anti-JAK3 for JAK3 kinase assay or with anti-PKC for a PKC kinase assay). For the specific isoforms of PKC, each sample was incubated with 10 μg of anti-myc-agarose, anti-PKCα-agarose, anti-PKCβ-agarose or anti-PKCδ-agarose at 4 °C for 2 hr using a tube rotisserie. Each immunoprecipitate was washed and resuspended into 2 × 30 μl volumes of SLB and used in the kinase assays as listed above using no peptide substrate or the PKC peptide substrate only. To further account for specificity, each kinase reaction contained a relevant, specific kinase substrate. The concentration of those are as follows: no peptide negative control, 500 μM MBP as the MAPK substrate, 62.5 μM S6 Kinase substrate peptide-2, 10 μM active MAPK for mTOR, 62.5 μM PKC substrate and 42 μM JAK3tide substrate. All kinase reactions were incubated at 30 °C for 20 min. Reactions were stopped by spotting 20 μl reactions onto 2 × 2.5 cm2 pieces of P81 Whatman filter paper for duplicate determinations. After washing all samples in filters were counted in a Beckman LS 6000TA liquid scintillation counter using the [32P] protocol for 1 min each. Results were quantified as DPMs and expressed in terms of –fold activation.
2.6 Phosphorylation of H2B
Immunoprecipitations with anti-PKCα antibodies were conducted similar to [27]. After immunoprecipitation, kinase assay was performed by incubating anti-PKCα-agarose for 1 h at 37°C in the presence of 20 μl kinase assay buffer (25 mM HEPES, pH 7.3, 10 mM MnCl2, 1 mM MgCl2, 1 mM DTT, 0.5 mM cold ATP and 5 μCi [γ-32P] ATP. To each reaction, 5 μg of histone H2B was added as exogenous substrate. Reactions were stopped by the addition of 10 μl 5× Laemmli buffer. Samples were boiled for 5 min and loaded onto a SDS-polyacrylamide gel.
2.7 Conditions for inhibition with apigenin
Approximately 5 × 106 human neutrophils or dHL-60 cells were incubated with the appropriate concentration of apigenin (in 1,000-concentrated stock) for 20 min in a 37°C water bath with vigorous shaking. After incubation with the inhibitor, cells were either mock-treated or treated with 20 nM L-8 for 14 min at 37 °C. After stimulation, cells were sedimented and washed and lysed via sonication in 200 μl SLB containing protease inhibitors.
2.8 Statistical Analysis
Data are presented as the mean + S.E. The difference between means was assessed by the single factor analysis of variance (ANOVA) test. Probability of p < 0.05 was considered to indicate a significant difference.
3. Results
3.1. IL-8 Stimulates JAK3 Activity of Human PMN and Leukemic Cells
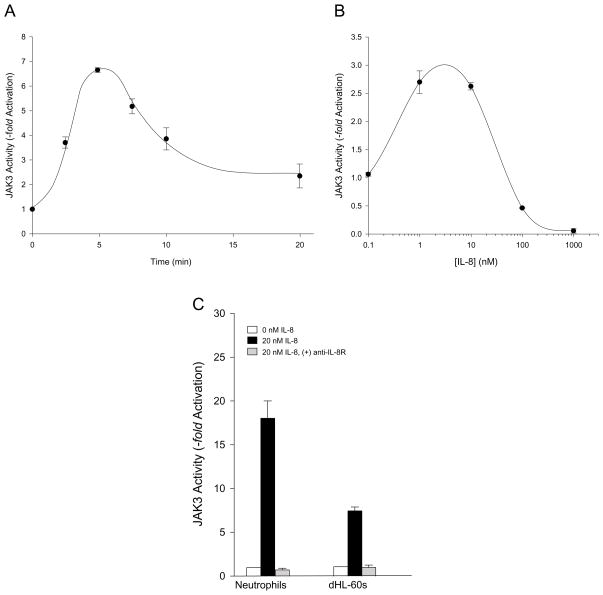
IL-8 stimulates the activity of JAK3 in human neutrophils in a time (Fig. 1A) and concentration dependent (Fig. 1B) fashion. Maximal phosphorylation of the JAK3 peptide substrate occurred at 5–7 min when using 20 nM IL-8 (Fig. 1A). Additionally, between 1–10 nM IL-8 provided optimal JAK3 phosphorylation at 7 min of time (Fig. 1B). The combination of immunoprecipitation with anti-JAK3 antibodies and the use of the JAK3 specific synthetic peptide substrate, GGEEEEYFELVKKKK, made it possible for us to ascertain that the isoform being conserved is indeed JAK3 (over JAK1 or JAK2). JAK3tide as an exclusive peptide substrate for JAK3 has been utilized by other authors [28], as it is phosphorylated by JAK3 at Tyr-7 and not by any other JAK family kinase.
