Abstract
The interferon (IFN)-inducible 2′-5′-oligoadenylate synthetase (OAS)/RNase L pathway blocks infections by some types of viruses through cleavage of viral and cellular single-stranded RNA. Viruses induce type I IFNs that initiate signaling to the OAS genes. OAS proteins are pathogen recognition receptors for the viral pathogen-associated molecular pattern, double-stranded RNA. Double-stranded RNA activates OAS to produce px5′A(2′p5′A)n; x = 1–3; n > 2 (2-5A) from ATP. Upon binding 2-5A, RNase L is converted from an inactive monomer to a potently active dimeric endoribonuclease for single-stranded RNA. RNase L contains, from N- to C-terminus, a series of 9 ankyrin repeats, a linker, several protein kinase-like motifs, and a ribonuclease domain homologous to Ire1 (involved in the unfolded protein response). In the past few years, it has become increasingly apparent that RNase L and OAS contribute to innate immunity in many ways. For example, small RNA cleavage products produced by RNase L during viral infections can signal to the retinoic acid-inducible-I like receptors to amplify and perpetuate signaling to the IFN-β gene. In addition, RNase L is now implicated in protecting the central nervous system against viral-induced demyelination. A role in tumor suppression was inferred by mapping of the RNase L gene to the hereditary prostate cancer 1 (HPC1) gene, which in turn led to discovery of the xenotropic murine leukemia-related virus. A broader role in innate immunity is suggested by involvement of RNase L in cytokine induction and endosomal pathways that suppress bacterial infections. These newly described findings about RNase L could eventually provide the basis for developing broad-spectrum antimicrobial drugs.
Introduction
The 2′-5′-oligoadenylate synthetase (OAS)/RNase L system was one of the first antiviral pathways to be discovered during investigations in the mid-1970s on how interferon (IFN) inhibits viral infections (Hovanessian and others 1977; Clemens and Williams 1978; Kerr and Brown 1978; Ratner and others 1978; Slattery and others 1979). IFNs induce transcription of a family of OAS genes in cells of higher vertebrates (see article by H. Kristiansen and others 2010, this issue). In addition, RNase L is IFN inducible in mouse cells, but only minimally if at all in human cells (Jacobsen and others 1983; Rusch and others 2000). OAS proteins are pathogen recognition receptors (PRR) for the viral pathogen-associated molecular pattern, double-stranded RNA (dsRNA). Once stimulated by dsRNA, OAS uses ATP to synthesize 2-5A molecules of the formula [px5′A(2′p5′A)n; x = 1–3; n > 2], yielding mainly the 5′-triphosphorylated triadenylate (Fig. 1). At subnanomolar levels, 2-5A activates RNase L to cleave single-stranded RNA (ssRNA) in U-rich sequences, typically after UU or UA dinucleotides leaving a 5′-OH and 3′-monophosphate (Floyd-Smith and others 1981; Wreschner and others 1981b). Murine OAS1b, also known as the flavivirus resistance gene (Flvr), does not synthesize 2-5A but has an alternative antiviral mechanism of action (Kakuta and others 2002; Mashimo and others 2002; Perelygin and others 2002). In addition, the classical OAS/RNase L pathway has an antiviral effect in cells from both Flvr (resistant) and Flvs (susceptible) mice (Scherbik and others 2006). RNase L is negatively regulated by RNase L inhibitor/ATP-binding cassette, sub-family E member 1 [RLI (ABCE)], which inhibits rRNA cleavage by RNase L (Bisbal and others 1995; Malathi and others 2004) and by the 2′-phosphodiesterase that degrades 2-5A (Kubota and others 2004). Beyond its antiviral function, RNase L has recently been implicated in innate immunity against bacterial infections (Li and others 2008). We begin with a review of the structure and function of RNase L. From there we highlight and discuss new findings on the role of RNase L in innate immunity and tumor suppression published in the last 3 years (for a review of prior literature see Silverman 2007).
FIG. 1.
