Abstract
Originally identified by their unusual ability to bind guanosine monophosphate (GMP) nucleotide agarose, the guanylate-binding proteins (GBPs) were used extensively to promote our understanding of interferon-induced gene transcription and as markers of interferon responsiveness. Structural and biochemical analyses of human GBP-1 subsequently demonstrated that the GBPs are a unique subfamily of guanosine triphosphatase (GTPases) that hydrolyze guanosine triphosphate (GTP) to both guanosine diphosphate (GDP) and GMP. As members of the larger dynamin superfamily of GTPases, GBPs exhibit such properties as nucleotide-dependent oligomerization and concentration-dependent GTPase activity. Recently, progress has been made in assigning functions to members of the GBP family. While many of these functions involve protection against intracellular pathogens, a growing number of them are not directly related to pathogen protection. It is currently unclear how the unusual properties of GBPs contribute to this growing list of functions. As future studies uncover the molecular mechanism(s) of action of the GBPs, we will gain a greater understanding of how individual GBPs can mediate what currently appears to be a divergent set of functions.
Introduction
Four major families of large guanosine triphosphatase (GTPases) contribute to interferon (IFN) responses in a variety of organisms (reviewed in MacMicking 2004). These are the Mx family, the very large inducible GTPases, the p47 immunity-related GTPases (IRGs), and the guanylate-binding proteins (GBPs). The function of very large inducible GTPase-1 is still unknown, but it is clear that the 280 kDa GTPase is induced by both type I and type II IFNs (Klamp and others 2003). The Mx proteins are induced by type I and type III IFNs and are best known for their antiviral activities (Haller and others 2007a, 2007b). They will be covered in detail in this issue. The response of murine cells to IFN-γ is dominated by the p47 IRGs and GBPs (Boehm and others 1998; Shenoy and others 2008). Members of both families are induced by type I IFNs but are much more robustly expressed after IFN-γ exposure. Studies in mice have elegantly characterized the activity of p47 IRGs against intracellular pathogens (reviewed in Howard 2008). However, the finding that humans lack IFN-induced members of the IRG family (Bekpen and others 2005) has shifted interest to the GBPs as the candidate proteins involved in the cellular resistance to these same intracellular pathogens in humans. Evidence will be presented that GBPs may be very important in resistance to pathogens. However, GBPs also mediate IFN responses that are not directly related to defense against pathogens.
Introduction to the GBPs
The GBPs are a family of large cytokine-induced GTPases that, based on their structural and biochemical properties, are a large subfamily within the dynamin superfamily of large GTPases. In mice there are 11 GBPs (designated mGBP-1 through −11), distributed within 2 clusters over 2 chromosomes (Olszewski and others 2006; Degrandi and others 2007; Kresse and others 2008). Humans are believed to have 7 GBPs (designated hGBP-1 through −7], all located within a single cluster on chromosome 1 (Olszewski and others 2006). Unfortunately, similarly numbered GBPs are not the most closely related. For example, hGBP-5 is not necessarily the ortholog of mGBP-5. All of the murine GBPs can be induced by IFN-γ (Degrandi and others 2007). It is unclear whether all of the murine promoters possess interferon-sensitive response elements (ISREs) and are inducible by type I IFNs. Some of the murine GBPs are also transcriptionally induced by interleukin-1α (IL-1α), IL-1β, and tumor necrosis factor-α (TNF-α). While interferon-gamma activated sequence (GAS) and ISRE elements are found in the promoters of many GBPs, not all of the hGBPs have either 1 or both of these elements (Olszewski and others 2006), so it remains unclear whether all of the hGBPs are induced by either type I or type II IFNs. However, hGBPs 1 through 5 can be induced by IFN-γ in cultured endothelial cells. In addition, hGBP-1, −2, and −3 can also be induced by IL-1β and TNF-α (Tripal and others 2007).
Isoprenylation of GBPs
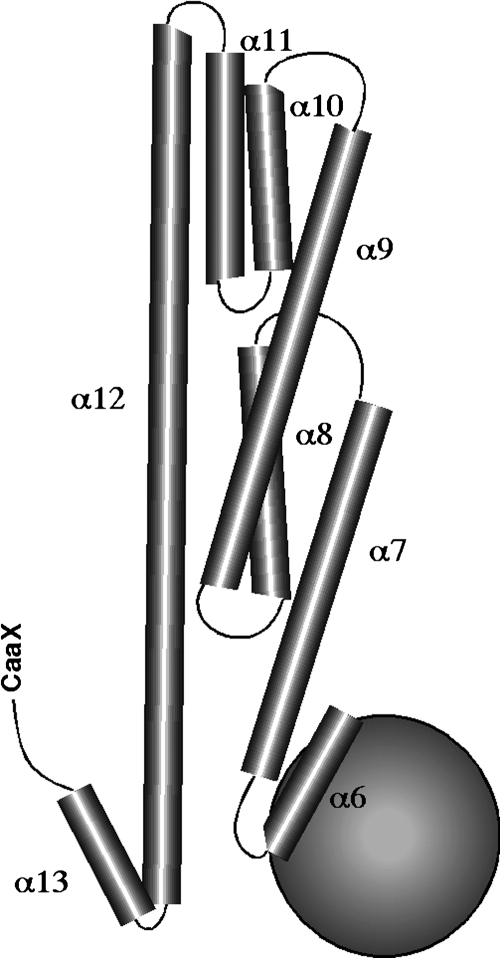
Isoprenylation is the addition of either a C-15 farnesyl or C-20 geranylgeranyl lipid moiety to the extreme carboxy terminus of a protein (reviewed in Rando 1996; Gelb and others 1998; Sinensky 2000) (Fig. 1). The addition of lipid and the choice of which of the 2 lipids to add is directed by a motif involving the last 4 amino acids at the C-terminus of the protein, called a CaaX sequence. A CaaX sequence is composed of a cysteine (C) followed by 2 amino acids that are usually aliphatic (aa). It is the terminal amino acid (X) that dictates which lipid is added to the protein. The terminal amino acid for hGBP-2, mGBP-2, mGBP-1, and hGBP-5 is leucine, which would predict addition of the C-20 geranylgeranyl lipid (Table 1). hGBP-1 and mGBP-5 have serine as the terminal amino acid, which should direct the addition of the farnesyl lipid (Table 1). Just because a protein has a CaaX sequence does not mean that it will be isoprenylated in vivo. There are known exceptions (Marshall 1993; Pfeffer and others 1995). Isoprenylation in vivo has been confirmed for hGBP-1 and mGBP-2 (Nantais and others 1996; Vestal and others 1998). Despite an identical CaaX sequence as mGBP-2, mGBP-1 is poorly prenylated, if at all (Stickney and Buss 2000). The remaining GBPs do not have CaaX sites and therefore cannot be prenylated.
FIG. 1.
Schematic of hGBP-1 structure. The large G domain of hGBP-1 is represented by a ball, and the α-helical regions from helix 6 through 13 are represented by barrels (reprinted from Vestal 2005). The schematic was based on the structural information from the study by Prakash and others (2000a).
Table 1.
