Abstract
Background
Medication errors and adverse drug events are common after hospital discharge, due to changes in medication regimens, suboptimal discharge instructions, and prolonged time to follow-up. Pharmacist-based interventions may be effective in promoting the safe and effective use of medications, especially among high risk patients such as those with low health literacy.
Methods and Results
The Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) study is a randomized controlled trial conducted at 2 academic centers – Vanderbilt University Hospital and Brigham and Women’s Hospital. Patients admitted with acute coronary syndrome or acute decompensated heart failure were randomized to usual care or intervention. The intervention consisted of pharmacist-assisted medication reconciliation, inpatient pharmacist counseling, low-literacy adherence aids, and tailored telephone follow-up after discharge. The primary outcome is the occurrence of serious medication errors in the first 30 days after hospital discharge. Secondary outcomes are health care utilization, disease-specific quality of life, and cost effectiveness. Enrollment was completed September 2009. A total of 862 patients were enrolled, and 430 patients were randomized to receive the intervention. Analyses will determine whether the intervention was effective in reducing serious medication errors, particularly in patients with low health literacy.
Conclusions
The PILL-CVD study was designed to reduce serious medication errors after hospitalization through a pharmacist-based intervention. The intervention, if effective, will inform health care facilities on the use of pharmacist-assisted medication reconciliation, inpatient counseling, low-literacy adherence aids, and patient follow-up after discharge.
Clinical Trial Registration
Keywords: coronary disease, heart failure, patients
Background
The period after hospital discharge is a vulnerable time for patients.1 Medication errors are common as a result of changes in the regimen during hospitalization, suboptimal discharge instructions, and prolonged time to follow-up.2, 3 Such errors can cause harm, including side effects, poor disease control, hospital readmission, and death.4
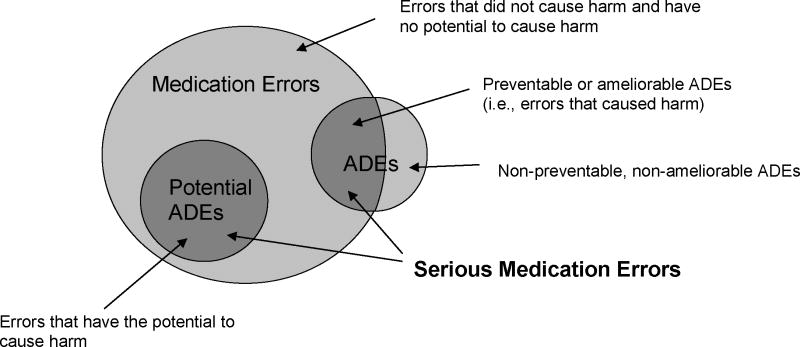
The relationships among medication errors, adverse drug events (ADEs), and potential ADEs are shown in Figure 1. ADEs are broadly defined as any injury due to medication.5 If an ADE resulted from a medication error, it is considered preventable or ameliorable. A preventable ADE is one in which absence of the error would have prevented the ADE. An ameliorable ADE is one in which absence of the error would have decreased the severity and/or duration of the ADE. A potential ADE is an error that could lead to an adverse event but has not yet caused harm (e.g., because the error was caught or because of patient variability in response to the error). Potential ADEs include unintentional medication discrepancies (differences between what patients think they should be taking and the actual regimens ordered by physicians) and medication non-adherence (differences between what patients think they should be taking and what they actually take).6 We define “serious medication error” (SME) as any preventable or ameliorable ADE, or a potential ADE due to non-adherence or an unintentional discrepancy.
Figure 1.