Fig. 1. IL-8 stimulates JAK3 activity of human neutrophils and differentiated HL-60 cells.
Neutrophils were incubated without or with 20 nM IL-8, lysates prepared, immunoprecipitated with specific anti-JAK3 antibodies and JAK3 kinase reactions performed as in Materials and Methods. (A) Time and concentration (B) response curves of JAK3 activity in neutrophils. (C) Effect of anti-IL-8R antibodies preincubated with 20 nM IL-8 prior to activation of JAK3 activity in neutrophils and dHL-60 cells.
We next compared the effect of IL-8 on JAK3 kinase activity between freshly isolated neutrophils and differentiated HL-60 that express the neutrophilic phenotype. We wanted to do this because subsequent experiments used molecular biology approaches (silencing with dsRNA and incubation for several days) that are not possible to evaluate with short-lived neutrophils. As indicated in Fig. 1C, IL-8 was able to stimulate the activity of JAK3 in neutrophilic dHL-60 albeit at a lower extent than that seen in fresh neutrophils, but still in a significant way over controls. When IL-8 was preincubated with antibodies to the IL-8 receptor (IL-8R) and subsequently utilized in the stimulation of cells, JAK3 phosphorylation was completely abrogated in both neutrophils and dHL-60s (Fig. 1C), indicating that the stimulation effect is mediated by the receptor of IL-8 specifically. Thus, we conclude that we are documenting a novel finding that JAK3 is present in neutrophils and can be specifically activated in vitro by IL-8 in both human neutrophils and differentiated leukemic dHL-60 cells.
3.2 IL-8-stimulated chemotaxis is mediated by JAK3
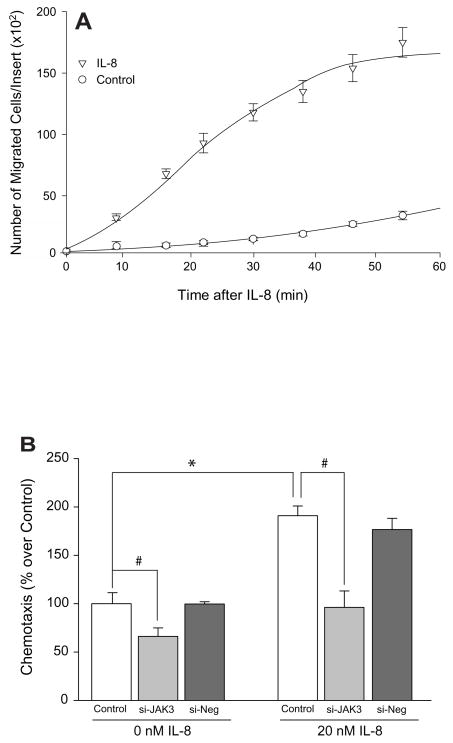
As known, IL-8 is a potent chemoattractant for neutrophils. Fig. 2A shows that it is also a chemottractant for neutrophilic dHL-60 leukemic cells. The number of cells that migrated in response to 20 nM IL-8 increased in proportion to increasing time of migration (open triangles) compared to the non-stimulated cells (open circles). As a negative control, Fig. 2A also shows IL-8-independent cell migration, chemokinesis, was detected in these cells but to a very limited and small extent (only about 20% of IL-8 stimulated cells at maximal time) (circles). Importantly, this effect was dependent on JAK3, as silencing with siRNA specific for JAK3 inhibited both basal chemokinesis by ~30% (Fig. 2B, left group of bars) and IL-8 activated chemotaxis by ~50% (Fig. 2B, right group of bars). This serves to indicate that IL-8-induced chemotaxis has a strong component that relies on JAK3 for intracellular signaling.
Fig. 2. IL-8-stimulated dHL-60 cell chemotaxis is mediated by JAK3.
(A) Time Course of IL-8-stimulated chemotaxis. Number of migrated cells/Transwell insert was measured as time elapsed from the addition of 20 nM IL-8 to the lower chambers of Transwell inserts. Results are the mean ± S.E. from three fields of view at 20× magnification. Control, untreated cells (open circles), cells treated with 20 nM IL-8 only (open triangles). (B) Effect of silencing JAK3 with dsRNA for fours days on dHL-60 cell chemotaxis. Si-Neg is a scrambled dsRNA.