CaaX Sites of Guanylate-Binding Proteins
| CaaX | Lipid | |
|---|---|---|
| mGBP-1 | CTIL | C-20a |
| mGBP-2 | CTIL | C-20b |
| mGBP-5 | CVIS | C-15 |
| hGBP-1 | CTIS | C-15b |
| hGBP-2 | CNIL | C-20 |
| hGBP-5 | CVLL | C-20 |
The GBPs containing CaaX sites at their C-termini are listed, as well as the amino acids contained within the CaaX site and the lipid that would be directed to this site.
GBP that is poorly prenylated, if at all.
GBPs that have been demonstrated to be prenylated.
GBPs, guanylate-binding proteins.
Isoprenylation is important in targeting proteins to intracellular membranes and/or to facilitate protein/protein interactions (Maltese and others 1996; Sinensky 2000). Where examined, prenylation is critical for at least one of the functions of any given prenylated protein. Whether prenylated or not, GBPs are predominantly cytosolic and have, at most, a relatively small portion of the total amount of the protein associated with membranes (Nantais and others 1996; Vestal and others 1998; Stickney and Buss 2000). However, as will be described below, some of the GBPs do associate with intracellular membranes. The ability to associate with membranes is an additional property that GBPs share with other members of the dynamin superfamily.
Structural and Biochemical Properties of GBPs
Even before the sequence analyses of the first cloned GBPs were completed, it was apparent that these GTPases would be unusual members of the superfamily. GBPs were originally identified by virtue of their ability to bind guanine nucleotide-conjugated agaroses (Cheng and others 1983, 1985). This ability is rare. Not only were GBPs able to bind guanosine triphosphate (GTP)- and GDP-agarose, but they also bound GMP-agarose (Cheng and others 1985). GBPs were later found to have the first 2 motifs of the consensus sequence for guanine nucleotide binding by GTPases (GxxxxGK(S/T) and DxxG) but to lack the third conserved motif ((N/T)KxD) that binds to the guanine base (Cheng and others 1991). An unusual sequence of TLRD at amino acid residues 181–184 was later identified as the region of guanine base contact (Praefcke and others 1999). Consistent with this unusual nucleotide binding region, recombinant hGBP-1 hydrolyzes GTP to both GDP and GMP, with GMP being the most abundant product (Table 2) (Schwemmle and Staeheli 1994). While recombinant hGBP-2 also hydrolyzes GTP to both GDP and GMP, GDP is the most prominent of the 2 products (Table 2) (Neun and others 1996). Because of a very fast dissociation rate, recombinant hGBP-1 has a much lower affinity for GTP than other GTPases such as Ras or Gα (Praefcke and others 1999).
Table 2.
Products of GTP Hydrolysis
| GDP (%) | GMP (%) | |
|---|---|---|
| hGBP-1 | 15 | 85 |
| hGBP-1 lipid conj. | 70 | 30 |
| hGBP-2 | 89 | 11 |
| hGBP-5 | 100 | 0 |
The percentages of GDP and GMP generated during GTP hydrolysis by members of the GBP family are listed. hGBP-1 lipid conj. is farnesylated hGBP-1 associated with liposomes.
conj., conjugated; GDP, guanosine diphosphate; GMP, guanosine monophosphate; GTP, guanosine triphosphate.
The first crystal structure of hGBP-1 was solved in the absence of bound nucleotide and illuminated a number of unusual properties for hGBP-1 (Prakash and others 2000a). At the amino terminus of hGBP-1 there is a compact globular domain, which investigators named the large G (LG) domain (Fig. 1). This is followed by an elongated series of α-helices. The LG is connected to the C-terminal α-helical domain by a short intermediate region consisting of an α-helical domain and 2 β-sheets (Prakash and others 2000a). The α-helical domain can actually be subdivided into 2 α-helical subdomains. A series of 5 α-helical regions extend distally from the LG domain. These helices are followed by a turn, which allows the very long α12 helix to double back and interact with other regions of the protein, such as the LG domain (Prakash and others 2000a). The overall length of the protein is about 130 Å (Prakash and others 2000a), making it a long and somewhat rodlike protein.
A second crystal structure for hGBP-1 was solved in the presence of GppNHp, a nonhydrolyzable analog of GTP (Prakash and others 2000b). Additional novel features emerged. While the Mg2+ coordination and P-loop orientation of hGBP-1 is very similar to those in members of the Ras and heterotrimeric G-protein families, the orientation of the guanine base is very different (Prakash and others 2000b). In hGBP-1, the glycosidic bond of the guanine nucleotide is at a very different angle compared to the glycosidic bond in all other GTPases, which have a very conserved angle to their N-glycosidic bond. In addition, hGBP-1 has 2 unique regions in the area of the guanine base and phosphate-binding regions. These have been named the guanine and phosphate caps (Prakash and others 2000b). The phosphate cap is found in the region analogous to Switch I of Ras and is proposed to shield the phosphate groups from the surrounding solution in a manner that would prevent access by a potential GTPase-activating protein (GAP) (Prakash and others 2000b). These observations, together with the findings of internal GAPs in both Mx and dynamin, prompted the suggestion of a possible internal GAP for hGBP-1. Indeed, recently, the intermediate region α-helices have been shown to function as an internal GAP (Abdullah and others 2009).
Further analysis of the purified hGBP-1 protein showed that, unlike the small monomeric GTPases such as Ras, hGBP-1 can form dimers and tetramers in solution. Multimerization is nucleotide dependent. hGBP-1 is monomeric in the absence of nucleotide or bound to GDP, but dimerizes when bound to GppNHp, a nonhydolyzable analog of GTP (Prakash and others 2000a). In addition, in the presence of GDP and aluminum fluoride, the protein forms a tetramer in solution (Praefcke and others 2004). Binding to the combination of GDP and aluminum fluoride is believed to be a transition state for GTPases. GTP hydrolysis showed multiple-fold increases with increasing concentrations of hGBP-1, consistent with a cooperative mechanism of hydrolysis (Prakash and others 2000a). These properties of nucleotide-dependent oligomerization, concentration dependence of GTP hydrolysis rate, and the structure of hGBP-1 are consistent with hGBP-1 being a member of the dynamin superfamily of large GTPases.
To provide additional information on how hGBP-1 multimerizes, crystal structures were solved for the isolated LG-domain of hGBP-1 (Ghosh and others 2006). This study showed that homodimerization occurs through interactions of the switch regions of the LG domain of hGBP-1 (Ghosh and others 2006). Recently, the C-terminal α-helical region (helices α12/13) was shown to interact with the LG domain to facilitate tetramer formation (Vopel and others 2010).
hGBP-1 is farnesylated and is, under certain conditions, associated with intracellular membranes. hGBP-1 can localize to the Golgi in the presence of other IFN-γ-induced proteins but only when cells were treated with aluminum fluoride and the protein has its lipid modification (Modiano and others 2005). Recently, investigators have examined the effect of farnesylation of hGBP-1 and its association with lipid membranes on its GTPase activity (Fres and others 2010). To obtain farnesylated hGBP-1, hGBP-1 was expressed in bacteria together with human farnesyltransferase α and β. Modified protein could be separated cleanly from unmodified protein and their properties compared (Fres and others 2010). Lipid-modified hGBP-1 bound to liposomes only in the presence of GDP*AlFx, the mimic for the transition state (Fres and others 2010). Unmodified hGBP-1 did not bind to liposomes under physiological salt conditions and in the presence of GDP*AlFx, suggesting that membrane association requires both the lipid modification and the transition state (Fres and others 2010). Both modified and unmodified hGBP-1 showed behavior consistent with self-association (dimer or multimer formation). However, the modified form in the presence of liposomes showed a 2-fold higher dimer dissociation constant than the unmodified form. The 2 forms had similar maximum GTPase activites. However, in the presence of lipid the ratio of GMP produced by the modified form changed from 85% GMP to 30% GMP for the lipid modified version (Table 2) (Fres and others 2010). Thus, lipid modification and association with lipid membranes alters the GTPase activity of hGBP-1.
