Abstract
Objective
To quantify photoreceptor outer segment (PROS) length in patients with diabetic macular edema (DME) using spectral domain optical coherence tomography (OCT), and to describe the correlation between PROS length and visual acuity in this group of patients.
Design
Prospective study.
Participants
Twenty-seven consecutive patients (30 eyes) with DME.
Methods
Three SD-OCT scans were performed on all eyes during each session using Cirrus™ HD-OCT. A prototype algorithm was developed for quantitative assessment of PROS length. Retinal thicknesses and PROS lengths were calculated for three parameters; macular grid (6mm × 6mm), central subfield (1mm), and center foveal point (0.33mm). Intrasession repeatability was assessed using coefficient of variation (CVW) and intraclass correlation coefficient (ICC). Association between retinal thickness and PROS length with visual acuity was assessed using linear regression and Pearson correlation analyses.
Main Outcome Measure
Intrasession repeatability of macular parameters, and correlation of these parameters with visual acuity.
Results
Mean retinal thickness and PROS length were 298-381 μm and 30-32 μm, respectively, for macular parameters assessed in this study. CVW values were 0.75-4.13% for retinal thickness, and 1.97-14.01% for PROS length. ICC values were 0.96-0.99 and 0.73-0.98 for retinal thickness and PROS length, respectively. Slopes from linear regression analyses assessing the association of retinal thickness and visual acuity were not significantly different from zero (p>0.20), whereas the slopes of PROS length and visual acuity were significantly different from zero (p<0.0005). Correlation coefficients for macular thickness and visual acuity ranged from 0.13 to 0.22, while coefficients for PROS length and visual acuity ranged from -0.61 to -0.81.
Conclusions
PROS length can be quantitatively assessed using Cirrus™ HD-OCT. Although the intrasession repeatability of PROS measurements was less than that of macular thickness measurements, the stronger correlation of PROS length with visual acuity suggests that PROS measures may be more directly related to visual function. PROS length may be a useful physiologic outcome measure, both clinically and as a direct assessment of treatment effects.
Introduction
Diabetic macular edema (DME) is a major cause of vision loss in the United States1, 2. Over the past decade, several clinical trials have assessed the effect of various treatments for DME. Macular thicknesses obtained using optical coherence tomography (OCT) are often used as outcomes measures in such trials3-8. The correlation between OCT-measured macular thickness and visual acuity obtained in various clinical studies, however, has been modest and variable. Furthermore, in some cases paradoxical changes in visual acuity occur in response to changes in OCT-measured thickening. These findings suggests that although OCT can serve as a valuable tool in the clinical evaluation of patients with DME, OCT-derived macular thickness measurements may not be appropriate as surrogate markers of visual acuity8. The clinical utility of other OCT-derived measurements, such as measures of photoreceptor length, remains to be determined.
Time domain OCT using the Stratus™ OCT instrument (Carl Zeiss Meditec, Inc., Dublin, CA) has been extensively used for the determination of macular measurements in the clinical setting. Qualitative assessment of the inner segment/outer segment (IS/OS) junction using Stratus™ OCT has demonstrated that the integrity of the IS/OS junction is valuable in predicting visual acuity in patients with retinitis pigmentosa (RP) and birdshot chorioretinopathy (BCR), as well as in patients following macular hole repair9-12. Prototype software algorithms used with Stratus™ OCT and other OCT systems have allowed for quantitative assessment of photoreceptor structure13-16. In patients with RP, such quantitative measures of photoreceptor structure have been shown to correlate with visual acuity17. A qualitative or quantitative examination of the photoreceptor layer in patients with DME remains to be performed.
Spectral (Fourier) domain technology has allowed for the development of a new generation of commercial OCT instruments with higher axial resolution (≈ 5μm) compared to time domain instruments (≈ 10μm). One such instrument that is currently available is the Cirrus™ HD-OCT (Carl Zeiss Meditec, Inc., Dublin, CA). Several reports have recently described morphological characteristics of the photoreceptor layer in various macular diseases using Cirrus™ HD-OCT18-22. We have developed a prototype software algorithm for the Cirrus™ HD-OCT that allows for quantification of photoreceptor outer segment (PROS) length by measuring the distance between the IS/OS junction and the RPE. In this report we describe the correlation of both PROS length and retinal thickness measurements obtained using Cirrus™ HD-OCT with visual acuity in patients with DME.