Conceptual model of serious medication errors
ADEs occur in 13–17% of patients after hospital discharge.4, 7 Studies suggest that improvements in provider communication could prevent or ameliorate 50–72% of ADEs.4, 7 Potential ADEs are also common and arise from unintentional discrepancies between admission and discharge regimens, such as changes in dose, route, or frequency, and/or introduction of new medications.8 Consequently, patients may have difficulty reconciling their new and old regimens upon returning home.1 In fact, drug additions/deletions or errors in dosing occur in 50–90% of patients one month post-discharge.9, 10
Patients with cardiovascular diseases may be particularly prone to SMEs. These patients are often elderly and prescribed complex medication regimens. Cardiovascular drugs are commonly implicated in adverse events, comprising 14% of all post-discharge ADEs.9 The consequences of poor medication management in cardiovascular disease, particularly acute coronary syndrome and heart failure,10, 11 can lead to readmission and complications.12 Often these complications are due to non-adherence to and early discontinuation of cardiac medications.13, 14
Health literacy is “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”15 Patients with low health literacy may have increased risk of SMEs related to difficulty understanding prescription drug information,16 maintaining adherence,17, 18 and other barriers.19
Pharmacist-based interventions are effective in promoting safe and effective use of medications during and after hospitalization.6, 20 Moreover, multifactorial interventions which include patient education and follow-up regarding medications have demonstrated a decrease in hospital readmission.21, 22 To date, however, few multi-site studies have rigorously evaluated a standard pharmacist-based educational intervention to improve medication safety, nor have many programs specifically targeted patients with low health literacy. No such interventions have been evaluated in an era where medication reconciliation1 is standard.23 This is important because at least some of the benefit of past pharmacist interventions may be attributed to their role in reconciling medications.6
This paper describes the design of the Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) study. PILL-CVD is a randomized controlled trial conducted at 2 academic medical centers – Vanderbilt University Hospital (VUH) and Brigham and Women’s Hospital (BWH). Our hypothesis is that an intervention consisting of pharmacist-assisted medication reconciliation, inpatient pharmacist counseling, low-literacy adherence aids, and tailored telephone follow-up will decrease the incidence of SMEs in the first 30 days after hospital discharge compared with usual care. We will also study the effect of patients’ health literacy on the effectiveness of the intervention, as well as the effect of the intervention on health care utilization, disease-specific quality of life, and costs.
Methods
Patient Enrollment
Patient enrollment began in May 2008 and ended in September 2009. Eligible patients were at least 18 years of age and admitted for acute coronary syndrome (ACS) or acute decompensated heart failure (ADHF). ACS was defined according to national guidelines.10 ADHF was determined by the presence of at least 2 major, or 1 major and 2 minor, Framingham criteria.24 Patients with volume overload due to sepsis, arteriovenous shunts, renal failure, or administration of intravenous fluids or blood products were not considered to have ADHF.
Patients were excluded if they were receiving intravenous cardiac inotropic medications at home; intravenous pressors, a ventricular assist device, intra-aortic balloon pump in the hospital; aggressive medication management as a result of recent or imminently planned transplantation; or another medication management program. Patients were excluded in the presence of corrected visual acuity worse than 20/200, severe hearing impairment, unintelligible speech, inability to communicate in English or Spanish, severe mental illness (psychosis or bipolar disorder) requiring active treatment, delirium or severe dementia (determined by diagnosis, altered consciousness, or lack of orientation to person/place/time), no telephone, a caregiver who managed their medications, planned discharge to a location other than home, police custody, or previous enrollment in the study. Finally, patients were excluded if discharge was anticipated within 3 hours of screening, or if study pharmacists would be unavailable to deliver the intervention if the patient were randomized to the intervention arm.
At each hospital, research assistants (RAs) identified potential subjects within 24 hours of admission through medical record review, as permitted by each site’s Institutional Review Board (IRB). The RAs approached each patient to confirm eligibility, provide study information, and seek written informed consent, including permission to obtain all medical records from the patient’s outpatient physicians and pharmacies within 30 days of hospital discharge. The consent process included a teach-back of key points to confirm patient comprehension prior to enrollment.25 All study procedures and materials at VUH and BWH were approved by the Vanderbilt University IRB and the Partners Human Research Committee, respectively.
Following informed consent, RAs conducted an intake interview which included questions about demographics, health literacy (short form of the Test of Functional Health Literacy in Adults, s-TOFHLA),26 cognitive function (Mini-Cog),27 medication self-management strategies,28 and self-reported medication adherence.29 Understanding of the preadmission medication regimen was measured using a tool we developed based on prior work.30, 31
Randomization
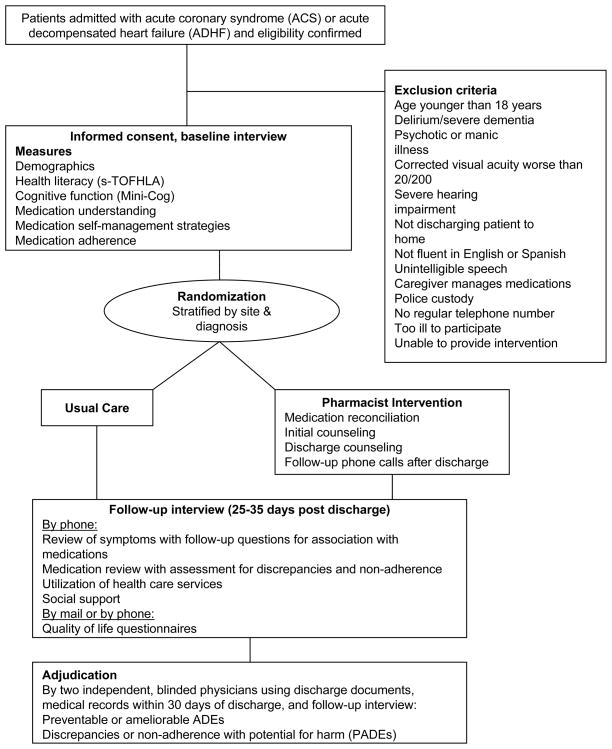
Consented patients were randomized to receive usual care or usual care plus the intervention (Figure 2). The randomization sequence was computer generated in permuted blocks of 2 to 6 patients, stratified by study site and diagnosis (ACS or ADHF). Randomization was managed with concealment of treatment allocation by a member of the study team at each site who was unblinded to patient assignment and had no role in outcome assessment. All investigators and outcome assessors were blinded.