3.3 JAK3 activity is inhibited by Apigenin
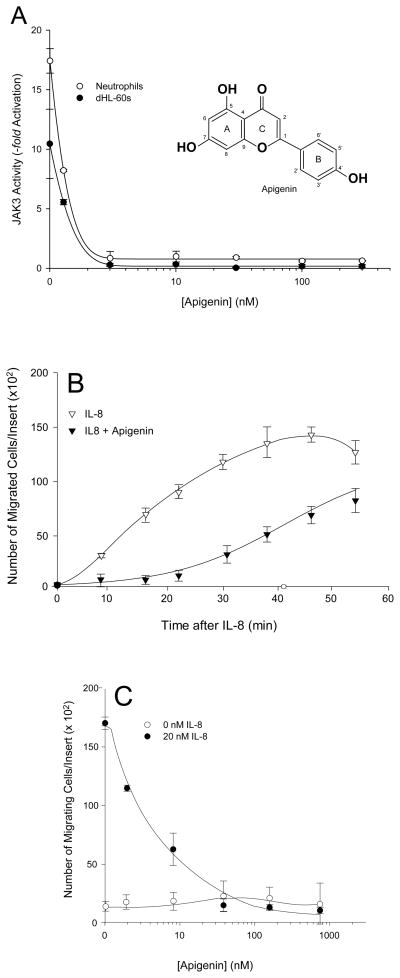
Next, we endeavored to further manipulate the IL-8-stimulated kinase activity with a small molecule inhibitor compound that could inhibit tyrosine kinases. We chose to use 4′,5,7-trihydroxyflavone (apigenin, Fig. 3A, inset) a plant polyphenol, flavonoid aglycone derived from green leafy vegetables [29–32]. Apigenin mediates inhibition of tyrosine kinase activities via interaction with the ATP-binding site of the specific kinase, which results in suppression of oncogene expression [27,33–40]. It also inhibits cell proliferation by arresting the cell cycle at G2/M phase [41–44]. Using either neutrophils or dHL-60 cells, we show the positive effect of 20 nM IL-8 stimulation on JAK3 (Fig. 3A) activity in the absence of apigenin. Following incubation with increasing concentrations of apigenin, JAK3 phosphorylation activity drastically decreased to near baseline in both cell models. IC50 apigenin concentrations for JAK3 were 1.3 nM for neutrophils and 1.1 nM for dHL-60 cells. Although an IC50 concentration of apigenin for JAK3 has not been reported to date by any other group, the IC50 concentration of apigenin for immunopurified phosphatidylinositol-3-kinase (PI3K) from human blood platelets has been determined to be much higher at 12 μM [36].
Fig. 3. Apigenin is a powerful inhibitor of both JAK3 activity and chemotaxis.
(A insert) Schematic drawing of apigenin structure. Neutrophils or dHL-60 cells were treated with increasing apigenin for 20 min at 37 °C followed by 20 nM IL-8 stimulation for 10 min at 37°C. (A) Effect of apigenin on JAK3 activity of neutrophils (open circles) or dHL-60 cells (filled circles). (B) Time Course of IL-8-stimulated dHL-60 cell chemotaxis in the presence (filled triangles) or absence (open triangles) of apigenin. (C) Concentration dependent response of apigenin concentrations and effect on neutrophil chemotaxis. Unstimulated neutrophils (open circles), neutrophils stimulated with 20 nM IL-8 (filled circles).
After measuring the effect of apigenin on JAK3 in vitro, we found that apigenin could inhibit IL-8-mediated dHL-60 cell chemotaxis in a time dependent manner, as evidenced in Fig. 3B. The number of cells that migrated in response to 20 nM IL-8 increased in proportion to increasing time of migration (open triangles). However, in the presence of 50 nM apigenin, IL-8-mediated cell migration was reduced by >70%, specifically at early times (10–30 min of chemotaxis) (filled triangles).
Next, we performed a concentration dependent experiment to ascertain the IC50 of apigenin in vitro during chemotaxis (Fig. 3C). Using increasing concentrations of apigenin up to 1 μM, we determined the IC50 concentration of apigenin to be 20 nM for human neutrophils cells (Fig. 3C) in response to 20 nM IL-8. The decrease in chemotaxis was drastic and significant. Additionally, the IC50 value for neutrophils was in the subnanomolar range indicating the extreme potency of apigenin to inhibit cell migratory functions. We also determined the IC50 concentration of apigenin to be 3.5 nM for dHL-60 cells in response to 20 nM IL-8 (data not shown). We report the effect of subnanomolar concentrations of apigenin on cell migration, while other groups have reported similar negative effects on cell migration assays but at ~1000-fold more apigenin (submicromolar range)Add refs. These data suggest apigenin is a powerful inhibitor of chemoattractant-mediated cell migrations.