Methods
Study Patients
Consecutive patients with DME who were seen at the National Eye Institute retina clinic over a two-month period were enrolled in this prospective study. Best-corrected visual acuity was measured using ETDRS charts. Eyes with significant media opacities which can result in poor OCT signal were excluded. Eyes with other conditions that can cause macular thickening such as venous occlusion, epiretinal membrane, and/or vitreomacular traction were also excluded. All individual B-scans from each OCT session were manually inspected to ensure proper delineation of the internal limiting membrane (ILM) and retinal pigment epithelium (RPE). Eyes with subretinal fluid were not included, nor were scans with significant hard exudates or intraretinal fluid that caused improper delineation of the ILM/RPE by the Cirrus™ HD-OCT software. In the presence of subretinal fluid, the distance between the IS/OS and the RPE would give an erroneously high measurement of outer segment length. A correct measurement would require subtracting the thickness of the fluid pocket, which is not possible without a separate subretinal fluid segmentation algorithm. Hard exudates and intraretinal fluid can obscure the underlying retina with their shadows. If the shadowed area is small enough, an RPE or IS/OS algorithm can reasonably interpolate across it, but for a very large shadowed regions it is unwise to do so. Only one eye in each patient was studied per study visit; if both eyes of a patient were eligible, the study eye was randomly selected and the fellow eye was allowed to be enrolled on an alternate study visit. The study was performed with informed patient consent and conducted under a protocol approved by the local institutional review board (IRB) and in accordance with the ethical standards stated in the 1964 Declaration of Helsinki.
Optical Coherence Tomography
Scanning with the Cirrus™ HD-OCT was performed using the 512 × 128 scan pattern where a 6mm × 6mm macular grid was scanned with 128 horizontal B-scan lines, each consisting of 512 A-scans per line (total of 65,536 sampled points). Each study eye was pharmacologically dilated prior to OCT scanning. All scans were performed by the same certified OCT technician. A total of three “high-quality” scans were obtained; these were defined as scans with a signal strength ≥6 that exhibit correct delineation of the ILM and RPE as detected automatically by the intrinsic software segmentation algorithm. The macular grid was centered on the intrinsic fixation target during OCT scanning, and decentration of the grid by the technician in order to attempt to center the grid on the fovea was not allowed. Hence, the center of the macular grid was maintained at the patients' point of fixation.
Determination of Macular Thickness and Photoreceptor Outer Segment Length
Cirrus™ HD-OCT data was processed for macular thickness and PROS length measurements without post-processing image alignment. These measurements were obtained for three macular parameters: macular grid (6mm × 6mm), the central subfield (1mm diameter), as well as for the central foveal point (0.33mm diameter). Macular thickness measurements were derived from the software (Cirrus™ 3.0, Carl Zeiss Meditec, Inc., Dublin, CA) provided by the manufacturer. In order to calculate PROS length, a prototype software algorithm was developed. The PROS was identified as the region between the RPE segmentation provided in the Cirrus™ HD-OCT and a prototype segmentation of the IS/OS boundary on each individual B-scan, and IS/OS segmentations were performed for all the B-scans on each macular grid. The prototype IS/OS segmentation software filtered the image data to reduce speckle and identified consistent bands near the RPE where the image transitioned from dark to bright. Once the interior edge of the IS/OS was thus located, the point of maximum brightness just below this edge was identified as the preliminary location of the IS/OS. Lateral smoothing was then applied to give the final result. No manual manipulation of OCT data was performed in the calculation of PROS length. Exclusion of OCT scans was only done based on the B-scans obtained from the Cirrus™ HD-OCT software, and no further exclusion was performed based on IS/OS segmentation by the prototype algorithm.