On the basis of the findings that both GDP and GMP were the reaction products for hGBP-1 and hGBP-2 and that there is tremendous conservation in the GTP binding region of all of the hGBPs, it had been suggested that all GBPs would produce both GDP and GMP. That was before the study of hGBP-5. hGBP-5 is the only hGBP known to have splice variants (Fellenberg and others 2004). There are 3 RNA versions of hGBP-5 that result in expression of 2 different proteins (Fellenberg and others 2004). Normal cells express the full-length protein (hGBP-5a/b), but melanoma and lymphoma cell lines also express a C-terminal truncated version that lacks the final 97 amino acids (hGBP-5ta). This tumor-specific variant has an identical GTP binding region to the wild-type protein, but the C-terminal truncation removes the CaaX site for isoprenoid addition (Fellenberg and others 2004). hGBP-5a/b differs from hGBP-1 in that it hydrolyzes GTP to only GDP. It does not even bind GMP (Table 2). As observed with hGBP-1, the rate of GTP hydrolysis is concentration dependent and there is weak self-activation. The truncation of the C-terminus in hGBP-5ta resulted in only minor changes in oligomerization, nucleotide binding, and hydrolysis compared to hGBP-5a/b (Wehner and Herrmann 2010). Because the residues involved in GTP binding and hydrolysis are conserved between hGBP-1 and hGBP-5a/b, this suggests that there is more to be learned about how GTP hydrolysis is regulated by the GBPs.
Functions of GBPs
Antiviral and antimicrobial activities
Studies using mice lacking either the type I IFN receptor or the type II IFN receptor suggested that type I IFNs are responsible for defense against many viruses, while type II IFN is required for defense against intracellular bacteria and parasites (van den Broek and others 1995). Since GBPs are induced by both type I and type II IFNs, it seemed logical to examine whether they are involved in host defense.
Antiviral activities
hGBP-1 has modest antiviral activity against the negative strand RNA Rhabdovirus, vesicular stomatitis virus, and the positive strand RNA Picornovirus and encephalomyocarditis virus in cultured cells (Anderson and others 1999). The putative murine ortholog of hGBP-1, mGBP-2, also shows modest activity against those same viruses (Carter and others 2005). Consistent with an antiviral activity for hGBP-1, it was identified as one of the ISGs whose RNA levels are repressed in Huh7 cells by the hepatitic C virus (HCV) replicon (Itsui and others 2006). Forced expression of hGBP-1 in Huh7 cells containing the HCV replicon inhibited replicon replication by 40% (Itsui and others 2006). hGBP-1 was shown to interact with HCV NS5B when co-transfected into HEK-293T cells (Itsui and others 2009). This interaction inhibited hGBP-1's GTPase activity and is proposed to inhibit the antiviral activity of hGBP-1 (Table 3). How expression of hGBP-1 is repressed by HCV is still unknown.
Table 3.
Putative Functions of Individual Guanylate-Binding Proteins
| Antimicrobial activities |
|---|
| hGBP-1 |
| Inhibits replication of VSV and EMCV |
| Inhibits replication of the HCV replicon in Huh-7 cells |
| Interacts with HCV NS5B protein in vitro |
| Inhibits growth of Chlamydia |
| Localizes to chlamydial inclusion membranes |
| hGBP-2 |
| Inhibits growth of Chlamydia |
| mGBP-2 |
| Inhibits replication of VSV and EMCV |
| Localizes to the parasitophorous vacuole in Toxoplasma gondii infected cells |
| mGBP-5 |
| Promotes Salmonella enterica seravar Typhimurium-induced pyroptosis |
| Other activities |
|---|
| hGBP-1 |
| Inhibits growth factor-induced proliferation of endothelial cells |
| Inhibits expression of MMP-1 in endothelial cells |
| Reduced ability of HUVECs to form capillaries in culture |
| Inhibits HUVEC spreading on fibronectin |
| Localizes with CAR in the tight junctions of intestinal epithelial cells and regulates barrier function |
| Provides modest resistance to paclitaxel-induced death in vitro |
| mGBP-2 |
| Promotes proliferation of NIH 3T3 cells |
| Inhibits spreading of NIH 3T3 and B16 melanoma cells on fibronectin |
| Inhibits Rac activation by integrin engagement and PDGF treatment |
| Inhibits PI3-K activation by integrin engagement |
| Inhibits paclitaxel-induced death in vitro |
The functions ascribed to members of the GBP family are divided into those that are antimicrobial and those that are not.
CAR, Coxsackie-and adenovirus receptor; EMCV; encephelomyocarditis virus; HCV, hepatitis C virus; HUVEC, human umbilical vein endothelial cells; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; TNF, tumor necrosis factor; VSV, vesicular stomatitis virus.
Inhibition of Chlamydia
Chlamydia trachomatis is a highly prevalent gram-negative obligate intracellular bacteria (reviewed in Roan and Starnbach 2008). Some serovars of C. trachomatis infect ocular tissues, while others infect genital tissues. While highly treatable, C. trachomatis infections are frequently asymptomatic until they lead to such problems as ectopic pregnancy, infertility, and trachoma, the leading cause of preventable blindness worldwide. A significant component of the pathology of these human diseases is the consequence of damage to mucosal tissues from inflammatory reactions that attempt to clear the pathogen but are unable to. Chlamydia has 2 developmental forms, the elementary body (EB) and the reticulate body (RB) (Moulder 1991). The relatively metabolically inactive EBs are the infective forms. EBs induce their own uptake by cells and the formation of structures called inclusions. EBs differentiate into metabolically active RBs within a couple of hours of entering the cell. RBs then replicate within the inclusions (which expands during this replication) and after about 18 h they will change back into EBs before cell lysis and release of the EBs (Moulder 1991).
Both hGBP-1 and hGBP-2 appear to be involved in the inhibition of C. trachomatis growth by IFN-γ (Tietzel and others 2009). In HeLa cells infected with C. trachomatis and transfected with either hGBP-1 or hGBP-2, both GBPs localize to the chlamydial inclusion membrane. For hGBP-1, localization to the inclusion membrane does not require the GTP binding domain but is mediated by the C-terminal α-helices containing the CaaX site for lipid addition (Tietzel and others 2009). Either hGBP-1 or hGBP-2 alone can delay the growth of C. trachomatis and this inhibition does require the GTPase activity of hGBP-1 (Tietzel and others 2009). This inhibition of parasite growth is accompanied by decreased size of Chlamydia inclusions. Together these data suggest that hGBP-1 and hGBP-2 are involved in the IFN-γ response against Chlamydia (Table 3).