Figure 2.
Study schema
Intervention
There were 4 intervention components (Table 1), developed according to the following principles:
Table 1.
Components of Pharmacist Intervention
| Activity | Details |
|---|---|
| 1. Medication reconciliation |
Pharmacist:
|
|
| |
| 2. Initial counseling |
Pharmacist:
|
|
| |
| 3. Discharge Counseling |
Pharmacist:
|
|
| |
| 4. Follow-up phone calls (1 to 4 days after discharge) |
Study Coordinator:
|
Pharmacist (if needed):
|
|
Pharmacist oversight can mitigate the potential harm caused by medication errors during care transitions, even with mandated medication reconciliation, because many institutions have difficulty implementing reconciliation and errors still remain.30
Tailored patient education can increase knowledge of medications and improve medication use.32 Educational interventions may benefit patients with low health literacy if they adhere to clear health communication guidelines.33
Phone calls within 72 hours of discharge can identify and mitigate problems related to filling prescriptions, early side effects, and misunderstanding of the regimen.34 A team-based approach leveraging non-clinical personnel can reduce program costs while maintaining effectiveness, reserving hospital pharmacists for specific activities that require a higher level of skill and training.
Understanding the patient subgroups that benefit most from the intervention, as well as program costs, will enable hospitals to use resources more judiciously.
In-hospital components
Pharmacists performed medication reconciliation upon enrollment, at discharge, and during in-hospital transfers. When unintentional discrepancies were identified, the pharmacist contacted the patient’s inpatient team to resolve the differences.
Intervention patients received tailored counseling by a pharmacist at enrollment and discharge. Pharmacists were informed of the patient’s health literacy and cognitive function so they could tailor their approach. During the initial counseling session, the pharmacist assessed the patient’s understanding of the pre-admission regimen and medication labels; medication adherence; barriers to adherence; and level of social support. A hospital social worker was notified if it appeared that the patient might need assistance obtaining discharge medications.
During the discharge counseling session, the pharmacist provided tailored counseling based on the initial assessment of that individual’s medication management needs and the discharge regimen determined by the treating physicians. The pharmacist emphasized differences between the preadmission medication regimen and the one prescribed at discharge, reviewed strategies to enhance medication adherence and techniques to prevent or ameliorate side effects. Extra counseling was provided for medications with special instructions (e.g., warfarin).
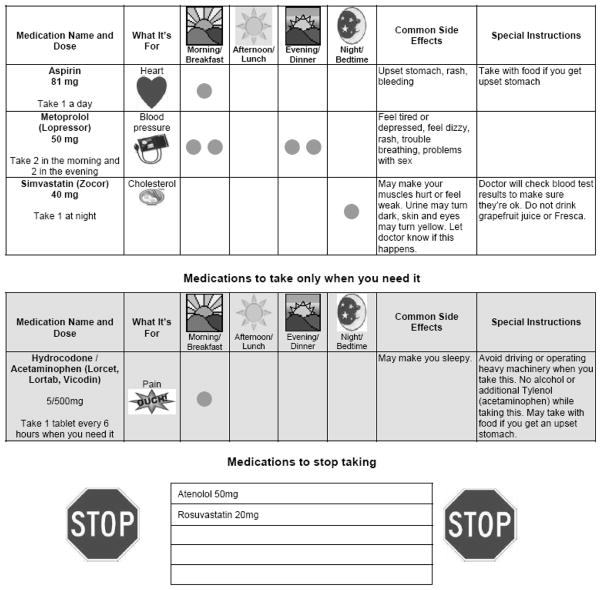
Patients received a personalized illustrated medication schedule demonstrating why, when and how they should take their medications, as well as major and common side effects (Figure 3).35 The medication schedule facilitated the pharmacist-patient interaction and served as a take-home educational aid. The schedule was prepared by a pharmacist using a master template created by the study team that contained pictures for indications, standard directions, and common side effects. Patients were asked to “teach-back” key aspects of their medication regimen to confirm understanding.36 Patients were offered a pill box and taught to fill it using the medication schedule as a guide. When discharge occurred on the day of enrollment, the admission and discharge counseling sessions were combined, with an emphasis on discharge elements.