Statistical Analysis
In order to assess and compare the intrasession repeatability of the macular thickness and PROS length measurements, the intrasession within-subject SD (SW), coefficient of variation (CVW)(100% × SW/overall mean), and intraclass correlation coefficient (ICC) were calculated for each macular parameter23, 24. For the ICC calculation, the two-way mixed effects model for measures of absolute agreement and single ratings was used. Pairwise comparisons of CVW values were performed using the two-tailed paired t-test with Bonferroni adjustment for multiple comparisons, while ICC values and linear regression slopes were compared by examination of respective confidence intervals (CIs). Linear regression and Pearson correlation analysis was performed in order to examine the relationship between macular thickness and PROS length measurements and visual acuity. Analysis of variance (ANOVA) with Newman-Keuls post-test was used to examine differences in retinal thicknesses and PROS lengths. All analyses, except for ICC, calculation, were performed using GraphPad Prism (version 4). ICC calculation was performed using SPSS (version 15.0).
Results
A total of 35 eyes from 32 patients were entered into this study. Three scans from 3 separate patients were excluded due to the presence of significant hard exudates that resulted in improper delineation by the Cirrus™ HD-OCT software algorithm, and 2 scans from 2 patients were excluded because of subretinal fluid. No scans were excluded based on improper delineation due to the presence of significant intraretinal fluid. Many of the included eyes had some hard exudates, but these were not significant enough to disrupt the ILM/RPE segmentation. The scans from the remaining 30 eyes of 27 patients were analyzed further. The mean age was 61.7 years (± 11.0 years), with a total of 12 females. The mean visual acuity of the eyes used in our analysis was 0.35 log MAR units (Snellen equivalent 20/45, range: 20/20 to 20/160), and the mean signal strength of the HD-OCT scans used was 7.0 (± 1.0). Results of the retinal thickness and PROS length measurements are summarized in Table 1. Retinal thickness measurements for the macular grid were significantly lower than those for the central subfield and center foveal point (p<0.001). No significant differences in PROS length were seen between the three macular parameters.
Table 1.
Mean Macular Thickness and Photoreceptor Outer Segment (PROS) Length Measurements in Diabetic Macular Edema Obtained Using Cirrus™ HD-OCT.
| Retinal Thickness (μm) (± SD) | PROS Length (μm) (± SD) | |
|---|---|---|
| Macular Grid | 298 (± 23) | 30 (± 6) |
| Central Subfield | 380 (± 95) | 30 (± 9) |
| Center Foveal Point | 381 (± 111) | 32 (± 10) |
Mean measurements are shown for the macular grid (6mm × 6mm), the central subfield (1mm), and the center foveal point (0.33mm).
SD = standard deviation.
Potential differences in intrasession repeatability of retinal thickness and PROS length measurements were assessed by comparison of calculated CVW and ICC values (Table 2). CVW values were 0.75- 4.13% and 1.97-14.01% for macular thickness and PROS length, respectively. Corresponding ICC values for macular thickness and PROS length were 0.96-0.99 and 0.73-0.98, respectively. The highest variation was seen in the foveal point for both macular thickness and PROS length. Pairwise comparisons of CVW values demonstrated lower values, and hence better repeatability, for retinal thickness measurements compared to PROS length measurements. All pairwise comparisons between CVW values for retinal thickness and PROS length measures were statistically significant (p<0.002). Comparison of ICC values demonstrated higher values, and hence better repeatability, for retinal thickness measures compared to PROS length measures. Confidence intervals for the ICC values of retinal thickness and PROS length overlapped for the macular grid, however they were non-overlapping for the central 1mm subfield and foveal point thickness. These ICC comparisons, along with those made for the CVW values, indicate that intrasession repeatability of macular thickness measurements was higher than the repeatability of PROS length measurements.
Table 2.