Salmonella induction of pyroptosis
Pyroptosis is a unique form of cell death induced by a range of microbial infections and is believed to be important in their control (reviewed in Bergsbaken and others 2009). In contrast to apoptosis, pyroptosis, also called caspase-1-dependent cell death, is proinflammatory. Caspase-1 activation is unique to this form of programmed cell death. Caspase-1 null mice have no defects in apoptosis. Activation of caspase-1 by bacteria results in a very rapid cell death as a consequence of plasma membrane rupture and release of the cell's proinflammatory contents (Fink and Cookson 2006; Bergsbaken and others 2009). While pyroptosis is an important control mechanism for these infectious agents, many bacteria have evolved mechanisms to inhibit pyroptosis. The fate of an infection can then be determined by the balance between the actions of the bacteria and the host cell on pyroptosis.
Salmonella enterica can infect a number of different cell types (reviewed in Fink and Cookson 2007). The form of cell death induced by Salmonella infection varies between the cell types. Infection of intestinal epithelial cells induces apoptosis, whereas infection of macrophages results in pyroptosis (Fink and Cookson 2007). The rapid pyroptosis induced by Salmonella in macrophages requires the pathogenicity island-1 type III secretion system and flagella. This secretion of flagellar fragments into the cell cytoplasm is what initiates caspase-1 activation. Bone marrow-derived macrophages die by pyroptosis within 1 h of Salmonella entry, whereas macrophage cell lines require a bit longer (Fink and Cookson 2006).
To characterize the IFN-γ-induced or -repressed proteins on isolated phagosomes of RAW 264.7 macrophages and possibly identify novel proteins involved in regulating pathogen internalization and survival, phagosomes were induced by internalization of latex beads (Jutras and others 2008). One of the proteins robustly induced by IFN-γ and incorporated into phagosomes was mGBP-5 (Jutras and others 2008). Because of the enrichment of mGBP-5 in phagosomal membranes, a role for mGBP-5 in S. enterica seravar Typhimurium–induced pyroptosis of RAW 264.7 cells was explored (Rupper and Cardelli 2008). RAW 264.7 macrophages are relatively resistant to Salmonella-induced pyroptosis unless primed with IFN-γ. mGBP-5 potentiates Salmonella-induced pyroptosis, and, not unexpectedly, this potentiation requires the activition of caspase-1, in part by the secretion of flagellin subunits into the cell cytoplasm through the type III secretion system. In addition, mGBP-5 localized to structures formed by the invading S. enterica serovar Typhimurium (Rupper and Cardelli 2008). Before invasion of Salmonella, mGBP-5 colocalizes with actin to the sites on the membrane where Salmonella is attached and it is later localized to the vacuolar membranes surrounding the bacteria (Rupper and Cardelli 2008) (Table 3). The mechanisms for how mGBP-5 enhances Salmonella-induced pyroptosis still require elucidation.
Listeria
Listeria monocytogenes is the intracellular bacteria responsible for the most virulent of the foodborne diseases, Listeriosis (reviewed in Barbuddle and Chakraborty 2009). The primary cells infected are intestinal epithelial cells. While internalization normally results in the bacteria being present initially in a phagosome, the bacteria must escape to the cytoplasm before fusion with the lysosome occurs to survive. Consistent with the possible involvement of GBPs in the protection against multiple intracellular pathogens, infection of mice with L. monocytogenes resulted in the upregulation of all of the RNAs for murine GBPs in the liver and spleen of the animals (Degrandi and others 2007). Whether or not the GBPs inhibit Listeria proliferation remains to be shown.
Toxoplasma
Toxoplasma gondii is an intracellular protozoan that causes the disease toxoplasmosis (reviewed in Laliberte and Carruthers 2008; Costa da Silva and Langoni 2009; Leng and others 2009). Toxoplasma is transmitted from animals to humans through contaminated food. It is estimated that 20%–50% of people are infected. However, after initial infection the organism normally is latent in the CNS and skeletal muscle in a cystic form. In normal individuals, the immune system recognizes any parasites that change back to the active form and maintains the latency, except under conditions of compromised immune system or congenital transmission. During the invasion of cells, T. gondii generates a special membrane enclosed structure called the parasitophorous vacuole (PV) (Laliberte and Carruthers 2008; Leng and others 2009). Simplistically, the PV serves to shield the organism from the cytoplasm and to block fusion with the endosomal/lysosomal compartment. In reality, the PV contains a number of parasite proteins that allow the recruitment of nutrients from the cell, while altering a number of cellular signal transduction cascades. The PV also recruits a number of cellular proteins.
Infection of mice with T. gondii also results in the upregulation of all of the murine GBP RNAs (Degrandi and others 2007). When cells were infected with T. gondii in culture, within 30 min, mGBP-1, 2, 3, 6, 7, and 9 were found around the PV (Degrandi and others 2007). It is appealing to speculate that 1 or more GBPs will be involved in the immune response against T. gondii.
Bacterial meningitis
hGBP-1 is found in the spinal fluid of patients with bacterial meningitis at levels greater than observed in the absence of infection (Naschberger and others 2006). The exact mechanism for how hGBP-1 gets to the spinal fluid is not clear. However, studies showed that hGBP-1 is secreted from cultured human umbilical vein endothelial cells (HUVECs) but not appreciably from HeLa, fibroblasts, smooth muscle cells, or the keritinocyte line, HaCaT (Naschberger and others 2006). It has been suggested that hGBP-1 might prove to be a marker of meningitis.
Other activities
Effects on proliferation
hGBP-1 inhibits the proliferation of endothelial cells induced by the combination of basic fibroblast growth factor and vascular endothelial growth factor in culture (Guenzi and others 2001). This inhibition is mediated by the C-terminal α-helices of hGBP-1 and does not require the presence of the globular GTP binding domain (Guenzi and others 2001) (Table 3).
While IFN-γ treatment inhibits the proliferation of growth factor-stimulated endothelial cells, it was defined as a mitogen for fibroblasts many years ago. Indeed, IFN-γ treatment of NIH 3T3 fibroblasts promotes their proliferation (Gorbacheva and others 2002). This enhancement of proliferation can be mimiced by the forced expression of mGBP-2 and the promotion of proliferation in fibroblasts appears to require its GTPase activity (Gorbacheva and others 2002). IFN-β, which also induces mGBP-2, does not promote NIH 3T3 fibroblast proliferation. However, IFN-β induces mGBP-2 to a lower level than IFN-γ and NIH 3T3 cell clones expressing mGBP-2 at levels comparable to IFN-β induction also show no difference in proliferation from control cells (Gorbacheva and others 2002) (Table 3). This prompts the question of whether different functions for GBPs require different expression levels. While much more would need to be done to demonstrate this unequivocally, this might not be surprising given the concentration dependence of the GTPase activity of GBPs.