Figure 3.
Example of a personalized medication schedule (reduced in size).
Post-discharge components
At each site, the unblinded project coordinator called each intervention patient 1–4 days after hospital discharge. The coordinator 1) reviewed the medications to identify any discrepancies from the discharge medication list, confirm that prescriptions had been filled, and verify that the patient understood how to take each medication; 2) performed a review of systems with follow-up questions to screen for early medication side effects; 3) documented discrepancies, non-adherence, new or worsening symptoms, and issues related to physician follow-up; and 4) communicated significant information to the study pharmacist. Pharmacists managed any significant issues in collaboration with the patient’s treating physicians, and made additional phone calls as needed with a frequency determined by the nature of the issue.
If the study pharmacist was unavailable at the time of discharge (i.e., evenings or weekends), the pharmacist called the patient at home within 1 to 4 days to perform discharge counseling and the follow-up call. In these situations, the personalized medication schedule and pill box were mailed.
Intervention fidelity
Pharmacists helped develop the content and delivery of the intervention, from which a procedure manual and reference sheet were created. Pharmacists were trained to deliver the intervention in a standardized fashion, while tailoring it to the patient’s needs. This training included one-on-one feedback using practice patients. Pharmacists and study staff received training in clear verbal communication using previously developed educational programs.37–39 Finally, with patient permission, we audio recorded a sample of counseling sessions at each site and provided pharmacists with individual feedback.
Usual Care
Patients in the usual care arm received routine counseling by the nurse and treating physicians at hospital discharge. In accordance with Joint Commission requirements,23 each hospital has a protocol for performing medication reconciliation within 24 hours of admission, at transfers of care, and discharge. In these academic centers, medical residents are responsible for medication reconciliation, although they can consult a nurse or clinical pharmacist for assistance. Nurses routinely verify reconciled medication information at hospital discharge in preparation to counsel patients on their discharge prescriptions. The process of obtaining a medication history for reconciliation at each hospital is facilitated by electronic medication records which contain complete prescribing information from the hospital and affiliated outpatient clinics. BWH also benefits from an internally developed, medication reconciliation application which has been shown to decrease potential ADEs.6
Outcome Assessment
Primary and secondary outcomes are shown in Table 2. Outcomes were based upon patient responses to a follow-up telephone interview conducted by research staff approximately 30 days (range 25–35 days) after discharge and a review of all available medical records during the 30 days after discharge. Up to 10 call attempts were made, primarily at times that each patient had previously indicated would be most convenient. The follow-up interview included a detailed review of symptoms, a medication review, an assessment of planned and unplanned health care utilization, and a quality of life evaluation.40, 41 For new or worsening symptoms, follow-up questions elicited details such as the onset, duration, and effect on the patient. The interviewer inquired if symptoms were related to medications, if a physician implicated a medication, and if symptoms responded to any adjustments in the medication regimen. RAs asked patients to state which medications they were supposed to be taking and then compared this to the discharge medication list (or a more recent list, if available). Discrepancies (e.g., change in dose or frequency, omissions, additional medications) were noted and reasons for these discrepancies were explored with the patient to determine if they were intentional or unintentional. Intentional medication changes were those reportedly made by a physician or due to completion of a prescribed course; physician changes to a regimen were confirmed with medical record review when possible. Finally, RAs assessed adherence by asking the number of days in the previous week that each medication was not taken as prescribed. This method of assessing self-reported adherence has been validated previously and associated with cardiovascular risk factor control.42
Table 2.
Outcome measures and definitions
| Primary Outcome | Description |
|---|---|
| Serious Medication Errors (SME) | Number of SMEs per patient in the first 30 days after hospital discharge |
| Composite of Preventable or Ameliorable ADEs or Potential ADEs | |
|
| |
| Secondary Outcomes | Description |
|
| |
| Health care utilization | Number of all emergency department visits and unplanned hospital readmissions within 30 days of discharge |
| Quality of life measure | |
| KCCQ | Quality of life assessment using the Kansas City Cardiomyopathy Questionnaire for all patients admitted with ADHF |
| SAQ | Quality of life assessment using Seattle Angina Questionnaire for all patients admitted with ACS |
| Cost effectiveness | Replication costs of the intervention and costs per SME averted |
Primary Outcome: Serious Medication Errors
The primary outcome is the occurrence of SMEs within 30 days after hospital discharge. SME is a composite of 1) preventable or ameliorable ADEs, and 2) potential ADEs as previously defined. To determine the presence of a SME, two blinded physician adjudicators independently reviewed the discharge summary and medication orders from the index hospitalization, the final 30-day interview report, and all available medical records within 30 days after discharge. The adjudicators followed a standardized procedure based on previously validated methods.5, 6, 43, 44 Differences between adjudicators were resolved by discussion or with assistance from a third adjudicator when needed. Early adjudication sessions were supervised to ensure consistency in adjudication practices, with teaching points documented and communicated to all adjudicators across sites.