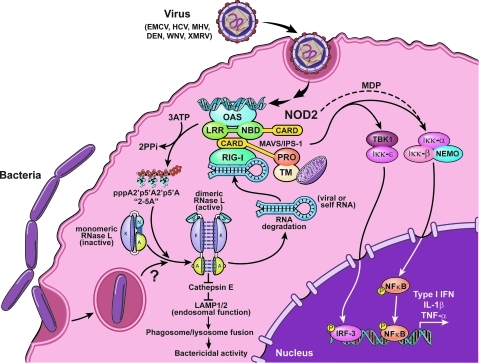
Role of RNase L in innate immunity against viruses and bacteria. The diagram illustrates how RNase L is activated in response to viral double-stranded RNA activating OAS to produce 2-5A from ATP. The RNase L domains shown are “A,” ARD; “K,” PK-like; and “N,” RNASE. 2-5A binding to the ARD causes inactive RNase L monomers to form activated dimers that degrade viral and cellular single-stranded RNA. RNA cleavage products activate RIG-I to amplify production of IFN-β. Bacterial infections signal through RNase L to inhibit Cathepsin E synthesis enhancing levels of lysosome-associated membrane proteins, LAMP 1 and 2, required for the terminal step in phagosome maturation and destruction of the microbial contents. NOD2 interacts with OAS2, whereas NOD2 overexpression leads to enhanced RNase L activity in the presence of double-stranded RNA. NOD2 responds to bacterial MDP triggering NF-κB activation contributing to type I IFN, IL-1β and TNF-α synthesis. ARD, ankyrin repeat domain; IFN, interferon; MDP, muramyl dipeptide; NOD2, nucleotide-binding and oligomerization domain-2; OAS, 2′-5′-oligoadenylate synthetase; PK, protein kinase; RIG-I, retinoic acid-inducible gene-I.
Biochemistry of RNase L
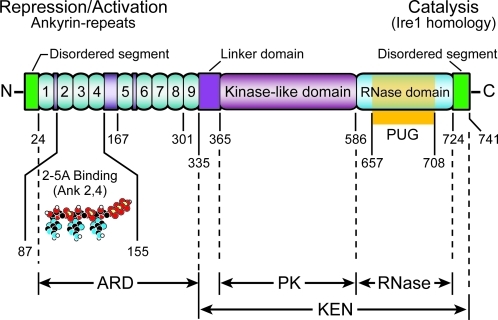
RNase L is a highly regulated, latent endoribonuclease (hence the “L” in RNase L) first cloned in 1993 when it was referred to as the 2-5A-dependent RNase (Zhou and others 1993). It is widely expressed in most, if not all, mammalian tissues (Zhou and others 2005), lying in wait for virus to infect and produce dsRNA, which triggers the pathway through OAS activation. RNase L is composed of 3 major domains: an N-terminal regulatory ankyrin repeat domain (ARD), a protein kinase (PK)-like domain, and an C-terminal ribonuclease domain (RNASE) (Fig. 2). The PK and RNASE domains [collectively referred to as the kinase-extenstion-nuclease (KEN) domain] have homology with Ire1, both a kinase and an endoribonuclease, that functions in the unfolded protein response (UPR) from yeast to humans (Sidrauski and Walter 1997; Lee and others 2008; Korennykh and others 2009). Analogous to the ∼120-amino-acid long linker domain that tethers the KEN domain to the transmembrane region in Ire1, there exist a much shorter, ∼30 amino acid, linker between ARD and PK homology domain of RNase L. Within the RNASE domain there is also a PUG (or PUB) domain similar to that in peptide N-glycanase, which removes glycans from misfolded glycoproteins, also present in some ubiquitin-protease system proteins (Allen and others 2006) and a possible protein–protein interaction domain (Suzuki and others 2001) (Fig. 2). There are other proteins that have both ARD and PK domains, such as integrin-linked kinase (Hannigan and others 1996), and death associate PK, DAP kinase (Bialik and others 2004). As mentioned, Ire1 proteins have both PK and RNASE domains (Sidrauski and Walter 1997), but RNase L is the only protein yet identified that has all 3 domains (ARD, PK, and RNASE). RNase L sequences are currently known for a wide range of mammalian species and 1 avian species (chicken, Gallus gallus), allowing for comparisons (Table 1). In general, the ARD is more highly conserved than the KEN domain. For instance, the Gallus RNase L has 47% and 32% similarity to the ARD and KEN domain of human RNase L, respectively. Sequence analysis of RNase L using GlobPlot 2 (Linding and others 2003) from different species predicted intrinsic disordered segments, ∼20 amino acids, at both the N- and C-termini (Fig. 1). These disordered segments are dispensable for human RNase L function (ie, 2-5A binding and ribonuclease activity) (Dong and Silverman 1997).