Inhibition of matrix metalloproteinase expression
hGBP-1 inhibits expression of matrix metalloproteinase 1 (MMP-1) by endothelial cells in culture (Guenzi and others 2003). hGBP-1 appears to be the protein responsible for MMP-1 inhibition by IL-1β, TNF-α, and IFN-γ (Guenzi and others 2003). The GTPase activity of hGBP-1 is required for the inhibition of MMP-1 levels (Guenzi and others 2003). Consistent with hGBP-1 inhibition of MMP-1, the transmigration of endothelial cells expressing hGBP-1 on substrates of relatively low concentrations of collagen I or collagen IV is less than control cells. This suggests that hGBP-1 inhibits the migratory ability of these cells. Transmigration of hGBP-1-expressing endothelial cells through higher concentrations of Col I or Col IV suggested that on Col I the invasive activity of the cells was inhibited (Guenzi and others 2003). Expression of hGBP-1 in HUVECs also resulted in reduced ability to form capillaries in Matrigel (Guenzi and others 2003). Expression of a single amino acid substitution that inhibits GTPase activity (D184N) and does not downregulate MMP-1 in HUVECs, also does not inhibit tube formation (Guenzi and others 2003) (Table 3).
mGBP-2, the putative ortholog of hGBP-1, also downregulates an MMP (Balasubramanian et al., unpublished data). Either IFN-γ treatment, which induces mGBP-2, or forced expression of mGBP-2 alone results in the downregulation of MMP-9 in NIH 3T3 fibroblasts (Table 3).
Inhibition of cell spreading
hGBP-1 inhibits HUVEC spreading on fibronectin (Weinlander and others 2008). In these cells forced expression of hGBP-1 upregulates integrin α4, which can inhibit cell spreading in some cell types. As expected, IL-1β and TNF-α, which modestly induce hGBP-1 in HUVECs, also induced α4 and reduced cell spreading. While the more robust inducer of hGBP-1 in HUVECs, IFN-γ, also inhibits cell spreading, this inhibition does not involve upregulation of α4 (Weinlander and others 2008) (Table 3). The mechanisms by which IFN-γ inhibits cell spreading in HUVECs are yet to be fully resolved.
IFN-γ treatment of NIH 3T3 fibroblasts inhibits cell spreading on fibronectin (FN) (Messmer-Blust and others 2010). Forced expression of mGBP-2 is sufficient for this inhibition in both NIH 3T3 cells and B16 melanoma cells. mGBP-2 does not upregulate α4 integrin in NIH 3T3 cells, but inhibits cell spreading by inhibiting integrin-mediated activation of the small GTPase Rac1. This inhibition of Rac activation by mGBP-2 is also observed after treatment of these cells with platelet-derived growth factor (Messmer-Blust and others 2010). A role for PI-3K in the inhibition of Rac by mGBP-2 is suggested by the observation that Rac inhibition during cell spreading is accompanied by the association of mGBP-2 with the catalytic subunit of PI-3K, p110, and the inhibition of PI-3K activity (Messmer-Blust and others 2010) (Table 3).
Intestinal epithelial cell barrier function
IFN-γ treatment of epithelial cells can result in apoptosis and loss or reduction in barrier function. Proinflammatory cytokines have been implicated in the pathogenesis of inflammatory bowel disease (IBD). Investigators have found that IFN-γ treatment of intestinal epithelial cells results in the upregulation of hGBP-1 (Schnoor and others 2009). hGBP-1 expression was also increased in intestinal mucosa from patients with IBD (Schnoor and others 2009). Consistent with a possible role in IBD, hGBP-1 is localized to the tight junction in intestinal epithelia but not in other epithelia (Schnoor and others 2009) (Table 3). In tight junctions, hGBP-1 co-localizes with coxsackie- and adenovirus receptor (CAR). Knockdown of hGBP-1 during IFN-γ treatment of SK-CO15 intestinal epithelial cells resulted in reduction in transepithelial electrical resistance (Schnoor and others 2009). It also enhanced caspase activation and apoptosis. Caspase inhibition in the presence of siRNA against hGBP-1 was able to reverse the reduction in barrier function (Schnoor and others 2009). How these results fit into a role for IFN-γ in the pathogenesis of IBD will require further study. On the basis of these in vitro data, the investigators propose that early after the exposure to IFN-γ, hGBP-1 is protective. Certainly, there are in vitro data with endothelial cells that IFN-α exposure or hGBP-1 expression protects endothelial cells against apoptosis upon short exposure (Pammer and others 2006). However, long-term exposure resulted in senescence (Pammer and others 2006). The authors of the study on intestinal epithelial cells speculate that prolonged exposure to IFNs and hGBP-1 may also have different effects in these cells (Pammer and others 2006).
Intestinal epithelial development
Human and murine infants are born with sterile intestinal systems. How those systems are populated with commensal bacteria determines their healthy development. Population of the intestinal tract of 2-week-old mice with commensal Escherichia coli induced expression of IFN-αA and GBP-1 (Mirpuri and others 2010). This upregulation of IFN-αA and GBP-1 is accompanied by resistance to staurosporine-induced apoptosis. Treatment of immature human epithelial cells in vitro or intestinal cells ex vivo with IFN-αA also upregulated GBP-1 and protected the cells from staurosporine-induced apoptosis (Mirpuri and others 2010). This protection by IFN-αA requires GBP-1 as evidenced by the ability of siRNA against GBP-1 to abrogate the protection (Mirpuri and others 2010). These studies suggest that IFN-αA induction by commensal bacteria protects the developing intestinal epithelia from apoptosis and possibly necrotizing enterocolitis through the expression of GBP-1 (Mirpuri and others 2010).
Paclitaxel resistance
hGBP-1 was identified as 1 of 5 genes upregulated in 3 different cancer cell lines as they became resistant to paclitaxel (Duan and others 2005b). Forced expression of hGBP-1 in OVCAR8 cells conferred some level of resistance to paclitaxel treatment (Duan and others 2005a). Forced expression of the ortholog, mGBP-2, in NIH 3T3 cells also protected against paclitaxel-induced death (Balasubramanian and others 2006) (Table 3).
Endothelial cell-related effects
hGBP-1 expression is associated with endothelial cells in human tissues (Lubeseder-Martellato and others 2002). It was also detected on mononuclear cells in a variety of tissues, but less frequently in other cell types or tissues in vivo (Lubeseder-Martellato and others 2002). hGBP-1 was not detected on endothelial cells in healthy skin. However, it was strongly expressed in Kaposi sarcoma (KS) lesions and other inflammatory skin diseases such as adverse drug reactions of the skin and psoriasis (Lubeseder-Martellato and others 2002). As such it is proposed as an activation marker during inflammatory diseases.
IFN-α can also induce hGBP-1 in HUVECs or human dermal microvascular endothelial cells in culture (Indraccolo and others 2001; Pammer and others 2006). In HUVECs the time course of hGBP-1 RNA expression is consistent with previous promoter studies on the induction of hGBP-1 where induction by type I IFNs was shown to be transient, while IFN-γ induction was more sustained (Decker and others 1989, 1991). Treatment of HUVECs with IFN-α for 5 h resulted in the induction of hGBP-1 RNA within 2 h and continued expression through 5 h. However, the RNA levels returned to uninduced levels within 18 h (Indraccolo and others 2001). After 5 h of IFN-γ treatment the induction was maintained through 96 h (Indraccolo and others 2001). Either IFN-α treatment or forced expression of hGBP-1 in HUVECs protects them from serum and growth factor starvation (Pammer and others 2006). However, addition of IFN-α to cultures of HUVEC in the presence of serum and growth factors results in cellular senescence. On the basis of this set of experiments the authors propose that the anti-angiogenetic activities of IFN-α may be due to the induction of senescence rather than apoptosis.
