Abstract
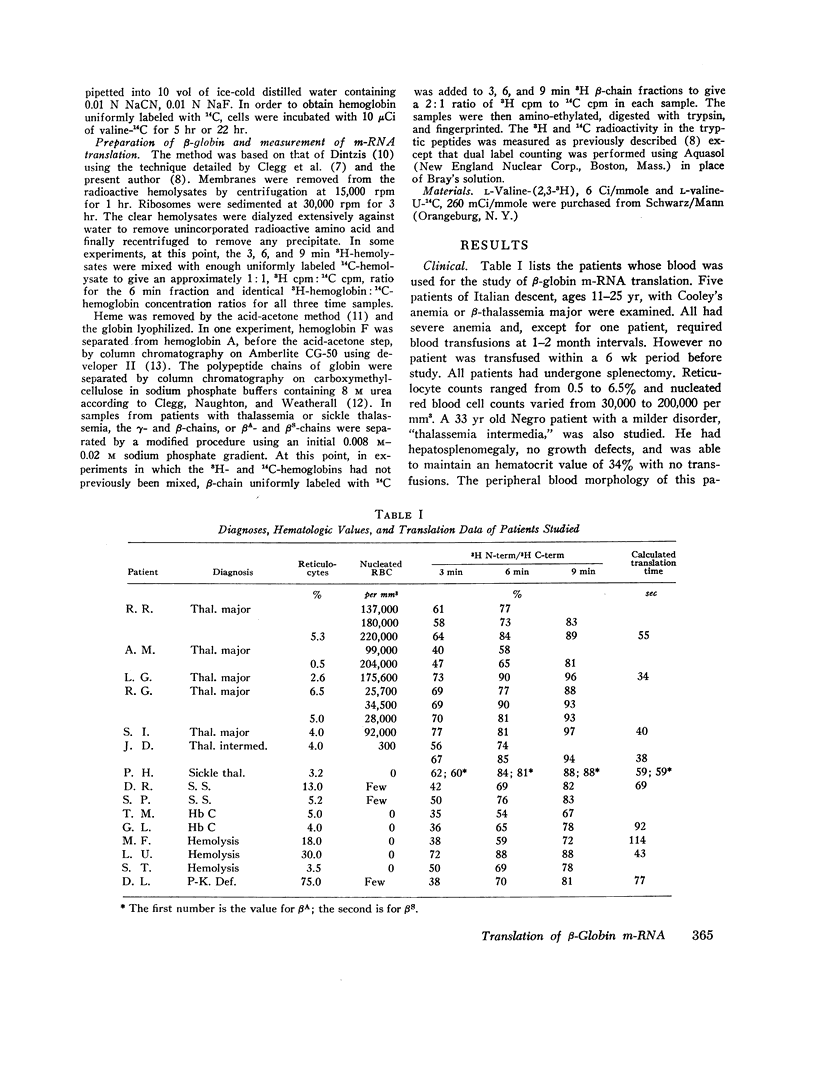
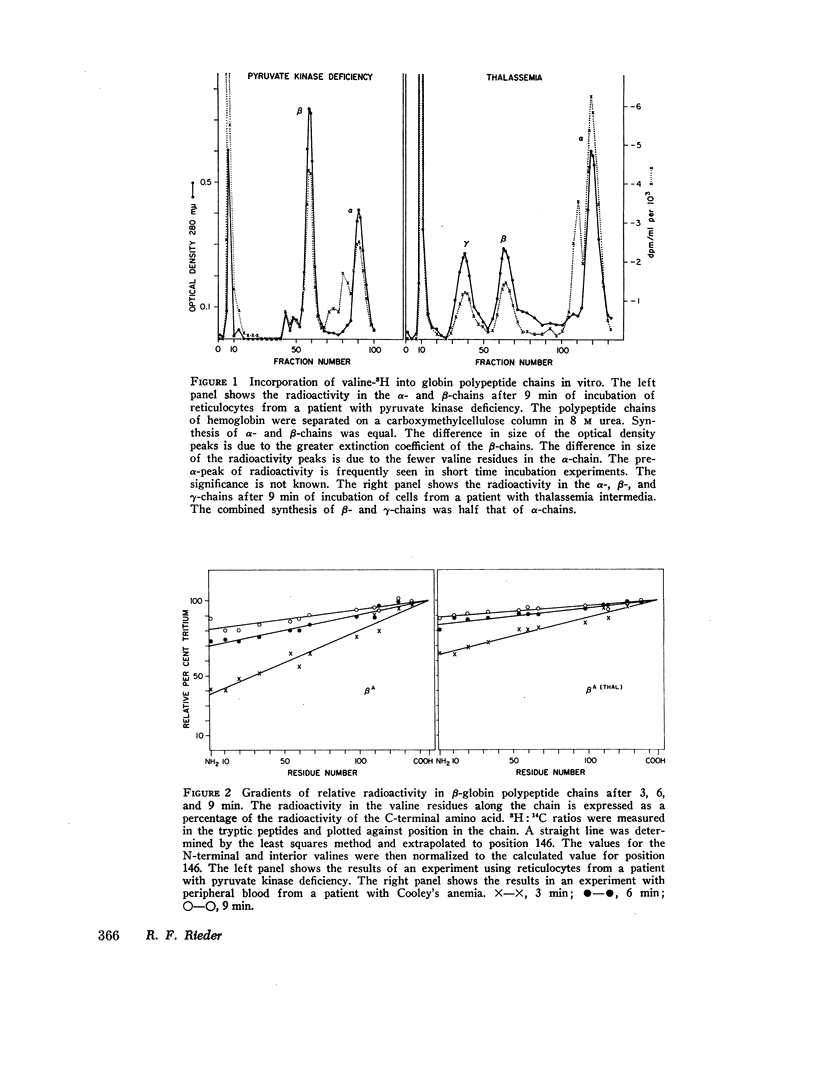
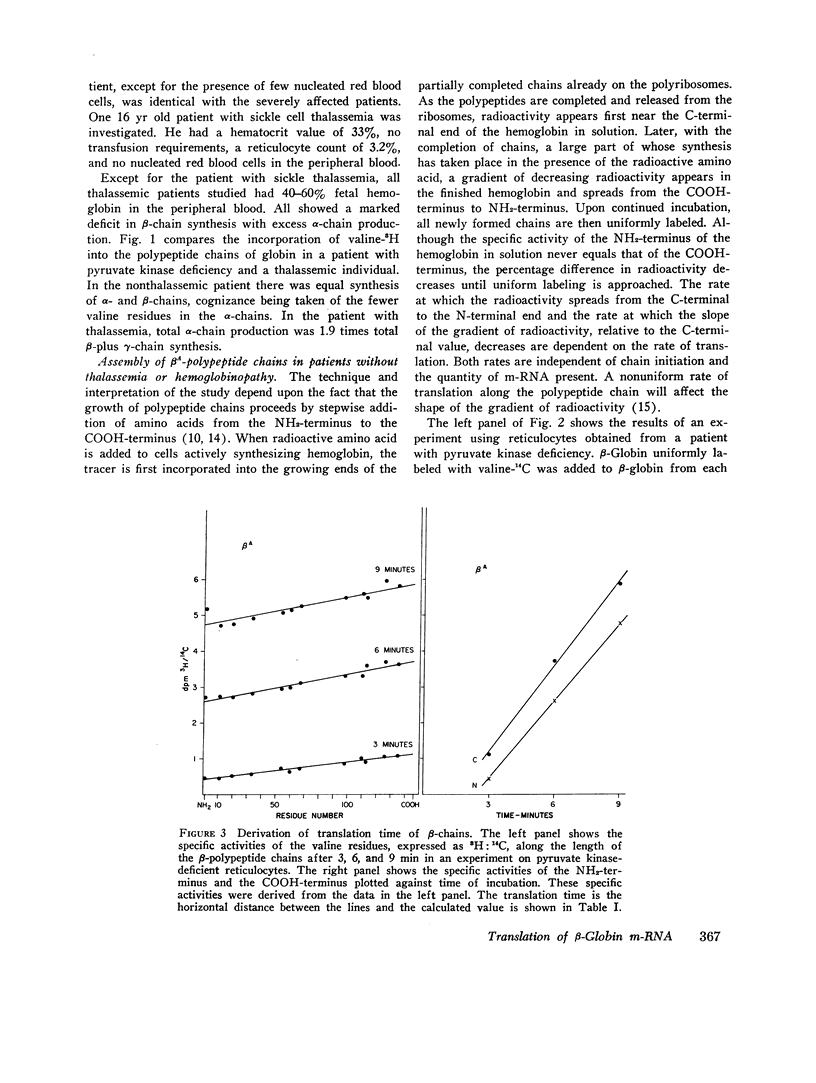
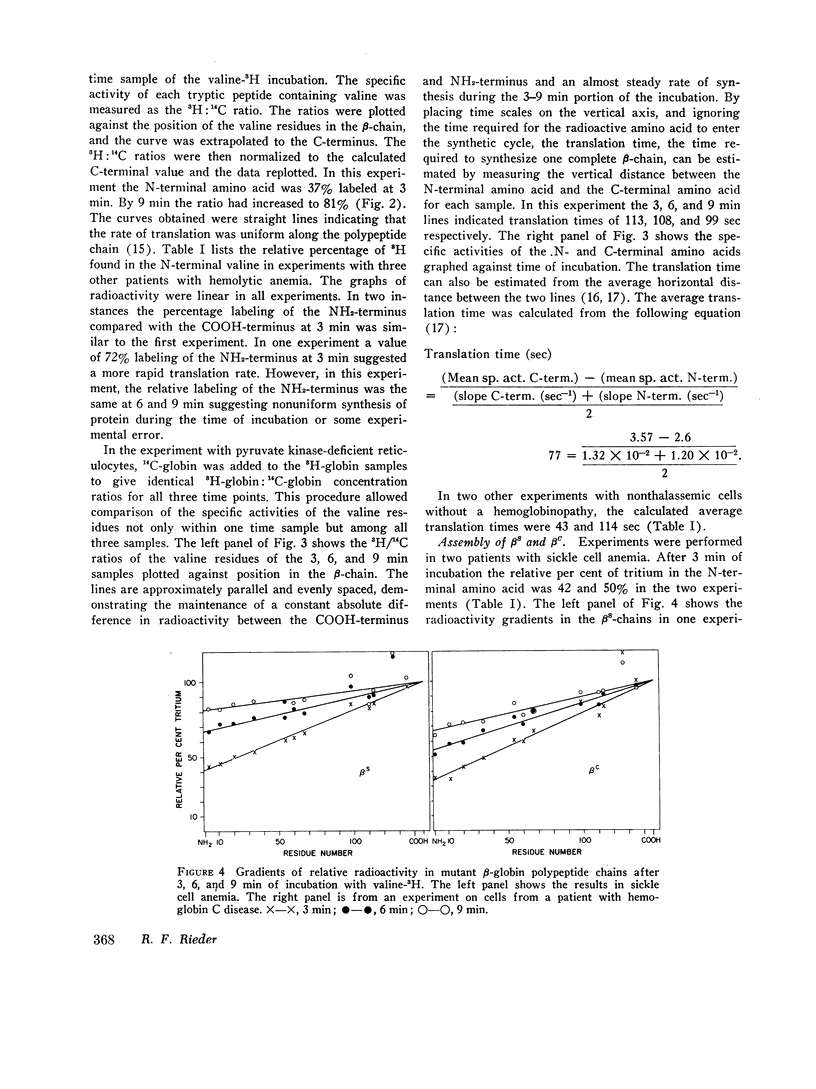
Genetic and biochemical evidence indicates that in β-thalassemia there is impaired synthesis of the β-globin chains of hemoglobin A. In patients heterozygous for the hemoglobinopathies, hemoglobin S and hemoglobin C, the mutant β-chain is produced in smaller amounts than normal βA. Defective m-RNA translation has been suggested as a possible cause of decreased β-globin polypeptide synthesis in thalassemia and the hemoglobinopathies. In the present study, the ribosomal assembly of β-globin chains was examined in the peripheral, nucleated red blood cells and reticulocytes of patients with Cooley's anemia, thalassemia intermedia, sickle thalassemia, sickle cell anemia, hemoglobin C disease, and in hemolytic anemias not associated with a hemoglobinopathy. The translation times of βA, βS, and βC did not differ significantly (average times; βA = 75 sec, range 43-114, βS = 69 sec, βC = 92 sec). In thalassemia, no evidence was found for a delay in translation as the cause of the marked impairment of β-globin synthesis. In several specimens of peripheral blood from thalassemic patients, the translation time of the