FIG. 2.
Domain structure of RNase L. The main structural and functional domains of RNase L are shown, including ankyrin repeat domain, ARD; protein kinase-like domain, PK; ribonuclease domain, RNASE; kinase-extension-nuclease domain, KEN, and peptide N-glycanase/UBA or UBX-containing protein domain, PUG or PUB. The region responsible for binding 2-5A is indicated.
Table 1.
Homology Between Different Mammalian and Avian Species of RNase L Protein
| |
Identity in domains of RNase L from different species compared with human RNase L (%) |
||
|---|---|---|---|
| Species | Full length (aa 1–741)a | ARD (aa 1–335)a | KEN (aa 336–741) |
| Homo sapiens | 100 | 100 | 100 |
| Pan troglodytes | 97 | 95 | 98 |
| Pongo abelii | 95 | 94 | 96 |
| Macaca mulatta | 92 | 91 | 93 |
| Equus caballus | 77 | 78 | 77 |
| Canis lupus familiaris | 71 | 72 | 70 |
| Bos taurus | 71 | 73 | 70 |
| Sus scrofa | 70 | 69 | 71 |
| Loxodonta africana | 67 | 68 | 66 |
| Cavia porcellus | 65 | 64 | 66 |
| Mus musculus | 64 | 63 | 65 |
| Rattus norvegicus | 62 | 64 | 62 |
| Gallus gallus | 39 | 47 | 32 |
Numbers of amino acid (aa) are from human RNase L Acc No. Q05823 (www.ncbi.nlm.nih.gov/protein/Q05823.2). The protein sequences were obtained from the NCBI database (www.ncbi.nlm.nih.gov/sites/entrez?db=cdd), and align using Clustlaw 2.0 (www.ebi.ac.uk/Tools/clustalw2/index.html). The percent identities were rounded to the nearest whole number.
ARD, ankyrin repeat domain; KEN, kinase-extenstion-nuclease.
Ankyrin repeats are one of the most common amino acid motifs, typically functioning in mediating protein–protein interactions, and are conserved in all kingdoms of life (reviewed in Barrick and others 2008). They are 33 amino acid units of paired antiparallel α-helices connected by a β-hairpin turn (Gorina and Pavletich 1996). RNase L has 8 complete ankyrin repeats and 1 partial repeat appearing as a disordered segment in the crystal structure of the N-terminal (amino acid 1–333) of human RNase L (Tanaka and others 2004). Ankyrin repeats 2 to 4 in RNase L constitute the 2-5A binding domain, a unique function among ARDs. In the absence of 2-5A, the ARD represses the RNASE domain. 2-5A binding to the ARD is believed to alter the conformation of RNase L, thereby exposing protein–protein interaction domains and releasing the RNASE domain from internal inhibitory sequences (Dong and others 2001) (Fig. 1). However, the precise activation mechanism will not be known until structures of full-length RNase L (as a free protein and in a complex with 2-5A) are solved. Removing ankyrin repeat 7 or 8, or Lys to Asn mutations at positions 240 and 274 in ARDs 7 and 8, respectively (Zhou and others 1993; Dong and Silverman 1997), result in loss of 2-5A binding and enzyme activities, suggesting that these changes affect the structural integrity of the 2-5A binding domain. Amino acid residues W60, N65, Q68, and K89 in repeat 2 and F126, E131, and R155 in repeat 4 directly interact with 2-5A either via H-bonding or by stacking interactions (Tanaka and others 2004). All the above residues are conserved among all known protein sequences of RNase L, except asparagine-65, which is substituted with a serine in Gallus RNase L. Mutagenesis studies in ARD 2 and 4, coupled with biochemical analysis, indicated that each of K89A, F126A, E131A, and R155A results in loss of 2-5A binding and enzyme activity, whereas N65A, Q68A, and Y135A had reduced 2-5A binding activity (Nakanishi and others 2005). Although RNase L has the only ARD known to bind to an oligonucleotide, the ARDs in transient receptor potential vanilloid (TRPV) channel proteins TRPV1, TRPV3, and TRPV4 have a multiligand binding site for ATP and calmodulin (Lishko and others 2007; Phelps and others 2010). Asparagine-233 in ankyrin repeat number 6 of human RNase L is hydroxylated by factor inhibiting hypoxia-inducible factor (HIF-1) alpha, factor inhibiting hypoxia-inducible factor (FIH), suggesting that RNase L stability and/or activity could be regulated in response to oxygen-related signals (Cockman and others 2009).