Because hGBP-1 had been shown to be involved in angiostasis, expression of hGBP-1 was examined in colorectal carcinomas (Naschberger and others 2008). For this study, 388 sporadic colorectal carcinoma tissue samples were examined for hGBP-1 expression. Expression of hGBP-1 was detected in 32% of colorectal carcinomas. This expression was in the stroma, not in the tumor cells proper. Expression of hGBP-1 correlated statistically with improved (16.2%) 5-year survival (Naschberger and others 2008). This suggests that hGBP-1 expression can be a prognostic marker in colorectal cancers.
Conclusions and Future Directions
Our understanding of the biochemical properties and structure of GBPs has increased tremendously in the last several years. In addition, a growing number of functions are being ascribed to the GBPs. Future experiments will confirm the role of GBPs in these aspects of the diverse responses to IFNs and begin to dissect the mechanism(s) used by GBPs to facilitate these changes.
Acknowledgments
The authors wish to apologize to all colleagues whose work is not included in this review because of space limitations. This work was supported in part by grants from the University of Toledo and NIH/NCI (1R21CA132016) to D.J.V.
Authors Disclosure Statement
No competing financial interests exist.
References
- Abdullah N. Srinivasan B. Modiano N. Cresswell P. Sau AK. Role of individual domains and indentification of internal gap in human guanylate binding protein-1. J Mol Biol. 2009;368:690–703. doi: 10.1016/j.jmb.2008.12.060. [DOI] [PubMed] [Google Scholar]
- Anderson SL. Carton JM. Lou J. Xing L. Rubin BY. Interferon-induced guanylate binding protein-1 (GBP-1) mediates an antiviral effect against vesicular stomatitis virus and encephalomyocarditis virus. Virology. 1999;256:8–14. doi: 10.1006/viro.1999.9614. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S. Nada S. Vestal DJ. The interferon-induced GTPase, mGBP-2, confers resistance to paclitaxel-induced cytotoxicity without inhibiting multinucleation. Cell Mol Biol. 2006;52:43–49. [PubMed] [Google Scholar]
- Barbuddle SB. Chakraborty T. Listeria as an enteroinvasive gastointestinal pathogen. Curr Top Microbiol Immunol. 2009;337:173–195. doi: 10.1007/978-3-642-01846-6_6. [DOI] [PubMed] [Google Scholar]
- Bekpen C. Hunn JP. Rohde C. Parvanova I. Guethlein L. Dunn DM. Glowalla E. Leptin M. Howard JC. The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 2005;6:R92. doi: 10.1186/gb-2005-6-11-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsbaken T. Fink SL. Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm U. Guethlein L. Klamp T. Ozbek K. Schaub A. Futterer A. Pleffer K. Howard JC. Two families of GTPases dominate the complex cellular response to IFN-γ. J Immunol. 1998;161:6715–6723. [PubMed] [Google Scholar]
- Carter CC. Gorbacheva VY. Vestal DJ. Inhibition of VSV and EMCV replication by the interferon-induced GTPase, mGBP-2: differential requirement for wild-type GTP binding domain. Arch Virol. 2005;150:1213–1220. doi: 10.1007/s00705-004-0489-2. [DOI] [PubMed] [Google Scholar]
- Cheng YE. Colonno RJ. Yin Fay H. Interferon induction of fibroblast proteins with guanylate binding activity. J Biol Chem. 1983;258:7746–7750. [PubMed] [Google Scholar]
- Cheng YS. Patterson CE. Staeheli P. Interferon-induced guanylate-binding proteins lack an N(T)KXD consensus motif and bind GMP in addition to GDP and GTP. Mol Cell Biol. 1991;11:4717–4725. doi: 10.1128/mcb.11.9.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y-SE. Becker-Manley MF. Chow TP. Horan DC. Affinity purification of an interferon-induced human guanylate-binding protein and its characterization. J Biol Chem. 1985;260:15834–15839. [PubMed] [Google Scholar]
- Costa da Silva R. Langoni H. Toxoplasma gondii: host-parasite interaction and behavior manipulation. Parasitol Res. 2009;105:893–898. doi: 10.1007/s00436-009-1526-6. [DOI] [PubMed] [Google Scholar]
- Decker T. Lew DJ. Cheng YS. Levy DE. Darnell JE., Jr. Interactions of alpha- and gamma-interferon in the transcriptional regulation of the gene encoding a guanylate-binding protein. Embo J. 1989;8:2009–2014. doi: 10.1002/j.1460-2075.1989.tb03608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T. Lew DJ. Darnell JE., Jr. Two distinct alpha-interferon-dependent signal transduction pathways may contribute to activation of transcription of the guanylate-binding protein gene. Mol Cell Biol. 1991;11:5147–5153. doi: 10.1128/mcb.11.10.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrandi D. Konermann C. Beuter-Gunia C. Kresse A. Wurthner J. Kurig S. Beer S. Pfeffer K. Extensive characterization of IFN-induced GTPases mGBP1 to mGBP10 involved in host defense. J Immunol. 2007;179:7729–7740. doi: 10.4049/jimmunol.179.11.7729. [DOI] [PubMed] [Google Scholar]
- Duan Z. Foster R. Brakora KA. Yusuf RZ. Seiden MV. GBP1 overexpression is associated with a paclitaxel resistance phenotype. Cancer Chemother Pharmacol. 2005a;57:25–33. doi: 10.1007/s00280-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Duan Z. Lamendola DE. Duan Y. Yusaf R. Seiden MV. Description of paclitaxel resistance-associated genes in ovarian and breast cancer cell lines. Cancer Chemother Pharmacol. 2005b;55:277–285. doi: 10.1007/s00280-004-0878-y. [DOI] [PubMed] [Google Scholar]
- Fellenberg F. Hartmann TB. Dummer R. Usener D. Schadendorf D. Eichmuller S. GBP-5 splicing variants: new guanylate-binding proteins with tumor-associated expression and antigenicity. J Invest Dermatol. 2004;122:1510–1517. doi: 10.1111/j.0022-202X.2004.22613.x. [DOI] [PubMed] [Google Scholar]
- Fink SL. Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- Fink SL. Cookson BT. Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol. 2007;9:2562–2570. doi: 10.1111/j.1462-5822.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- Fres JM. Muller S. Praefcke GJK. Purification of the CaaX modified dynamin-related large GTPase hGBP-1 by co-expression with farnesyltransferase. J Lipid Res. 2010;51:2454–2459. doi: 10.1194/jlr.D005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb MH. Scholten JD. Sebolt-Leopold JS. Protein prenylation: from discovery to prospects for cancer treatment. Curr Opin Chem Biol. 1998;2:40–48. doi: 10.1016/s1367-5931(98)80034-3. [DOI] [PubMed] [Google Scholar]
