Abstract
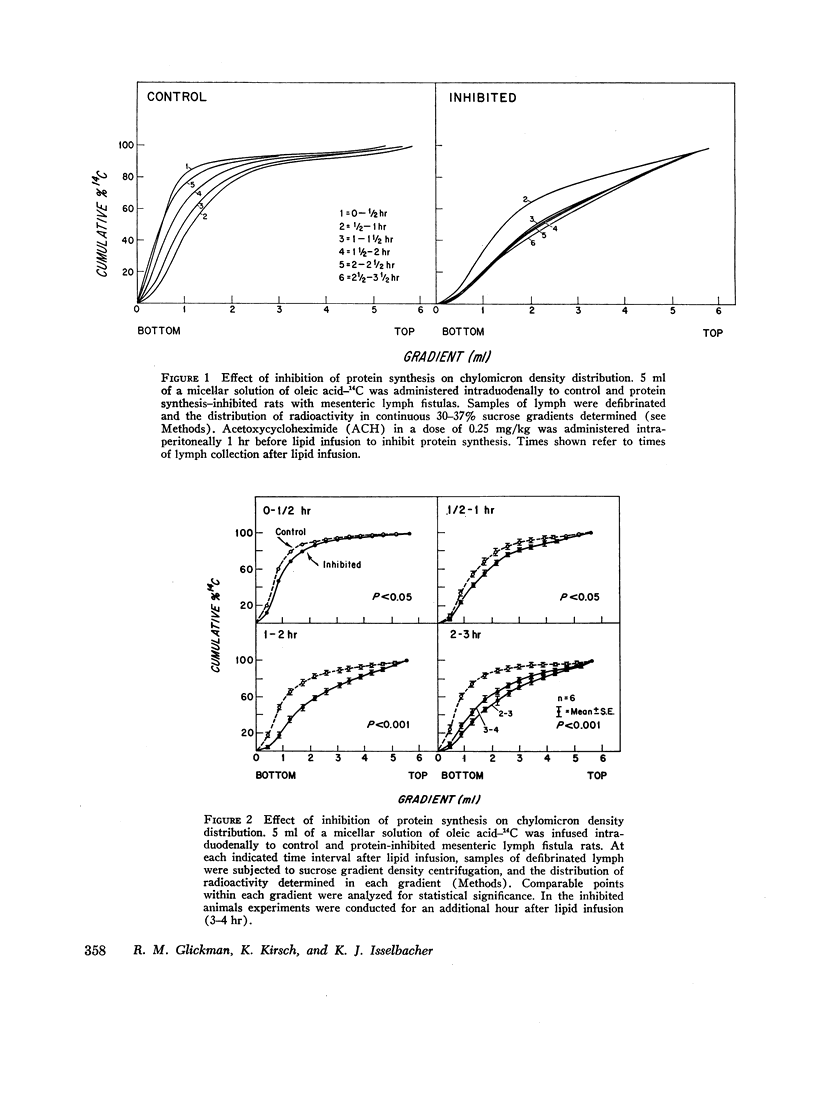
The effect of protein synthesis inhibition on the absorption of oleic acid from micellar solution was studied in mesenteric lymph fistula rats. A micellar solution of oleic acid labeled with tracer doses of oleic acid-14C was administered by intraduodenal infusion to rats with indwelling mesenteric lymph cannulas. Protein synthesis was inhibited by intraperitoneal acetoxycycloheximide (ACH), 0.25 mg/kg, 1 hr before lipid infusion. Lymph chylomicrons labeled with oleic acid-14C were collected from control and protein inhibited animals at various times after lipid infusion and subjected to sucrose density gradient centrifugation to determine changes in size. In control animals there was a transient increase in chylomicron size during maximal triglyceride absorption; however, in protein-inhibited animals there was a marked and sustained increase in chylomicron size as late as 4 hr after lipid infusion.