3.4. IL-8 Stimulates other kinases: PKCα and PKCδ,albeit at a lower extent than JAK3
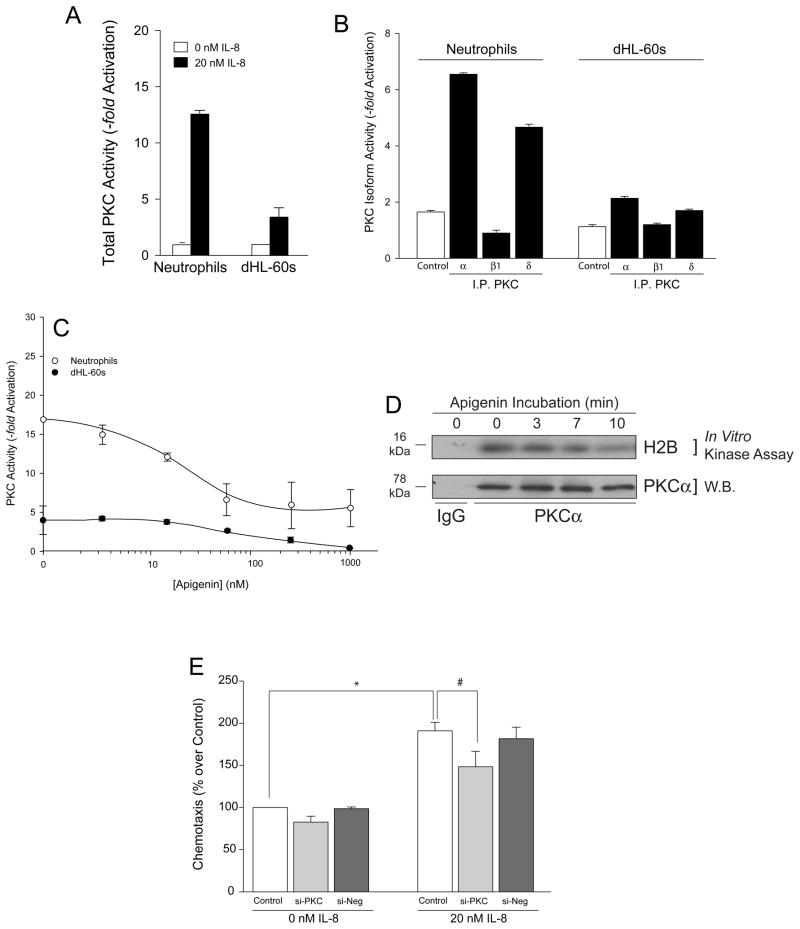
We have shown here for the first time that IL-8 stimulates JAK3 kinase activity in human neutrophils and the neutrophil-like human promyelocytic dHL-60 cells. Additionally, we wanted to determine how specific the effect of IL-8 stimulation was on JAK3. We performed similar kinase assays as those utilized for JAK3 (immunoprecipitation using specific kinase substrates as indicated in Material and Methods) and found that other kinases were activated by IL-8 in neutrophils; all but PKC exerted a lower extent of activation than JAK3. Further, we calculated the neutrophilic IC50 concentrations for apigenin inhibition for other kinases as follows: MAPK (1 μM), S6K (14 μM), mTOR (1 μM) and PKC (20 nM for neutrophils, ~50 nM for dHL-60 cells). All are 1–2 orders of magnitude greater than that of JAK3 as reported here.
Out of the four kinases, both the enhancing effect of IL-8 mediated by PKC and the inhibition by apigenin were somewhat close to that observed with JAK3, and we further explored PKC. Fig. 4A shows that the effect of IL-8 is in the range of what we have observed for JAK3 (Fig. 1C) albeit slightly lower. We next investigated which specific PKC subunits were involved in IL-8 signaling in human PMN and leukemic cells. Cell lysates were prepared and immunoprecipitated using antibodies specific for α-myc (a negative control reaction) and three different subunits of PKC: PKCα, PKCβ1 and PKCδ. As indicated in Fig. 4B, PKCα and PKCδ were detected in both IL-8-stimulated neutrophils and dHL-60 cells. PKCδ and PKCα were about 3.5-fold greater in neutrophils and 1.5-fold greater in dHL-60 cells when each was compared to their respective negative controls, respectively. Peak activity of each kinase was achieved at 10–14 min of incubation time (data not shown). In general, PKCα and PKCδ activities were more robustly measured in human neutrophils when compared to dHL-60 cells. PKCβ1 kinase activity was equal to or fell below that of the negative control α-myc immunoprecipitates and, therefore, was not readily quantifiable in either cell line using our experimental design.
Fig. 4. IL-8 also activates PKC, albeit at a lower extent that it does JAK3.