The KEN domain in RNase L functions in dimerization and catalysis (Dong and Silverman 1995, 1997; Carroll and others 1997). The RNASE domain becomes constitutively active upon removal of the ARD (although at 6-fold reduced activity compared with activated full-length protein) (Dong and Silverman 1997). Residues 365 to 586 in the KEN domain contain PK homology motifs (Fig. 1). RNase L is a pseudokinase due to lack of several amino acids required for an active kinase (including lack of critical residues in activation loop, substrate binding site, and active site). However, RNase L has highly conserved residues responsible for ATP binding and stimulation of enzyme activity (Wreschner and others 1982; Dong and others 1994; Lee and others 2008; Korennykh and others 2009). In the human kinome, the PK domain of RNase L is most closely related to that of protein kinase R (PKR) (Manning and others 2002). In addition to being related in their respective PK domains, PKR and RNase L are both antiviral enzymes whose levels and/or activities are regulated by IFN and viral dsRNA. Could this mean that the PK domains of PKR and RNase L evolved from a common ancestral precursor gene (Silverman and Williams 1999)?
Although RNase L shares some domain architecture with Ire1, there are both similarities and differences between these proteins. The N-terminal regions are unrelated, but both receive the activation signal (Korennykh and others 2009). In Ire1, activation is triggered by unfolded proteins through its endoplasmic reticulum (ER)-luminal domain that titrate off BiP, an ER chaperone, whereas RNase L activation occurs in the cytoplasm in response to 2-5A produced during viral infections. Both proteins are regulated at the level of dimerization (or oligomerization). Whereas a high-order assembly has been shown for Ire1, RNase L has thus far been shown to only form dimers (Dong and Silverman 1995; Carroll and others 1997; Korennykh and others 2009). In a highly unusual RNA splicing pathway, Ire1 cleaves pre-mRNAs for transcription factors HAC1 (yeast) or XBP1 (mammals) at unique sites (Korennykh and others 2009). HAC1 and XBP1 regulate UPR gene expression. In addition, Ire1 causes degradation of host mRNAs (mostly for membrane proteins), in a process named “regulated Ire1-dependent decay” (RIDD) (Hollien and others 2009). RIDD requires the nuclease activity of Ire1, but does not appear to be dependent on its kinase activity (Hollien and others 2009); in this respect, Ire1 is similar to RNase L. RNase L lacks PK activity and does not require phosphorylation for activation of its ribonuclease activity (Dong and Silverman 1999). Ire1 could have antiviral functions, beyond its role in the UPR, that overlap with RNase L. For example, RIDD was suggested to play a role in attenuating viral protein synthesis during hepatitis C virus (HCV) infections (Tardif and others 2004; Hollien and others 2009). Could RNase L be involved in RIDD during viral infections or could Ire1 play a role in the RNase L antiviral effect?
Role in IFN Induction
RNase L blocks different types of viruses (mostly RNA viruses) by different mechanisms depending the specific RNA substrates and the extent of ribonuclease activity (discussed in Silverman 2007). For instance, sustained RNase L activity is thought to eliminate virus-infected cells through apoptosis (Castelli and others 1997; Zhou and others 1997). Even cleavage of some cellular RNAs, such as rRNA in intact ribosomes, likely contributes to the antiviral activity of RNase L (Wreschner and others 1981a; Silverman and others 1983). Some of the RNA cleavage products resulting from RNase L activity, named “suppressor of virus RNA,” either viral or cellular in origin, can contribute to production of type I IFNs (Malathi and others 2007; Malathi and others 2010).