Intrasession Repeatability Indices for Macular Thickness and Photoreceptor Outer Segment (PROS) Length in Diabetic Macular Edema Obtained Using Cirrus™ HD-OCT.
| Coefficient of Variation (CVW) (± SD) | |||
|---|---|---|---|
| Macular Grid | Central Subfield | Center Foveal Point | |
| Retinal Thickness | 0.75 % (± 0.50 %) | 2.38 % (± 2.21 %) | 4.13 % (± 4.08 %) |
| PROS Length | 1.97 % (± 1.48 %) | 6.20 % (± 6.04 %) | 14.01 % (± 9.57 %) |
| Intraclass Correlation Coefficient (ICC) (95% CI) | |||
| Macular Grid | Central Subfield | Center Foveal Point | |
| Retinal Thickness | 0.99 (0.98, 0.99) | 0.98 (0.97, 0.99) | 0.96 (0.93, 0.98) |
| PROS Length | 0.98 (0.97, 0.99) | 0.91 (0.84, 0.95) | 0.73 (0.58, 0.85) |
Repeatability indices are shown for the macular grid (6mm × 6mm), the central subfield (1mm), and the center foveal point (0.33mm).
SD = standard deviation.
CI = confidence interval.
Representative examples of IS/OS segmentation produced by the software algorithm used in this study, as well as topographical PROS length maps, are shown in Figure 1. Examination of the IS/OS segmentation produced by the software algorithm demonstrated good delineation of the IS/OS junctions, which was seen as a hyperreflective line in between the hyperreflective lines corresponding to the external limiting membrane (ELM) and the RPE. Inspection of the topographical PROS length maps demonstrated a qualitative association between the PROS length and visual acuity; eyes with poor visual acuity had more areas of shorter PROS length over the entire macular grid. This association was particularly true in the foveal region, where longer PROS length was associated with better visual acuity despite short PROS length throughout the macula.
Figure 1.

Inner Segment/Outer Segment (IS/OS) Segmentation and Photoreceptor Outer Segment (PROS) Topographical Maps in Diabetic Macular Edema.
Representative examples of B-scans without (A, D, F) and with (B, E, H) IS/OS segmentation and topographical PROS (C, F, I) maps are shown. Representative examples are shown for patients with visual acuities of 20/20 (A-C), 20/100 (D-F), and 20/160 (G-I). The IS/OS junction (denoted with blue line) is visualized as a hyperreflective line between the retinal pigment epithelium (denoted with red line) and the external limiting membrane (ELM). Center foveal point thickness (0.33mm) is denoted by green circles, and the central subfield (1mm) is denoted by blue circles. Color scale adjacent to topographical PROS maps indicates scale in microns (μm).
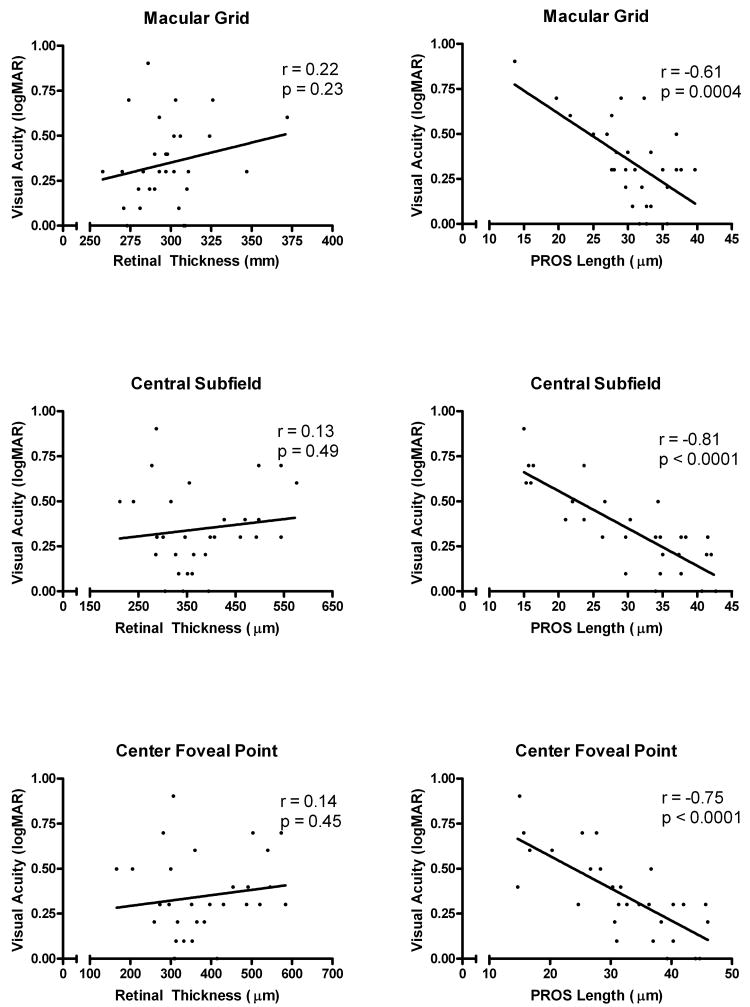
In order to quantitatively assess the relationship between macular thickness and PROS length and visual acuity, linear regression and Pearson correlation analyses were performed (Figure 2). Slopes of macular thickness vs. visual acuity were not statistically significantly different from zero; however, slopes of PROS length vs. visual acuity were all statistically significantly different from zero. In agreement with our qualitative observations of PROS maps, the PROS length in the foveal region appeared to be more highly correlated with visual acuity than overall PROS length