ADEs
The presence of ADEs was based on the 30-day review of symptoms and chart review. For each adverse event, adjudicators decided whether the injury was related to a medication using a six-point confidence scale, based on clinical judgment and the Naranjo algorithm,45 a validated scoring system to assess causality. For all ADEs (i.e., greater than 50% confidence that the injury was due to a medication), clinicians determined the likelihood that it could have been prevented or ameliorated by any change in management. “Probably” or “definitely” preventable and/or ameliorable ADEs were counted in the outcome. Severity of ADEs was determined using two definitions: 1) “significant,” “serious,” “life-threatening,” or “fatal,” using previously established definitions,46 and 2) serious ADEs as defined by the Food and Drug Administration.47
Potential ADEs
Based on their review of the 30-day medication history and patient chart, adjudicators noted the presence of unintentional medication discrepancies and self-reported non-adherence. Based on the medication involved and the degree of deviation from what was prescribed, adjudicators determined the likelihood of it potentially causing harm. Cases with at least a 50% likelihood of potential harm were considered potential ADEs, and the severity of potential ADEs was graded as “significant,” “serious,” or “life-threatening” using definitions analogous to the severity of ADEs.30
Secondary Outcomes
Quality of life
Quality of life questionnaires were mailed to all patients prior to the 30-day follow-up call. Questionnaires could be completed by mail or, if preferred, administered by phone during the follow-up call. All patients admitted with ADHF received the Kansas City Cardiomyopathy Questionnaire.40 Those admitted with ACS received the Seattle Angina Questionnaire.41
Excess healthcare utilization
Healthcare utilization will be assessed as the number of Emergency Department (ED) visits and unplanned hospital readmissions within 30 days of discharge, including care received at the study hospitals and other facilities. Utilization rates will be based on all available hospital and ED records as well as self-report.
Cost effectiveness
We will estimate program replication costs for potential adopters of the intervention, as well as the program cost per SME averted.
Power and Sample Size
We based the initial sample size calculations on achieving a 25% reduction in the percentage of patients who would experience at least 1 SME after discharge.20 Assuming a control event rate of 40%,4, 5, 19, 48 80% power, α of 0.05, and 15% loss to follow-up, we planned to enroll 862 patients. Prior to study initiation, we reframed the primary outcome as the number of SMEs per patient, rather than the percentage of patients with at least 1 SME. A review of the frequency of SMEs among 50 patients demonstrated that the number of SMEs per patient is an over-dispersed count, with mean=1.0 (95% CI=0.63 to 1.37). Using simulations, we determined that with 862 patients we will be able to detect a 30% reduction in the primary outcome, with 80% power and α of 0.05.
Statistical Analysis
Data will be analyzed on the basis of intent-to-treat. We will also perform a sensitivity analysis (per protocol “as treated” analysis). We will examine patient characteristics in the two study arms using proportions for categorical variables, and means and standard deviations or medians and interquartile ranges for continuous variables, depending on their distribution. For univariable comparisons of the primary outcome, we will assess whether the number of SMEs per patient differs between the intervention arm and usual care using the Wilcoxon Rank Sum test. To adjust for potential confounding, we will conduct multivariable analysis using Poisson or negative binomial regression, depending on the characteristics of the outcome variable. Potential confounders, chosen a priori,49 will include health literacy, age, cognition, number of pre-admission medications, number of high-risk medications, and medication understanding score. The model will also include an interaction term of the intervention and health literacy to examine whether health literacy modifies the effect of the intervention. Other intervention effect modifiers to be evaluated using interaction terms will include number of pre-admission medications, number of high-risk medications, and medication understanding score. Secondary outcomes – including each component of SMEs (preventable or ameliorable ADEs, and potential ADEs), quality of life, and health care utilization – will be modeled similarly, with model type determined by characteristics of the outcome variable. All analysis will be performed in statistical language R, version 2.6.0 (http://www.r-project.org/). We will use a two-sided 5% significance level for all statistical inferences.