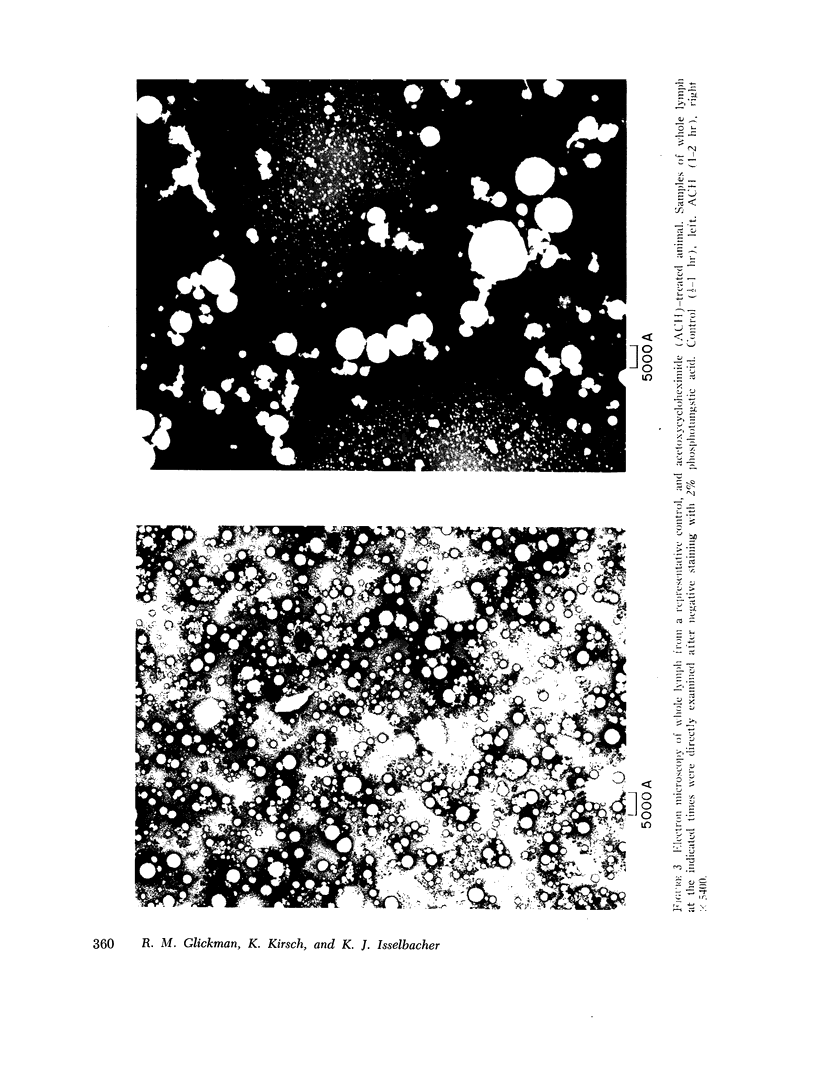
Triglyceride and phospholipid determinations on washed chylomicrons from both groups indicated a greater triglyceride/phospholipid ratio after protein synthesis inhibition supporting a greater chylomicron size. Electron microscopy of lymph from both groups further confirmed a markedly increased chylomicron size after protein synthesis inhibition. It is proposed that an increase in size conserves chylomicron surface components, i.e. apoprotein, during conditions of inhibition of protein synthesis.