of the macular grid. The correlation coefficients for PROS length and visual acuity were greater in the central subfield and center foveal point than the full macular grid region (Figure 2). As has been reported in other studies3-8, correlations between macular thickness and visual acuity in our study were relatively low, with correlation coefficients ranging from 0.13 to 0.22. The correlation between PROS length and visual acuity was stronger with correlation coefficients ranging from -0.61 to -0.81.
Figure 2.
Linear Regression and Correlation Analysis of Retinal Thickness and Photoreceptor Outer Segment (PROS) Length with Visual Acuity in Diabetic Macular Edema.
Graphical analyses are shown for the macular grid (6mm × 6mm), the central subfield (1mm), and the center foveal point (0.33mm). Pearson correlation coefficients (r) and p values for the slope of the regression line are noted.
Discussion
With the use of Cirrus™ HD-OCT and a prototype software algorithm, we have quantified PROS length in patients with DME. Furthermore, we report a relatively strong correlation between PROS length and visual acuity in these patients. To our knowledge, this is the first report demonstrating the use of an OCT-based approach for the quantification of PROS length in patients with DME. Previous histological studies of human retina have demonstrated PROS length of 25-63 μm in the macula25, 26. Recently, an OCT-based approach has demonstrated a mean cone OS length of 40.6 μm in the fovea of healthy subjects27. The mean PROS length of the central subfield for patients with DME in this study was found to be 30 ± 9 μm, demonstrating good agreement between our observations and those of other investigators.
For both macular thickness and PROS length, we found that variation in measurements increased as the size of area analyzed decreased. This is not surprising, as smaller areas will have less sampled points and hence increased variation between measurements. We found the intrasession repeatability for PROS measurements to be less than that for macular thickness measurements. Intrasession repeatability of macular thickness measurements in normal subjects using Cirrus™ HD-OCT has previously demonstrated CVW values ranging from 0.6%-2.4%, and ICC values ranging from 0.92-0.9928. Corresponding values for CVW and ICC using Cirrus™ HD-OCT in patients with DME have been reported as 0.58-2.87% and 0.84-1.00, respectively29. Our reported results of intrasession repeatability for macular thickness and PROS length are comparable to these previously published results, except for foveal point thickness which demonstrated higher intrasession variation. Repeatability of foveal point macular thickness has not been previously assessed using Cirrus™ HD-OCT28, 29. Studies of repeatability of macular thickness measurements in DME using the Stratus™ OCT system (Carl Zeiss Meditec, Inc., Dublin, CA) have demonstrated higher reproducibility of central subfield measures compared to center point foveal thickness30. A higher intrasession variation with center point foveal macular thickness and PROS length using the Cirrus™ HD-OCT is thus not unexpected.