- Ghosh A. Praefcke GJK. Renault L. Wittinghofer A. Herrmann C. How guanylate-binding proteins acheive assembly stimulated processive cleavage of GTP to GMP. Nature. 2006;440:101–104. doi: 10.1038/nature04510. [DOI] [PubMed] [Google Scholar]
- Gorbacheva VY. Lindner D. Sen GC. Vestal DJ. The IFN-induced GTPase, mGBP-2: role in IFN-γ induced murine fibroblast proliferation. J Biol Chem. 2002;277:6080–6087. doi: 10.1074/jbc.M110542200. [DOI] [PubMed] [Google Scholar]
- Guenzi E. Topolt K. Cornali E. Lubeseder-Martellato C. Jorg A. Matzen K. Zietz C. Kremmer E. Nappi F. Schwemmle M. Hohenadl C. Barillari G. Tschachler E. Monini P. Ensoli B. Sturzl M. The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines. EMBO J. 2001;20:5568–5577. doi: 10.1093/emboj/20.20.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzi E. Topolt K. Lubeseder-Martellato C. Jorg A. Naschberger E. Benelli R. Albini A. Sturzl M. The guanylate binding protein-1 GTPase controls the invasive and angiogenic capabiliy of endothelial cells through inhibition of MMP-1 expression. EMBO J. 2003;22:3772–3782. doi: 10.1093/emboj/cdg382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller O. Staeheli P. Gochs G. Interferon-induced Mx proteins in antiviral host defense. Biochimie. 2007a;6–7:812–818. doi: 10.1016/j.biochi.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Haller O. Stertz S. Kochs G. The Mx GTPase family of interferon-induced antiviral proteins. Microbes Infect. 2007b;14–15:1636–1643. doi: 10.1016/j.micinf.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Howard J. The IRG proteins: a function in search of a mechanism. Immunobiology. 2008;213:367–375. doi: 10.1016/j.imbio.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Indraccolo S. Pfeffer U. Minuzzo S. Esposito G. Roni V. Mandruzzato S. Ferrari N. Anfosso L. Dell'Eva R. Noonan DM. Idenfication of genes selectively regulated by IFNs in endothelial cells. J Immunol. 2001;178:1122–1135. doi: 10.4049/jimmunol.178.2.1122. and others. [DOI] [PubMed] [Google Scholar]
- Itsui Y. Sakamoto N. Kakinuma S. Nakagawa M. Sekine-Osajima Y. Tasaka-Fujita M. Nishimura-Sakurai Y. Suda G. Karakama Y. Mishima K. Antivral effects of the interferon-induced protein guanylate binding protein 1 and its interaction with the hepatitis C virus NS5B protein. Hepatology. 2009;50:1727–1737. doi: 10.1002/hep.23195. and others. [DOI] [PubMed] [Google Scholar]
- Itsui Y. Sakamoto N. Kurasaki M. Kanazawa N. Tanabe Y. Koyama T. Takeda Y. Nakagawa M. Kakinuma S. Sekine Y. Expressionl screening of interferon-stimulated genes for antiviral activity against hepatitis C virus replication. J Viral Hepat. 2006;13:690–700. doi: 10.1111/j.1365-2893.2006.00732.x. and others. [DOI] [PubMed] [Google Scholar]
- Jutras I. Houde M. Currie N. Boulais J. Duclos S. LaBoissiere S. Bonneil E. Kearney P. Thibault P. Paramithiotis E. Modulation of the phagosome proteome by interferon-γ. Mol Cell Proteomics. 2008;7:697–715. doi: 10.1074/mcp.M700267-MCP200. and others. [DOI] [PubMed] [Google Scholar]
- Klamp T. Boehm U. Schenk D. Pfeffer K. Howard JC. A giant GTPase, very large inducible GTPase-1, is inducible by IFNs. J Immunol. 2003;171:1255–1265. doi: 10.4049/jimmunol.171.3.1255. [DOI] [PubMed] [Google Scholar]
- Kresse A. Konermann C. Degrandi D. Beuter-Gunia C. Wuerthner J. Pfeffer K. Beer S. Analyses of murine GBP homology clusters based on in silico, in vitro and in vivo studies. BMC Genomics. 2008;9:158. doi: 10.1186/1471-2164-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberte J. Carruthers VB. Host cell manipulation by the human pathogen Toxoplasma gondii. Cell Mol Life Sci. 2008;65:1900–1915. doi: 10.1007/s00018-008-7556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng J. Butcher BA. Denkers EY. Dysregulation of macrophage signal transduction by Toxoplasma gondii: past progress and recent advances. Parasite Immunol. 2009;31:717–728. doi: 10.1111/j.1365-3024.2009.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubeseder-Martellato C. Guenzi E. Jorg A. Topolt D. Naschberger E. Kremmer E. Zietz C. Tschachler E. Hutzer P. Schwemmle M. Guanylate-binding protein-1 expression is selectively induced by inflammatory cytokines and is an activation marker of endothelial cells during inflammatory diseases. Am J Pathol. 2002;161:1749–1759. doi: 10.1016/S0002-9440(10)64452-5. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking JD. IFN-inducible GTPases and immunity to intracellular pathogens. Trends Immunol. 2004;25:601–609. doi: 10.1016/j.it.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Maltese WA. Wilson AL. Erdman RA. Prenylation-dependent interaction of Rab proteins with GDP dissociation inhibitors. Biochem Soc Trans. 1996;24:703–708. doi: 10.1042/bst0240703. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Protein prenylation: a mediator of protein-protein interactions. Science. 1993;259:1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- Messmer-Blust AF. Balasubramanian S. Gorbacheva VY. Jeyaratnam JA. Vestal DJ. The interferon-γ-induced murine guanylate-binding protein-2 (mGBP-2) inhibits Rac activation during cell spreading on fibronectin and platelet-derived growth factor (PDGF) treatment: role for phosphatidylinositol 3-kinase (PI3-K) Mol Biol Cell. 2010;21:2514–2528. doi: 10.1091/mbc.E09-04-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirpuri J. Brazil JC. Berardinelli AJ. Nasr TR. Cooper K. Schnoor M. Lin PW. Parkos CA. Louis NA. Commensal Escherichia coli reduced epithelial apoptosis through IFN-αA mediated induction of guanylate binding protein-1 in human and murine models of developing intestine. J Immunol. 2010;184:7186–7195. doi: 10.4049/jimmunol.0903116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modiano N. Lu YE. Cresswell P. Golgi targeting of human guanylate-binding protein-1 requires nucleotide binding, isoprenylation, and an IFN-γ-inducible cofactor. Proc Natl Acad Sci USA. 2005;102:8680–8685. doi: 10.1073/pnas.0503227102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantais DE. Schwemmle M. Stickney JT. Vestal DJ. Buss JE. Prenylation of an Interferon-γ-induced GTP-binding protein: the human guanylate binding protein, huGBP-1. J Leukoc Biol. 1996;60:423–431. doi: 10.1002/jlb.60.3.423. [DOI] [PubMed] [Google Scholar]
- Naschberger E. Croner RS. Merkel S. Dimmler A. Tripal P. Amann KU. Kremmer K. Brueckl WM. Papadopoulos T. Hohenadl C. Angiostatic immune reaction in colorectal carcinomas: impact on survival and perspectives for antiangiogenic therapy. Int J Cancer. 2008;123:2120–2129. doi: 10.1002/ijc.23764. and others. [DOI] [PubMed] [Google Scholar]