(A) IL-8 stimulates total PKC activity of human neutrophils and differentiated HL-60 cells. Cells were incubated without or with 20 nM IL-8, lysates prepared and immunoprecipitated and PKC kinase reactions performed as in Materials and Methods. (B) Neutrophil and dHL-60 cell lysates were immunoprecipitated with anti-myc-agarose (negative control) or with α-PKCα-, α-PKCβ1- or α-PKCδ-agarose and used in the PKC kinase assay. (C) Effect of apigenin on PKC activity of neutrophils (open circles) and dHL-60 cells (filled circles). (D) dHL-60 cell lysates were immunoprecipitated with anti-PKCα antibodies or IgG control and subjected to in vitro kinase assay using H2B as substrate in the presence of [γ-32P] ATP. Kinase reactions were resolved by SDS-PAGE, western-blot transferred and products visualized using autoradiography. Autoradiograph of increasing time of incubation with apigenin on PKCα phosphorylation of H2B (top panel). The same membrane was immunoblotted with anti-PKCα antibody to ensure equal protein loading control (bottom panel). (E) Effect of silencing PKC with dsRNA for fours days on dHL-60 cell chemotaxis. Si-Control is a scrambled dsRNA.
PKC phosphorylation activity decreased as well in the presence of apigenin but not to the same extent as JAK3 activity (Fig. 4C). IC50 apigenin concentrations for total PKC activity were 15 nM for neutrophils (open circles) and 40-fold greater (600 nM) for dHL-60 cells (filled circles). Previously, it has been reported by Huang et al. that the IC50 concentration of apigenin for PKC is 10 μM in murine embryonic fibroblasts [37], The data presented here indicate that neutrophils are more sensitive to apigenin than fibroblasts [36,37]. As PKCα was the PKC isoform that was activated to the greatest extent in both neutrophils and dHL-60 cells, we chose to determine the level of PKCα activity of an actual downstream protein substrate and not a synthetic peptide substrate (histone-2B, H2B). As shown in Fig. 4D, PKCα was able to phosphorylate the protein substrate H2B when stimulated with IL-8. When in the presence of 50 nM apigenin, markedly less phosphorylation of H2B by PKCα was evident. After 10 min of incubation with apigenin, H2B phosphorylation by PKCα decreased by more than 50% when compared to the PKCα loading control.
Lastly, the PKC-mediated component of PKC activation does not seem to play as important a role in chemotaxis as does JAK3, as silencing with dsRNA specific for PKC had a marginal effect on chemotaxis (Fig. 4E). Silencing with siRNA specific for PKC inhibited basal chemokinesis by only ~15% (Fig. 4E, left group of bars) and IL-8 activated chemotaxis by ~20% (Fig. 4E, right group of bars).
4. Discussion
The non-receptor tyrosine kinase JAK3 is vital to the regulation of T-cell signaling, lymphoid development and severe combined immunodeficiency (SCID), which contributes to it being considered a potential and worthwhile pharmacological target. Recently, JAK3 has specifically been implicated in myeloid cell development, as mutations to JAK3 have been found in acute megakaroblastic leukemia [45]. Thymocytes and bone marrow progenitor cells from Jak 3−/− mice have decreased chemotactic responses to the chemokines CXCL12 and CCL25 [7]. The N-terminal FERM and possibly the SH2-like domains of JAK proteins regulate catalytic activity and/or are involved in binding to and association with relevant cytokine receptors and are considered vital to the initial steps involved in cytokine-mediated signaling [9,46,47]. The conversion of the extracellular binding signal following binding to the receptor of interest into a cascade of tyrosine kinase activity is mediated predominantly by JAK proteins. JAK3 is specifically implicated in signaling pathways of several interleukin cytokines that are involved in allergic airway disease/pulmonary inflammation via phosphorylation of downstream STAT proteins [10–12].
It has been shown that airway epithelial cells secrete various chemokines, cytokines, extracellular matrix proteins and lipid mediators, one of which is IL-8 that is already known to attract neutrophils and other hematopoietic cells to the site of inflammation [48–50]. IL-8 is involved in several human diseases including inflammation, and its primary target is induction of chemotaxis in granulocytic neutrophils and leukocytes, which are appropriate cell motility models for inflammation, tumor migration or wound healing. JAK3 has been found to have a pivotal role in mast cell-mediated bacterial clearance and neutrophil recruitment via regulation of TNF from mast cells [51].
Unlike G-CSF which cannot activate JAK3 in human neutrophils [52], we have shown for the first time that JAK3 can be correlated to human neutrophilic function in a time and concentration dependent manner and via the IL-8 signal transduction pathway, which implicates a role for JAK3 in IL-8-mediated inflammatory processes and cancer. Upon further determination, both PKCα and PKCδ were found to be IL-8-activated to a greater extent in neutrophilic lysates than in leukemic cell lysates following immunoprecipitation with antibodies specific to either kinase. JAK3 is more robustly activated in neutrophils by IL-8 than other kinases like PKC, MAPK, mTOR or p70S6K, which further implicates a JAK3 mechanism in IL-8-stimulated chemotaxis.