In this study, we found a relatively low correlation between macular thickness and visual acuity. The correlation we found was lower than that obtained in previous studies (r=0.28-0.73) in patients with DME3-8. This likely reflects differences in patient selection, instrumentation, and experimental methods. Prior studies that examined the relationship between retinal thickness and visual acuity used OCT instruments with segmentation algorithms different than that of the Cirrus™ HD-OCT. Furthermore, these studies did not exclude scans with subretinal fluid as we did in the current study. OCT segmentation algorithms define the outer retinal border as the hyperreflective line corresponding to the RPE, thereby incorporating subretinal fluid into the retinal thickness measurement. This can result in retinal thickness and PROS length measurements that are erroneously larger than their true values. Exclusion of OCT scans with subretinal fluid focuses only on retinal thickness, not a combination of retinal thickness plus the amount of subretinal fluid, and the correlation coefficients we obtained may be more representative of the true relationship between retinal thickness and visual acuity. Due to the nature of our segmentation algorithm, it is not possible to know if the average PROS length shortening that we observed in DME is due to multiple points of localized breakdown of the IS/OS junction or from generalized thinning of the photoreceptor outer segments. Future work with OCT-based assessment of photoreceptor structure should try to assess these issues. The reasons for PROS length shortening in DME can be speculated upon based on what is known about the pathophysiology of this disease. Tissue ischemia, lipid and fluid exudation, as well as the accumulation of toxic metabolic waste products and inflammatory mediators may be contributing to photoreceptor pathology in DME.
The correlation that we observed between PROS length and visual acuity was comparable to or greater than that previously reported between macular thickness and visual acuity3-8. The modest correlation between retinal thickness and visual acuity that has been previously reported suggests that these thickness measurements are not particularly predictive of visual acuity8. We found PROS length measurements correlated more strongly with visual acuity than macular thickness measurement, suggesting that PROS length may represent a novel and reliable correlate of visual acuity in patients with DME. The correlation coefficients between PROS length and visual acuity were -0.61 to -0.81, indicating that a significant percentage of the variation in visual acuity can be explained by PROS length. However, PROS length cannot explain all of the variation in visual acuity and other factors like macular ischemia, retinal cell function, etc., may be important as well. Future observational studies and clinical trials of DME should consider the addition of OCT-based PROS length measurements, along with other modalities such as fluorescein angiography, as outcome measures.
Acknowledgments
We would like to thank Susan Vitale, PhD, for her advice and assistance with statistical analysis.
Financial Support: National Eye Institute intramural research program.
Footnotes
Conflict of Interest: Paul F. Stetson and Scott A. Meyer are both employees of Carl Zeiss Meditec, Inc.
References
- 1.Kempen JH, O'Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–63. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 2.Roy MS, Klein R, O'Colmain BJ, Klein BE, Moss SE, Kempen JH. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol. 2004;122:546–51. doi: 10.1001/archopht.122.4.546. [DOI] [PubMed] [Google Scholar]
- 3.Bandello F, Polito A, Del Borrello M, Zemella N, Isola M. “Light” versus “classic” laser treatment for clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:864–70. doi: 10.1136/bjo.2004.051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–7. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 5.Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–9. doi: 10.1136/bjo.2003.040949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozdemir H, Karacorlu M, Karacorlu SA. Regression of serous macular detachment after intravitreal triamcinolone acetonide in patients with diabetic macular edema. Am J Ophthalmol. 2005;140:251–5. doi: 10.1016/j.ajo.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Massin P, Duguid G, Erginay A, Haouchine B, Gaudric A. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003;135:169–77. doi: 10.1016/s0002-9394(02)01837-8. [DOI] [PubMed] [Google Scholar]
- 8.Browning DJ, Glassman AR, Aiello LP, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oishi A, Otani A, Sasahara M, et al. Photoreceptor integrity and visual acuity in cystoid macular oedema associated with retinitis pigmentosa. Eye. 2008 doi: 10.1038/eye.2008.266. [DOI] [PubMed] [Google Scholar]