β-chain was even shorter than in nonthalassemic specimens (average time = 45 sec, range 35-59). The results suggest that the defect in β-globin synthesis in β-thalassemia is due to impaired initiation of β-globin chain assembly or a quantitative deficiency in m-RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYER S. H., HATHAWAY P., GARRICK M. D. MODULATION OF PROTEIN SYNTHESIS IN MAN: AN IN VITRO STUDY OF HEMOGLOBIN SYNTHESIS BY HETEROZYGOTES. Cold Spring Harb Symp Quant Biol. 1964;29:333–346. doi: 10.1101/sqb.1964.029.01.036. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Campana T. Alpha-chain and globin: intermediates in the synthesis of rabbit hemoglobin. Eur J Biochem. 1967 Nov;2(4):480–492. doi: 10.1111/j.1432-1033.1967.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Bishop J., Leahy J., Schweet R. FORMATION OF THE PEPTIDE CHAIN OF HEMOGLOBIN. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1030–1038. doi: 10.1073/pnas.46.8.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Weatherall D. J., Na-Nakorn S., Wasi P. Haemoglobin synthesis in beta-thalassaemia. Nature. 1968 Nov 16;220(5168):664–668. doi: 10.1038/220664a0. [DOI] [PubMed] [Google Scholar]
- DINTZIS H. M. Assembly of the peptide chains of hemoglobin. Proc Natl Acad Sci U S A. 1961 Mar 15;47:247–261. doi: 10.1073/pnas.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander S. W., Page L. A. Interpretation of data on sequential labeling of growing polypeptides. Biochem Biophys Res Commun. 1965 May 18;19(5):565–570. doi: 10.1016/0006-291x(65)90375-x. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Herzberg M., Revel M., Danon D. The influence of ribosomal factors during the maturation of reticulocytes. Eur J Biochem. 1969 Nov;11(1):148–153. doi: 10.1111/j.1432-1033.1969.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Heywood D., Karon M., Weissman S. Asymmetrical incorporation of amino acids in the alpha and beta chains of hemoglobin synthesized by thalassemic reticulocytes. J Lab Clin Med. 1965 Sep;66(3):476–482. [PubMed] [Google Scholar]
- Hunt T., Hunter T., Munro A. Control of haemoglobin synthesis: distribution of ribosomes on the messenger RNA for alpha and beta chains. J Mol Biol. 1968 Aug 28;36(1):31–45. doi: 10.1016/0022-2836(68)90217-9. [DOI] [PubMed] [Google Scholar]
- Hunt T., Hunter T., Munro A. Control of haemoglobin synthesis: rate of translation of the messenger RNA for the alpha and beta chains. J Mol Biol. 1969 Jul 14;43(1):123–133. doi: 10.1016/0022-2836(69)90083-7. [DOI] [PubMed] [Google Scholar]