Viral RNAs are typically recognized by different PRRs (reviewed in Kumar and others 2009). For example, toll-like receptor 3 is a sensor for dsRNA in the endosomal compartment, whereas OAS is a PRR for viral dsRNA in the cytoplasm. Retinoic acid-inducible gene-I (RIG-I, also known as DDX58) and melanoma differentiation-associated gene-5 (MDA5, also known as IFIH1) are activated by 5′-triphosphorylated, double-stranded, or uridine and adenosine-rich viral RNAs in the cytoplasm (Saito and others 2008). Upon activation, the PRRs trigger signaling cascades that stimulate transcription of type I IFN genes (Kumar and others 2009). A role for RNase L in IFN induction was apparent from studies on mouse embryonic fibroblasts deficient in RNase L that showed reduced IFN-β production upon treatment with 2-5A, synthetic dsRNA [poly(rI):poly(rC)] and Sendai virus infection (Malathi and others 2007). The ribonuclease function of RNase L was essential for IFN induction with contributions from both RIG-I and MDA5. Total cellular RNA digested with RNase L or just the small cleaved RNA fragments, <200 nt, induced higher levels of IFN induction than uncleaved RNA. RNase L produces small RNA cleavage products with 3′-monophosphate (3′-p) and 5′-hydroxyl (5′-OH) at the termini. The 3′-p of the cleaved RNAs function in the recognition by RIG-I or MDA5, as calf alkaline phosphatase (CIP) treatment compromised their ability to induce IFN. Cellular observations were validated in mice where injection of 2-5A caused IFN-β induction in wild-type mice but not in mice deficient in RNase L. Moreover, mice lacking RNase L had several-fold reduced levels of IFN induction after infections with EMCV and Sendai virus. Therefore, effects of the OAS/RNase L pathway extend beyond initially infected cells to support a prolonged antiviral state in the organism. In essence, RNase L converted self-RNA into small RNA products that appeared to the host cell as nonself RNA. RNase L is also a critical component in IFN induction by a DNA virus, HSV-2 (Rasmussen and others 2009).
RNase L, Cancer, and Xenotropic Murine Leukemia-Related Virus
The cell growth inhibitory and proapoptotic effects of RNase L, and its chromosomal location at 1q25 mapping to some types of cancers, led to early speculation that RNase L could be a tumor suppressor (Hassel and others 1993; Lengyel 1993; Squire and others 1994; Castelli and others 1997; Zhou and others 1997). The idea gained support when the hereditary prostate cancer 1 (HPC1) gene was mapped to the RNase L gene (RNASEL) (Carpten and others 2002). HPC is apparent when there are 3 or more first degree relatives with the disease. Several germline mutations in RNASEL were observed in HPC, including M1I, E265X, 471ΔAAAG, and R462Q (reviewed in Silverman 2003). The missense variant, R462Q, mapping to the PK-like domain, reduces enzymatic activity by a factor of ∼3 (Xiang and others 2003) and doubled the risk of prostate cancer when homozygous (QQ) (Casey and others 2002). A homozyogous variant allele (rs12757998) downstream of the gene was associated with a significant increased risk of prostate cancer, including risk of higher grade tumors, in association with increased plasma biomarkers of inflammation, interleukin-6 (IL-6), C-reactive protein, and TNFR2 (Meyer and others 2010). It is unknown if this variant affects RNase L expression or if it is in linkage disequilibrium with a functional SNP that was not monitored. In the same study, the R462Q variant was not associated with advanced stage disease overall in the PSA era (post-1992); however, the QQ genotype was significantly associated with increased risk in the pre-PSA era, possibly reflecting diagnosis at a later stage in the disease (Meyer and others 2010).
Although some reports confirmed a role for RNASEL mutations in HPC or sporadic prostate cancer, others did not (see Silverman 2003; Meyer and others 2010 for discussion of recent literature). These studies suggest that environmental influences, such as microbial infections, might contribute to varying findings on RNase L/HPC1 in prostate cancer. Therefore, a clinical study was conducted to determine if a low activity variant of RNase L (R462Q) could be correlated to the presence of a viral infection in prostate cancer (Urisman and others 2006). One hundred fifty men with localized prostate cancer were genotyped for the RNase L variant, whereas RNA isolated from tumor-bearing prostate tissues was used to probe a pan-viral DNA microarray known as ViroChip (Wang and others 2002). A novel human retrovirus, the xenotropic murine leukemia virus-related virus (XMRV), was identified, mostly in men who were homozygous for the reduced activity variant of RNase L (Urisman and others 2006) and confirmed in (Arnold 2010) (reviewed in Silverman and others 2010). Another study on XMRV did not find an association with R462Q, although other mutations in the OAS/RNase L were not investigated (Schlaberg and others 2009). More recently, XMRV has been found in association with chronic fatigue syndrome in 1 study (Lombardi and others 2009), but not in others (Erlwein and others 2010; Groom and others 2010; van Kuppeveld and others 2010). Previously, RNase L was shown to be degraded in peripheral blood mononuclear cells (PBMC) of chronic fatigue syndrome (CFS) patients, providing a possible link between RNase L and XMRV infections in CFS (Suhadolnik and others 1997; Demettre and others 2002). RNase L was shown to be necessary for a complete IFN antiviral response against XMRV, thus supporting an association of RNase L mutation with XMRV in prostate cancer (Dong and others 2007). In addition, a 5′-untranslated region (UTR) mutation, rs3738579, in RNASEL associated with cancers of head and neck cancer, uterine cervix, and breast (Madsen and others 2008). The homozygous R462Q allele is also associated with disease aggressiveness and metastasis in familial pancreatic cancer (Bartsch and others 2005) and with age of onset of hereditary nonpolyposis colon cancer (Kruger and others 2005).