Intervention Fidelity and Follow-Up
A total of 430 patients (200 of 403 enrollees at VUH, and 230 of 459 at BWH) were randomized to receive the intervention. The majority (N=402, 93.5%) received tailored pharmacist counseling that included review of discharge medications, either in person or by phone (Table 3). Most patients (N=367, 85.3%) received the follow-up phone call to review medications and symptoms.
Table 3.
Intervention fidelity among the 430 patients randomized to intervention arm.
| Patient Intervention Delivery | Total N= 430 | Vanderbilt N=200 | BWH N=230 |
|---|---|---|---|
| 2 separate counseling sessions (initial and discharge) in hospital | 247 (57.4) | 106 (53.0) | 141 (61.3) |
| 1 combined counseling session in hospital | 45 (10.5) | 14 (7.0) | 31 (13.5) |
| Initial counseling in hospital and discharge counseling by phone | 95 (22.1) | 60 (30.0) | 35 (15.2) |
| Both initial and discharge counseling by phone | 15 (3.5) | 5 (2.5) | 10 (4.3) |
| Initial session in hospital and no discharge counseling* | 16 (3.7) | 11 (5.5) | 5 (2.2) |
| No intervention† | 12 (2.8) | 4 (2.0) | 8 (3.5) |
| Post discharge follow up phone call completed (1–4 days post discharge) | 367 (85.3) | 181 (90.5) | 186 (80.9) |
Values are presented as N (%)
Pharmacist unable to reach patients due to early discharge, inability to reach patient by phone, patient withdrawal from study, death, or patient not having any medications ordered at discharge.
Among these: 2 died in hospital, 2 withdrew consent, and 8 did not receive intervention for logistical and/or clerical reasons (e.g., pharmacist unavailable).
Three patients withdrew from the study and 6 died in the hospital prior to entering the follow-up period. A total of 690 (80.0%) were successfully contacted for 30-day follow-up.
Summary
The PILL-CVD study was designed to reduce SMEs after hospital discharge through a pharmacist-based intervention. If effective, the intervention will serve as a basis for developing guidelines for hospitals and health care facilities on pharmacist assisted medication reconciliation, inpatient pharmacist counseling, low-literacy adherence aids, and patient follow-up after discharge. Cost effectiveness analysis, coupled with identification of patients most likely to benefit from this intervention, will inform hospitals about how to improve medication safety while using resources judiciously.
Acknowledgments
FUNDING SOURCES
Funded by R01 HL089755 (NHLBI, Kripalani) and also by K23 HL077597 (NHLBI, Kripalani), K08 HL072806 (NHLBI, Schnipper), and VA Career Development Award 04-342-2 (Roumie).
APPENDIX
The PILL-CVD study group includes the following:
Investigators: Sunil Kripalani, MD, MSc (PI); Jeffrey L. Schnipper, MD, MPH (BWH site-PI); Christianne L. Roumie, MD, MPH; Anuj K. Dalal, MD; Terry A. Jacobson, MD; Kimberly J. Rask, MD, PhD; Viola Vaccarino, MD, PhD; Tejal K. Gandhi, MD, MPH; and David W. Bates, MD, MSc.
Biostatistics: Svetlana K. Eden, MS; Charles Dupont.
Research staff: Courtney Cawthon, MPH (coordinator); Alexandra Businger (coordinator); Ileko Mugalla, MS, PhD, MPH; Kurt J. Niesner; Abby Stufflebam; Meghan M. Higgins; Edith Swain; Jeffrey Kemnitz; Harry Reyes; Alison C. Pietras; Arianne Cordon; Catherine Liang, MPH.
Pharmacists: Dan Johnson, PharmD; Erin Bedard, PharmD; Stephanie Labonville, PharmD; Judy Cheng, PharmD, MPH; Heather Dell’Orfano, PharmD; Radmila Levinson, PharmD; Beth Anne Filkins, PharmD; Pershank Bamarni, PharmD; Eli Guadalupe, DPh; Jill Helmke, DPh, NPh; David Gregory, PharmD; Marketa Marvanova, PharmD, PhD.
Outcome assessors: Kelly E. Cunningham, MD; L. Jeff Harris, MD; Cecelia Theobald, MD; Robert L. Huang, MD, MPH; Danielle Scheurer, MD, MSc; Susan Hunt, MD.
External advisors: Mark V. Williams, MD; Daniel J. Cobaugh, PharmD
Footnotes
Medication Reconciliation is “the process of creating the most accurate list possible of all medications a patient is taking – including drug name, dosage, frequency, and route – and comparing that list against the physician’s admission, transfer, and/or discharge orders, with the goal of providing correct medications to the patient at all transition points within the hospital.”