- KNOPF P. M., LAMFROM H. CHANGES IN THE RIBOSOME DISTRIBUTION DURING INCUBATION OF RABBIT RETICULOCYTES IN VITRO. Biochim Biophys Acta. 1965 Mar 15;95:398–407. doi: 10.1016/0005-2787(65)90186-3. [DOI] [PubMed] [Google Scholar]
- Luppis B., Bargellesi A., Conconi F. Control of hemoglobin synthesis at the translation level. Nascent polypeptide chain distribution on rabbit reticulocyte polyribosomes. Biochemistry. 1970 Oct 13;9(21):4175–4179. doi: 10.1021/bi00823a020. [DOI] [PubMed] [Google Scholar]
- Rieder R. F. Synthesis of hemoglobin Gun Hill: increased synthesis of the heme-free beta-GH globin chain and subunit exchange with a free alpha-chain pool. J Clin Invest. 1971 Feb;50(2):388–400. doi: 10.1172/JCI106506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaeffer J. R., Trostle P. K., Evans R. F. Inhibition of the biosynthetic completion of rabbit hemoglobin by isolated human hemoglobin chains. J Biol Chem. 1969 Aug 25;244(16):4284–4291. [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B., Naughton M. A. Globin synthesis in thalassaemia: an in vitro study. Nature. 1965 Dec 11;208(5015):1061–1065. doi: 10.1038/2081061a0. [DOI] [PubMed] [Google Scholar]
- Winslow R. M., Ingram V. M. Peptide chain synthesis of human hemoglobins A and A2. J Biol Chem. 1966 Mar 10;241(5):1144–1149. [PubMed] [Google Scholar]