We have shown here JAK3 and PKCα phosphorylation activities in both neutrophils and leukemic cells following IL-8 stimulation are negatively affected by apigenin action. Additionally, we have associated the presence of nanomolar concentrations of apigenin to negative effects on random and chemoattractant-stimulated cell migration of neutrophils and leukocytes in a time and concentration dependent manner. It has been determined that submicromolar concentrations of apigenin suppressed by a factor of approximately 9-fold the release or production of proinflammatory cytokines, including IL-8, in human monocytes [53]. Apigenin has been found to severely hinder cell migratory processes, particularly adhesion, chemotaxis, invasion and metastasis in ovarian, breast, melanoma lung, cervical and prostate cancers [54–59]. Conventional wisdom suggests that apigenin is a pleiotropic effector that affects protease-dependent invasiveness and associated processes, proliferation of tumor cells and actin cytoskeleton organization, which exerts an anti-tumorigenic effect in vivo via inhibition of tumor cell penetration into healthy tissue.
In conclusion, our results provide documentation of the novel effect of IL-8-stimulated JAK3 activation in human neutrophils and leukemia. We have documented a previously defined molecular mechanism relevant to inflammation and cancer that also uniquely regulates JAK3. We have demonstrated that apigenin interferes with IL-8-mediated chemotaxis of human neutrophils and leukocytes that can be correlated to suppression of certain tyrosine kinases, namely JAK3, PKCα and PKCδ.
Additionally, we have been able to correlate a role for JAK3 in leukemic cell migration as transfection of JAK3 siRNA into dHL-60 cells severely inhibited chemotactic cells in response to IL-8. As apigenin effectively binds to JAK3 and suppresses this kinase moiety, information presented here could be used to design new chemotherapeutic strategies against IL-8-mediated inflammation and leukemia, potentially including use of the IL-8 receptor.
Acknowledgments
We thank Dr. Larry Ream for providing technical assistance. The grant HL056653 (J.G.-C.) from the National Institutes of Health has supported this work and NHLBI-HL 075040 (A.I.D.)
Abbreviations
- JAK3
Janus kinase 3
- IL-8
interleukin-8
- dHL-60
differentiated HL-60 cells
- MBP
myelin basic protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russell SM, et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994;266:1042–5. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- 2.Russell SM, et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 3.Rane SG, Reddy EP. JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene. 1994;9:2415–23. [PubMed] [Google Scholar]
- 4.Musso T, Johnston JA, Linnekin D, Varesio L, Rowe TK, O’Shea JJ, McVicar DW. Regulation of JAK3 expression in human monocytes: phosphorylation in response to interleukins 2, 4, and 7. J Exp Med. 1995;181:1425–31. doi: 10.1084/jem.181.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–7. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 6.Nosaka T, et al. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–2. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 7.Soldevila G, Licona I, Salgado A, Ramirez M, Chavez R, Garcia-Zepeda E. Impaired chemokine-induced migration during T-cell development in the absence of Jak 3. Immunology. 2004;112:191–200. doi: 10.1111/j.1365-2567.2004.01863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudbeck EA, Liu XP, Narla RK, Mahajan S, Ghosh S, Mao C, Uckun FM. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clin Cancer Res. 1999;5:1569–82. [PubMed] [Google Scholar]
- 9.Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–37. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- 10.Changelian PS, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–8. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 11.Behbod F, et al. Concomitant inhibition of Janus kinase 3 and calcineurin-dependent signaling pathways synergistically prolongs the survival of rat heart allografts. J Immunol. 2001;166:3724–32. doi: 10.4049/jimmunol.166.6.3724. [DOI] [PubMed] [Google Scholar]
- 12.Cortes JR, Perez GM, Rivas MD, Zamorano J. Kaempferol inhibits IL-4-induced STAT6 activation by specifically targeting JAK3. J Immunol. 2007;179:3881–7. doi: 10.4049/jimmunol.179.6.3881. [DOI] [PubMed] [Google Scholar]
- 13.Proost P, Wuyts A, van Damme J. The role of chemokines in inflammation. Int J Clin Lab Res. 1996;26:211–23. doi: 10.1007/BF02602952. [DOI] [PubMed] [Google Scholar]
- 14.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–21. [PubMed] [Google Scholar]