DISCLOSURES
Dr. Kripalani is a consultant to and holds equity in PictureRx, LLC, which makes patient education tools to improve medication management. PictureRx did not provide materials or funding for this study.
References
- 1.Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med. 2004;141:533–536. doi: 10.7326/0003-4819-141-7-200410050-00009. [DOI] [PubMed] [Google Scholar]
- 2.Coleman EA, Smith JD, Raha D, Min SJ. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165:1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 3.Clark PA, Drain M, Gesell SB, Mylod DM, Kaldenberg DO, Hamilton J. Patient perceptions of quality in discharge instruction. Patient Educ Couns. 2005;59:56–68. doi: 10.1016/j.pec.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 5.Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, Vander Vliet M, Nemeskal R, Leape L. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274:29–34. [PubMed] [Google Scholar]
- 6.Schnipper JL, Kirwin JL, Cotugno MC, Wahlstrom SA, Brown BA, Tarvin E, Kachalia A, Horng M, Roy CL, McKean SC, Bates DW. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006;166:565–571. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 7.Forster AJ, Clark HD, Menard A, Dupuis N, Chernish R, Chandok N, Khan A, van Walraven C. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170:345–349. [PMC free article] [PubMed] [Google Scholar]
- 8.Beers MH, Dang J, Hasegawa J, Tamai IY. Influence of hospitalization on drug therapy in the elderly. J Am Geriatr Soc. 1989;37:679–683. doi: 10.1111/j.1532-5415.1989.tb02227.x. [DOI] [PubMed] [Google Scholar]
- 9.Rask KJ, Wells KJ, Teitel GS, Hawley JN, Richards C, Gazmararian JA. Can an algorithm for appropriate prescribing predict adverse drug events? Am J Manag Care. 2005;11:145–151. [PubMed] [Google Scholar]
- 10.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DEJ, Chavey WEI, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, American College of Physicians, Society for Academic Emergency Medicine, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2007;50:e1–157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA American College of Cardiology, American Heart Association Task Force on Practice Guidelines. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 13.Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, Krumholz HM, Rumsfeld JS. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, Messenger JC, Khanal S, Peterson ED, Bach RG, Krumholz HM, Cohen DJ. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- 15.Selden CR, Zorn M, Ratzan S, Parker RM. Current bibliographies in medicine: health literacy. Bethesda, MD: National Library of Medicine; 2000. [Google Scholar]
- 16.Davis TC, Wolf MS, Bass PF, III, Thompson JA, Tilson HH, Neuberger M, Parker RM. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145:887– 894. doi: 10.7326/0003-4819-145-12-200612190-00144. [DOI] [PubMed] [Google Scholar]
- 17.Gazmararian J, Kripalani S, Miller MJ, Echt KV, Ren J, Rask KJ. Factors associated with medication refill adherence in cardiovascular-related diseases: a focus on health literacy. J Gen Intern Med. 2006;21:1215–1221. doi: 10.1111/j.1525-1497.2006.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray MD, Tu W, Wu J, Morrow D, Smith F, Brater DC. Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills. Clin Pharmacol Ther. 2009;85:651–658. doi: 10.1038/clpt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kripalani S, Henderson LE, Jacobson TA, Vaccarino V. Medication use among inner-city patients after hospital discharge: patient reported barriers and solutions. Mayo Clin Proc. 2008;83:529–535. doi: 10.4065/83.5.529. [DOI] [PubMed] [Google Scholar]
- 20.Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166:955–964. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 21.Coleman EA, Parry C, Chalmers S, Min S. The Care Transitions Intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 22.Jack BW, Chetty VK, Anthony D, Greenwald JL, Sanchez GM, Johnson AE, Forsythe SR, O’Donnell JK, Paasche-Orlow MK, Manasseh C, Martin S, Culpepper L. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joint Commission on Accreditation of Healthcare Organizations. [Accessed July 17, 2006.];Joint Commission National Patient Safety Goals. Available at: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/
- 24.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 25.Kripalani S, Bengtzen R, Henderson LE, Jacobson TA. Clinical research in low-literacy populations: using teach-back to assess comprehension of informed consent and privacy information. IRB. 2008;30:13–19. [PubMed] [Google Scholar]
- 26.Nurss JR, Parker RM, Williams MV, Baker DW. Short test of functional health literacy in adults. Snow Camp, NC: Peppercorn Books and Press; 1998. [Google Scholar]