- 10.Baba T, Yamamoto S, Arai M, et al. Correlation of visual recovery and presence of photoreceptor inner/outer segment junction in optical coherence images after successful macular hole repair. Retina. 2008;28:453–8. doi: 10.1097/IAE.0b013e3181571398. [DOI] [PubMed] [Google Scholar]
- 11.Aizawa S, Mitamura Y, Baba T, Hagiwara A, Ogata K, Yamamoto S. Correlation between visual function and photoreceptor inner/outer segment junction in patients with retinitis pigmentosa. Eye. 2008 doi: 10.1038/sj.eye.6703076. [DOI] [PubMed] [Google Scholar]
- 12.Monnet D, Levinson RD, Holland GN, Haddad L, Yu F, Brezin AP. Longitudinal cohort study of patients with birdshot chorioretinopathy. III. Macular imaging at baseline. Am J Ophthalmol. 2007;144:818–828. doi: 10.1016/j.ajo.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Shahidi M, Wang Z, Zelkha R. Quantitative thickness measurement of retinal layers imaged by optical coherence tomography. Am J Ophthalmol. 2005;139:1056–61. doi: 10.1016/j.ajo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Chan A, Duker JS, Ishikawa H, Ko TH, Schuman JS, Fujimoto JG. Quantification of photoreceptor layer thickness in normal eyes using optical coherence tomography. Retina. 2006;26:655–60. doi: 10.1097/01.iae.0000236468.33325.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan VJ, Monson BK, Wojtkowski M, et al. Characterization of outer retinal morphology with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49:1571–9. doi: 10.1167/iovs.07-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa H, Stein DM, Wollstein G, Beaton S, Fujimoto JG, Schuman JS. Macular segmentation with optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:2012–7. doi: 10.1167/iovs.04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witkin AJ, Ko TH, Fujimoto JG, et al. Ultra-high resolution optical coherence tomography assessment of photoreceptors in retinitis pigmentosa and related diseases. Am J Ophthalmol. 2006;142:945–52. doi: 10.1016/j.ajo.2006.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang LK, Koizumi H, Spaide RF. Disruption of the photoreceptor inner segment-outer segment junction in eyes with macular holes. Retina. 2008;28:969–75. doi: 10.1097/IAE.0b013e3181744165. [DOI] [PubMed] [Google Scholar]
- 19.Spaide RF, Koizumi H, Freund KB. Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol. 2008;146:111–20. doi: 10.1016/j.ajo.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Sayanagi K, Ikuno Y, Soga K, Tano Y. Photoreceptor inner and outer segment defects in myopic foveoschisis. Am J Ophthalmol. 2008;145:902–8. doi: 10.1016/j.ajo.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto H, Kishi S, Otani T, Sato T. Elongation of photoreceptor outer segment in central serous chorioretinopathy. Am J Ophthalmol. 2008;145:162–168. doi: 10.1016/j.ajo.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Piccolino FC, de la Longrais RR, Ravera G, et al. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. Am J Ophthalmol. 2005;139:87–99. doi: 10.1016/j.ajo.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Leung CK, Wong L, et al. Comparative Study of Central Corneal Thickness Measurement with Slit-Lamp Optical Coherence Tomography and Visante Optical Coherence Tomography. Ophthalmology. 2007 doi: 10.1016/j.ophtha.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 24.Polito A, Del Borrello M, Isola M, Zemella N, Bandello F. Repeatability and reproducibility of fast macular thickness mapping with stratus optical coherence tomography. Arch Ophthalmol. 2005;123:1330–7. doi: 10.1001/archopht.123.10.1330. [DOI] [PubMed] [Google Scholar]
- 25.Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vision Res. 1986;26:847–55. doi: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- 26.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292:497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan VJ, Adler DC, Chen Y, et al. Ultrahigh-speed Optical Coherence Tomography for Three-Dimensional and En Face Imaging of the Retina and Optic Nerve Head. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.08-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung CK, Cheung CY, Weinreb RN, et al. Comparison of Macular Thickness Measurements between Time Domain and Spectral Domain Optical Coherence Tomography. Invest Ophthalmol Vis Sci. 2008 doi: 10.1167/iovs.07-1326. [DOI] [PubMed] [Google Scholar]
- 29.Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT. Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci. 2008;49:4290–6. doi: 10.1167/iovs.08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krzystolik MG, Strauber SF, Aiello LP, et al. Reproducibility of macular thickness and volume using Zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology. 2007;114:1520–5. doi: 10.1016/j.ophtha.2006.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]