- 15.Benelli R, et al. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. Faseb J. 2002;16:267–9. doi: 10.1096/fj.01-0651fje. [DOI] [PubMed] [Google Scholar]
- 16.Beeh KM, Beier J, Kornmann O, Buhl R. Neutrophilic inflammation in induced sputum of patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:138–43. [PubMed] [Google Scholar]
- 17.Wang JM, et al. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1166–74. doi: 10.1161/01.atv.11.5.1166. [DOI] [PubMed] [Google Scholar]
- 18.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 19.Sozzani S, Agwu DE, Ellenburg MD, Locati M, Rieppi M, Rojas A, Mantovani A, McPhail LC. Activation of phospholipase D by interleukin-8 in human neutrophils. Blood. 1994;84:3895–901. [PubMed] [Google Scholar]
- 20.Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, Strieter RM. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174:1355–62. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudack C, Jorg S, Sachse F. Biologically active neutrophil chemokine pattern in tonsillitis. Clin Exp Immunol. 2004;135:511–8. doi: 10.1111/j.1365-2249.2003.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 23.Nufer O, Corbett M, Walz A. Amino-terminal processing of chemokine ENA-78 regulates biological activity. Biochemistry. 1999;38:636–42. doi: 10.1021/bi981294s. [DOI] [PubMed] [Google Scholar]
- 24.Lehman N, Di Fulvio M, McCray N, Campos I, Tabatabaian F, Gomez-Cambronero J. Phagocyte cell migration is mediated by phospholipases PLD1 and PLD2. Blood. 2006;108:3564–72. doi: 10.1182/blood-2006-02-005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshikawa T, Dent G, Ward J, Angco G, Nong G, Nomura N, Hirata K, Djukanovic R. Impaired neutrophil chemotaxis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:473–9. doi: 10.1164/rccm.200507-1152OC. [DOI] [PubMed] [Google Scholar]
- 26.Lehman JA, Paul CC, Baumann MA, Gomez-Cambronero J. MAP kinase upregulation after hematopoietic differentiation: role of chemotaxis. Am J Physiol Cell Physiol. 2001;280:C183–91. doi: 10.1152/ajpcell.2001.280.1.C183. [DOI] [PubMed] [Google Scholar]
- 27.Vargo MA, Voss OH, Poustka F, Cardounel AJ, Grotewold E, Doseff AI. Apigenin-induced-apoptosis is mediated by the activation of PKCdelta and caspases in leukemia cells. Biochem Pharmacol. 2006;72:681–92. doi: 10.1016/j.bcp.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Nara H, Rahman M, Juliana FM, Araki A, Asao H. Impaired IL-7 signaling may explain a case of atypical JAK3-SCID. Cytokine. 2010;49:221–8. doi: 10.1016/j.cyto.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Hempel J, Pforte H, Raab B, Engst W, Bohm H, Jacobasch G. Flavonols and flavones of parsley cell suspension culture change the antioxidative capacity of plasma in rats. Nahrung. 1999;43:201–4. doi: 10.1002/(SICI)1521-3803(19990601)43:3<201::AID-FOOD201>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Phippen WB, Simon JE. Anthocyanin inheritance and instability in purple basil (Ocimum basilicum L.)] J Hered. 2000;91:289–96. doi: 10.1093/jhered/91.4.289. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Simon JE, Aviles IF, He K, Zheng QY, Tadmor Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.) J Agric Food Chem. 2003;51:601–8. doi: 10.1021/jf020792b. [DOI] [PubMed] [Google Scholar]
- 32.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–12. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 33.Kim JY, Kwon EY, Lee YS, Kim WB, Ro JY. Eupatilin blocks mediator release via tyrosine kinase inhibition in activated guinea pig lung mast cells. J Toxicol Environ Health A. 2005;68:2063–80. doi: 10.1080/15287390500177024. [DOI] [PubMed] [Google Scholar]
- 34.Ahn BH, Min G, Bae YS, Min DS. Phospholipase D is activated and phosphorylated by casein kinase-II in human U87 astroglioma cells. Exp Mol Med. 2006;38:55–62. doi: 10.1038/emm.2006.7. [DOI] [PubMed] [Google Scholar]
- 35.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 36.Agullo G, Gamet-Payrastre L, Manenti S, Viala C, Remesy C, Chap H, Payrastre B. Relationship between flavonoid structure and inhibition of phosphatidylinositol 3-kinase: a comparison with tyrosine kinase and protein kinase C inhibition. Biochem Pharmacol. 1997;53:1649–57. doi: 10.1016/s0006-2952(97)82453-7. [DOI] [PubMed] [Google Scholar]
- 37.Huang YT, Kuo ML, Liu JY, Huang SY, Lin JK. Inhibitions of protein kinase C and proto-oncogene expressions in NIH 3T3 cells by apigenin. Eur J Cancer. 1996;32A:146–51. doi: 10.1016/0959-8049(95)00540-4. [DOI] [PubMed] [Google Scholar]