- 27.Borson S, Scanlan JM, Watanabe J, Tu SP, Lessig M. Simplifying detection of cognitive impairment: comparison of the Mini-Cog and Mini-Mental State Examination in a multiethnic sample. J Am Geriatr Soc. 2005;53:871–874. doi: 10.1111/j.1532-5415.2005.53269.x. [DOI] [PubMed] [Google Scholar]
- 28.Littenberg B, MacLean CD, Hurowitz L. The use of adherence aids by adults with diabetes: a cross-sectional survey. BMC Fam Pract. 2006;7:1. doi: 10.1186/1471-2296-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self- reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, Carty MG, Karson AS, Bhan I, Coley CM, Liang CL, Turchin A, McCarthy PC, Schnipper JL. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23:1414–1422. doi: 10.1007/s11606-008-0687-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farris KB, Phillips BB. Instruments assessing capacity to manage medications. Ann Pharmacother. 2008;42:1026–1036. doi: 10.1345/aph.1G502. [DOI] [PubMed] [Google Scholar]
- 32.Krueger KP, Felkey BG, Berger BA. Improving adherence and persistence: a review and assessment of interventions and description of steps toward a national adherence initiative. J Am Pharm Assoc. 2003;43:668–678. doi: 10.1331/154434503322642598. [DOI] [PubMed] [Google Scholar]
- 33.Rothman RL, DeWalt DA, Malone R, Bryant B, Shintani A, Crigler B, Weinberger M, Pignone M. Influence of patient literacy on the effectiveness of a primary care-based diabetes disease management program. JAMA. 2004;292:1711–1716. doi: 10.1001/jama.292.14.1711. [DOI] [PubMed] [Google Scholar]
- 34.Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Am J Med. 2001;111:26S–30S. doi: 10.1016/s0002-9343(01)00966-4. [DOI] [PubMed] [Google Scholar]
- 35.Kripalani S, Robertson R, Love-Ghaffari MH, Henderson LE, Praska J, Strawder A, Katz MG, Jacobson TA. Development of an illustrated medication schedule as a low-literacy patient education tool. Patient Educ Couns. 2007;66:368–377. doi: 10.1016/j.pec.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Leong-Grotz K, Castro C, Bindman AB. Closing the loop. Physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163:83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- 37.Kripalani S, Jacobson KL. A training program for pharmacy staff. (Curriculum guide prepared under contract No. 290-00-0011 T07.). AHRQ publication No. 07(08)-0051-1-EF. Rockville, MD: Agency for Healthcare Research and Quality; Oct, 2007. Strategies to improve communication between pharmacy staff and patients. Available at http://www.ahrq.gov/qual/pharmlit/pharmtrain.htm. [Google Scholar]
- 38.Kripalani S, Jacobson KL, Brown S, Manning K, Rask KJ, Jacobson TA. Development and implementation of a health literacy training program for medical residents. Med Educ Online. 2006;11:1–8. doi: 10.3402/meo.v11i.4612. [DOI] [PubMed] [Google Scholar]
- 39.Kripalani S, Weiss BD. Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21:888–890. doi: 10.1111/j.1525-1497.2006.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 41.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 42.Grant RW, Devita NG, Singer DE, Meigs JB. Polypharmacy and medication adherence in patients with type 2 diabetes. Diabetes Care. 2003;26:1408–1412. doi: 10.2337/diacare.26.5.1408. [DOI] [PubMed] [Google Scholar]
- 43.Gandhi TK, Weingart SN, Borus J, Seger AC, Peterson J, Burdick E, Seger DL, Shu K, Federico F, Leape LL, Bates DW. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 44.Poon EG, Cina JL, Churchill W, Patel N, Featherstone E, Rothschild JM, Keohane CA, Whittemore AD, Bates DW, Gandhi TK. Medication dispensing errors and potential adverse drug events before and after implementing bar code technology in the pharmacy. Ann Intern Med. 2006;145:426–434. doi: 10.7326/0003-4819-145-6-200609190-00006. [DOI] [PubMed] [Google Scholar]
- 45.Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, Hebert L, Newhouse JP, Weiler PC, Hiatt H. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–384. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 46.Folli HL, Poole RL, Benitz WE, Russo JC. Medication error prevention by clinical pharmacists in two children’s hospitals. Pediatrics. 1987;79:718–722. [PubMed] [Google Scholar]
- 47.Food and Drug Administration. [Accessed Sept 25, 2009.];What is a serious adverse event? Available at: http://www.fda.gov/Safety/MedWatch/HowToReport/ucm053087.htm.
- 48.Moore C, Wisnivesky J, Williams S, McGinn T. Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18:646–651. doi: 10.1046/j.1525-1497.2003.20722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Senn S. Testing for balance in clinical trials. Statistics in Medicine. 1994;13:1715–1726. doi: 10.1002/sim.4780131703. [DOI] [PubMed] [Google Scholar]