These studies clearly demonstrate that the intestinal inhibition of protein synthesis is associated with an increase in the size of intestinal lymph chylomicrons and support the concept that protein synthesis is important in the formation and transport of chylomicrons from the mucosal cell into the lymph.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Eggstein M., Kreutz F. H. Eine neue Bestimmung der Neutralfette im Blutserum und Gewebe. I. Prinzip, Durchführung und Besprechung der Methode. Klin Wochenschr. 1966 Mar 1;44(5):262–267. doi: 10.1007/BF01747716. [DOI] [PubMed] [Google Scholar]
- Fraser R., Cliff W. J., Courtice F. C. The effect of dietary fat load on the size and composition of chylomicrons in thoracic duct lymph. Q J Exp Physiol Cogn Med Sci. 1968 Oct;53(4):390–398. doi: 10.1113/expphysiol.1968.sp001984. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Alpers D. H., Drummey G. D., Isselbacher K. J. Increased lymph alkaline phosphatase after fat feeding: effects of medium chain triglycerides and inhibition of protein synthesis. Biochim Biophys Acta. 1970 Feb 24;201(2):226–235. doi: 10.1016/0304-4165(70)90296-5. [DOI] [PubMed] [Google Scholar]
- Gotto A. M., Levy R. I., John K., Fredrickson D. S. On the protein defect in abetalipoproteinemia. N Engl J Med. 1971 Apr 15;284(15):813–818. doi: 10.1056/NEJM197104152841503. [DOI] [PubMed] [Google Scholar]
- Kessler J. I., Stein J., Dannacker D., Narcessian P. Biosynthesis of low density lipoprotein by cell-free preparations of rat intestinal mucosa. J Biol Chem. 1970 Oct 25;245(20):5281–5288. [PubMed] [Google Scholar]
- Levy R. I., Fredrickson D. S., Laster L. The lipoproteins and lipid transport in abetalipoproteinemia. J Clin Invest. 1966 Apr;45(4):531–541. doi: 10.1172/JCI105367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockner R. K., Hughes F. B., Isselbacher K. J. Very low density lipoproteins in intestinal lymph: origin, composition, and role in lipid transport in the fasting state. J Clin Invest. 1969 Nov;48(11):2079–2088. doi: 10.1172/JCI106174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINTER G. G., ZILVERSMIT D. B. A gradient centrifugation method for the determination of particle size distribution of chylomicrons and of fat droplets in artificial fat emulsions. Biochim Biophys Acta. 1962 May 7;59:116–127. doi: 10.1016/0006-3002(62)90702-3. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G. Inhibition of protein synthesis and absorption of lipid into thoracic duct lymph of rats. Proc Soc Exp Biol Med. 1969 Mar;130(3):776–780. doi: 10.3181/00379727-130-33653. [DOI] [PubMed] [Google Scholar]
- Redgrave T. G., Zilversmit D. B. Does puromycin block release of chylomicrons from the intestine? Am J Physiol. 1969 Aug;217(2):336–340. doi: 10.1152/ajplegacy.1969.217.2.336. [DOI] [PubMed] [Google Scholar]
- SABESIN S. M., ISSELBACHER K. J. PROTEIN SYNTHESIS INHIBITION: MECHANISM FOR THE PRODUCTION OF IMPAIRED FAT ABSORPTION. Science. 1965 Mar 5;147(3662):1149–1151. doi: 10.1126/science.147.3662.1149. [DOI] [PubMed] [Google Scholar]
- SALT H. B., WOLFF O. H., LLOYD J. K., FOSBROOKE A. S., CAMERON A. H., HUBBLE D. V. On having no beta-lipoprotein. A syndrome comprising a-beta-lipoproteinaemia, acanthocytosis, and steatorrhoea. Lancet. 1960 Aug 13;2(7146):325–329. doi: 10.1016/s0140-6736(60)91478-1. [DOI] [PubMed] [Google Scholar]
- SENIOR J. R., ISSELBACHER K. J. Activation of long-chain fatty acids by rat-gut mucosa. Biochim Biophys Acta. 1960 Nov 4;44:399–400. doi: 10.1016/0006-3002(60)91594-8. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Horowitz M. I. Long-chain bases in the sphingomyelins of bovine serum. Biochim Biophys Acta. 1970 Sep 8;210(3):493–495. doi: 10.1016/0005-2760(70)90048-2. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Levy R. I. Production of beta-lipoprotein by intestine in the rat. J Biol Chem. 1968 Sep 25;243(18):4878–4884. [PubMed] [Google Scholar]
- Windmueller H. G., Lindgren F. T., Lossow W. J., Levy R. I. On the nature of circulating lipoproteins of intestinal origin in the rat. Biochim Biophys Acta. 1970 May 5;202(3):507–516. doi: 10.1016/0005-2760(70)90121-9. [DOI] [PubMed] [Google Scholar]
- Zilversmit D. B. Formation and transport of chylomicrons. Fed Proc. 1967 Nov-Dec;26(6):1599–1605. [PubMed] [Google Scholar]