- 38.Geahlen RL, Koonchanok NM, McLaughlin JL, Pratt DE. Inhibition of protein-tyrosine kinase activity by flavanoids and related compounds. J Nat Prod. 1989;52:982–6. doi: 10.1021/np50065a011. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita S, Yamashita T, Yamada K, Tachibana H. Flavones suppress type I IL-4 receptor signaling by down-regulating the expression of common gamma chain. FEBS Lett. 2010;584:775–9. doi: 10.1016/j.febslet.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 40.Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. Faseb J. 2005;19:342–53. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 41.Plaumann B, Fritsche M, Rimpler H, Brandner G, Hess RD. Flavonoids activate wild-type p53. Oncogene. 1996;13:1605–14. [PubMed] [Google Scholar]
- 42.Reiners JJ, Jr, Clift R, Mathieu P. Suppression of cell cycle progression by flavonoids: dependence on the aryl hydrocarbon receptor. Carcinogenesis. 1999;20:1561–6. doi: 10.1093/carcin/20.8.1561. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–10. [PubMed] [Google Scholar]
- 44.Sato F, Matsukawa Y, Matsumoto K, Nishino H, Sakai T. Apigenin induces morphological differentiation and G2-M arrest in rat neuronal cells. Biochem Biophys Res Commun. 1994;204:578–84. doi: 10.1006/bbrc.1994.2498. [DOI] [PubMed] [Google Scholar]
- 45.Malinge S, et al. Activating mutations in human acute megakaryoblastic leukemia. Blood. 2008;112:4220–6. doi: 10.1182/blood-2008-01-136366. [DOI] [PubMed] [Google Scholar]
- 46.Haan C, Kreis S, Margue C, Behrmann I. Jaks and cytokine receptors--an intimate relationship. Biochem Pharmacol. 2006;72:1538–46. doi: 10.1016/j.bcp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 47.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 48.Wilson SJ, Leone BA, Anderson D, Manning A, Holgate ST. Immunohistochemical analysis of the activation of NF-kappaB and expression of associated cytokines and adhesion molecules in human models of allergic inflammation. J Pathol. 1999;189:265–72. doi: 10.1002/(SICI)1096-9896(199910)189:2<265::AID-PATH415>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 49.Striz I, Mio T, Adachi Y, Carnevali S, Romberger DJ, Rennard SI. Effects of interferons alpha and gamma on cytokine production and phenotypic pattern of human bronchial epithelial cells. Int J Immunopharmacol. 2000;22:573–85. doi: 10.1016/s0192-0561(00)00020-5. [DOI] [PubMed] [Google Scholar]
- 50.Vliagoftis H, Befus AD, Hollenberg MD, Moqbel R. Airway epithelial cells release eosinophil survival-promoting factors (GM-CSF) after stimulation of proteinase-activated receptor 2. J Allergy Clin Immunol. 2001;107:679–85. doi: 10.1067/mai.2001.114245. [DOI] [PubMed] [Google Scholar]
- 51.Malaviya R, Navara C, Uckun FM. Role of Janus kinase 3 in mast cell-mediated innate immunity against gram-negative bacteria. Immunity. 2001;15:313–21. doi: 10.1016/s1074-7613(01)00184-4. [DOI] [PubMed] [Google Scholar]
- 52.Avalos BR, Parker JM, Ware DA, Hunter MG, Sibert KA, Druker BJ. Dissociation of the Jak kinase pathway from G-CSF receptor signaling in neutrophils. Exp Hematol. 1997;25:160–8. [PubMed] [Google Scholar]
- 53.Nicholas C, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-kappaB through the suppression of p65 phosphorylation. J Immunol. 2007;179:7121–7. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 54.Hu XW, Meng D, Fang J. Apigenin inhibited migration and invasion of human ovarian cancer A2780 cells through focal adhesion kinase. Carcinogenesis. 2008;29:2369–76. doi: 10.1093/carcin/bgn244. [DOI] [PubMed] [Google Scholar]
- 55.Lindenmeyer F, Li H, Menashi S, Soria C, Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr Cancer. 2001;39:139–47. doi: 10.1207/S15327914nc391_19. [DOI] [PubMed] [Google Scholar]
- 56.Lee WJ, Chen WK, Wang CJ, Lin WL, Tseng TH. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol Appl Pharmacol. 2008;226:178–91. doi: 10.1016/j.taap.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 57.Piantelli M, Rossi C, Iezzi M, La Sorda R, Iacobelli S, Alberti S, Natali PG. Flavonoids inhibit melanoma lung metastasis by impairing tumor cells endothelium interactions. J Cell Physiol. 2006;207:23–9. doi: 10.1002/jcp.20510. [DOI] [PubMed] [Google Scholar]
- 58.Czyz J, Madeja Z, Irmer U, Korohoda W, Hulser DF. Flavonoid apigenin inhibits motility and invasiveness of carcinoma cells in vitro. Int J Cancer. 2005;114:12–8. doi: 10.1002/ijc.20620. [DOI] [PubMed] [Google Scholar]
- 59.Franzen CA, et al. The chemopreventive bioflavonoid apigenin inhibits prostate cancer cell motility through the focal adhesion kinase/Src signaling mechanism. Cancer Prev Res (Phila Pa) 2009;2:830–41. doi: 10.1158/1940-6207.CAPR-09-0066. [DOI] [PubMed] [Google Scholar]