- Naschberger E. Lugeseder-Martellato C. Meyer N. Gessner R. Kremmer E. Gessner A. Sturzl M. Human guanylate-binding protein-1 is a secreted GTPase present in increased concentrations in the cerebrospinal fluid of patients with bacterial meningitis. Am J Pathol. 2006;169:1088–1098. doi: 10.2353/ajpath.2006.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neun R. Richter MF. Staeheli P. Schwemmle M. GTPase properties of the interferon-induced human guanylate-binding protein 2. FEBS Lett. 1996;390:69–72. doi: 10.1016/0014-5793(96)00628-x. [DOI] [PubMed] [Google Scholar]
- Olszewski MA. Gray J. Vestal DJ. In silico genomic analysis of the human and murine guanylate binding protein (GBP) gene clusters. J Interferon Cytokine Res. 2006;26:328–352. doi: 10.1089/jir.2006.26.328. [DOI] [PubMed] [Google Scholar]
- Pammer J. Reinisch C. Birner P. Pogoda K. Struzl M. Tschachler E. Interferon-α prevents apoptosis of endothelial cells after short-term exposure but induces replicative senescence after continuous stimulation. Lab Invest. 2006;86:997–1007. doi: 10.1038/labinvest.3700461. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Dirac-Svejstrup AG. Soldati T. Rab GDP dissociation inhibitor: putting Rab GTPases in the right place. J Biol Chem. 1995;270:17057–17059. doi: 10.1074/jbc.270.29.17057. [DOI] [PubMed] [Google Scholar]
- Praefcke GJK. Geyer M. Schwemmle M. Kalbitzer HR. Herrmann C. Nucleotide-binding characteristics of human guanylate-binding protein 1 (hGBP-1) and identification of the third GTP-binding motif. J Mol Biol. 1999;292:321–332. doi: 10.1006/jmbi.1999.3062. [DOI] [PubMed] [Google Scholar]
- Praefcke GJK. Kloep S. Benscheid U. Lillie H. Prakash B. Herrmann C. Identification of residues in the human guanylate-binding protein 1 critical for nucleotide binding and cooperative GTP hydrolysis. J Mol Biol. 2004;344:257–269. doi: 10.1016/j.jmb.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Prakash B. Praefcke GJK. Renault L. Wittinghofer A. Herrmann C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature. 2000a;403:567–571. doi: 10.1038/35000617. [DOI] [PubMed] [Google Scholar]
- Prakash B. Renault L. Praefcke GJK. Herrmann C. Wittinghofer A. Triphosphate structure of guanylate-binding protein 1 and implications for nucleotide binding and GTPase mechanism. EMBO J. 2000b;19:4555–4564. doi: 10.1093/emboj/19.17.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando RR. Chemical biology of protein isoprenylation/methylation. Biochim Biophys Acta. 1996;1300:5–16. doi: 10.1016/0005-2760(95)00233-2. [DOI] [PubMed] [Google Scholar]
- Roan NR. Starnbach MN. Immune-mediated control of Chlamydia infection. Cell Microbiol. 2008;10:9–19. doi: 10.1111/j.1462-5822.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- Rupper AC. Cardelli JA. Induction of guanylate binding protein 5 by gamma interferon increases susceptibility to Salmonella enterica serovar typhimurium-induced pyroptosis in RAW 264.7 cells. Infect Immun. 2008;76:2304–2315. doi: 10.1128/IAI.01437-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoor M. Betanzos A. Weber DA. Parkos CA. Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-γ and regulates barrier function through effects on apoptosis. Mucosal Immunol. 2009;2:33–42. doi: 10.1038/mi.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwemmle M. Staeheli P. The interferon-induced 67-kDa guanylate-binding protein (hGBP1) is a GTPase that converts GTP to GMP. J Biol Chem. 1994;269:11299–11305. [PubMed] [Google Scholar]
- Shenoy AR. Kim B-H. Choi H-P. Matsuzawa T. Tiwari S. MacMicking JD. Emerging themes in IFN-γ-induced macrophage immunity by p47 and p65 GTPase families. Immunobiology. 2008;212:771–784. doi: 10.1016/j.imbio.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Recent advances in the study of prenylated proteins. Biochim Biophys Acta. 2000;1484:93–106. doi: 10.1016/s1388-1981(00)00009-3. [DOI] [PubMed] [Google Scholar]
- Stickney JT. Buss JE. Murine guanylate-binding protein: incomplete geranylgeranyl isoprenoid modification of an interferon-γ-inducible guanosine triphosphate-binding protein. Mol Biol Cell. 2000;11:2191–2200. doi: 10.1091/mbc.11.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietzel I. El-Haibi C. Carabeo RA. Human guanylate binding proteins potentiate the anti-chlamydia effects of inteferon-γ. PLOS One. 2009;4:e6499. doi: 10.1371/journal.pone.0006499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripal P. Bauer M. Naschberger E. Mortinger T. Hohenadl C. Cornali E. Thurau M. Sturzl M. Unique features of different members of the human guanylate-binding protein family. J Interferon Cytokine Res. 2007;27:44–52. doi: 10.1089/jir.2007.0086. [DOI] [PubMed] [Google Scholar]
- van den Broek MF. Muller U. Huang S. Zinkernagel RN. Aquet M. Immune defense in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Vestal DJ. The guanylate binding proteins (GBPs): pro-inflammatory cytokine-induced members of the dynamin superfamily with unique GTPase activity. J Interferon Cytokine Res. 2005;25:435–443. doi: 10.1089/jir.2005.25.435. [DOI] [PubMed] [Google Scholar]
- Vestal DJ. Buss JE. McKercher SR. Jenkins NA. Copeland NG. Kelner GS. Asundi VK. Maki RA. Murine GBP-2: a new IFN-γ-induced member of the GBP family of GTPases isolated from macrophages. J Interferon Cytokine Res. 1998;18:977–985. doi: 10.1089/jir.1998.18.977. [DOI] [PubMed] [Google Scholar]
- Vopel T. Syguda A. Britzen-Laurent N. Kunzelmann S. Ludemann MB. Dovengerds C. Sturzl M. Herrmann C. Mechanism of GTPase activity induced self-assembly of human guanylate binding protein 1. J Mol Biol. 2010;400:63–70. doi: 10.1016/j.jmb.2010.04.053. [DOI] [PubMed] [Google Scholar]
- Wehner M. Herrmann C. Biochemical properties of the human guanylate binding protein 5 and a tumor-specific truncated splice variant. FEBS J. 2010;277:1597–1605. doi: 10.1111/j.1742-4658.2010.07586.x. [DOI] [PubMed] [Google Scholar]
- Weinlander K. Naschberger E. Lehmann MH. Tripal P. Paster W. Stockinger H. Hohenadl C. Sturzl M. Guanylate binding protein-1 inhibits spreading and migration of endothelial cells through induction of integrin a4 expression. FASEB J. 2008;22:4168–4178. doi: 10.1096/fj.08-107524. [DOI] [PubMed] [Google Scholar]