Protection Against Virus-Mediated Demyelination in the Central Nervous System
A novel protective role for RNase L in mitigating viral-induced demyelination in the central nervous system has been demonstrated showing that even when RNase L does not inhibit global viral replication it can still protect from virus-mediated disease. A sub-lethal, demyelinating mouse hepatitis virus (the neutropic coronavirus strain JHM) was lethal to a majority of RNase L-deficient mice by 12 days postinfection (Ireland and others 2009). Absence of RNase L in mice enhanced the morbidity rate without affecting overall viral replication in the brain. Further, RNase L deficiency did not impair type I IFN production or interferon stimulated gene (ISG) expression after viral infection, and neither did it alter the inflammatory response in central nervous system (CNS). Instead, there was early onset of severe demyelination with axonal damage in the brain stem and spinal cord of infected animals that lacked RNase L. Foci of infected microglia, sustained brain stem infection, and enhanced apoptosis were observed in the mice lacking RNase L. Therefore, RNase L prevents spread of virus to the microglia, leading to protection of the CNS from virus-induced demyelination. The authors suggest that by contributing to viral tropism in the CNS, RNase L affects the balance between neuroprotective and neutoxic effects of microglia.
A Region of Poliovirus RNA Is an RNase L Inhibitor
Poliovirus RNA is resistant to cleavage by RNase L due to a conserved RNA structure in the group C enteroviruses present in the 3CPro open reading frame (known as the “RNase L competitive inhibitor RNA” or RNase L ciRNA) (Han and others 2007; Townsend and others 2008a, 2008b). CiRNA does not affect 2-5A binding, but instead competitively inhibits the RNASE domain (Townsend and others 2008a). The RNase L inhibitory function of the ciRNA requires a putative loop E motif and an H-H kissing loop (Townsend and others 2008b).
Bacterial Targets
Although RNase L is usually thought of in terms of its antiviral functions, it also is protective in mice against infections by Bacillus anthracis and Escherichia coli (Li and others 2008) (Fig. 2). After infections with either type of bacteria, mice lacking RNase L had increased bacterial loads and higher mortality rates than identically infected WT mice. These findings were correlated to reduced levels of proinflammatory cytokines, IL-1β and TNF-α, and IFN-β after bacterial infection in the RNase L-deficient mice. Also, IRF3 dimerization was reduced 2-fold in E. coli–infected RNase L−/− macrophages. Microarray analysis and subsequent experiments revealed a role of RNase L in regulation of Cathepsin E (Cat E), an endolysosomal aspartyl proteinase. Cat E mRNA was stabilized in macrophages lacking RNase L leading to increased levels in the protein. Elevated Cat E levels correlated with reduction of lysosome-associated membrane proteins, LAMP1 and LAMP2, required for the terminal step in phagosome maturation in which late endosomes fuse with lysosomes to eliminate phagocytosed microbial cargo. Decreased expression of LAMP1/2 in macrophages from RNaseL−/− mice was correlated to accumulated phagocytic vacuoles and ineffective clearance of bacteria. In addition, overexpression of Cat E mimicked the absence of RNase L by impairing the induction of IL-1β after LPS treatment. The findings identify an essential role for RNase L in antibacterial immunity in which RNase L is required for the optimal induction of proinflammatory cytokines while also regulating Cat E, and associated endolysosomal functions, required for the elimination of phagocytosed bacteria. However, exactly how bacteria or LPS are signaling to RNase L, or whether, if fact, the ribonuclease activity of RNase L is required for its anti-bacterial role has not been reported. Curiously, while the IFN-inducible OAS/RNase L pathway is antibacterial, the type I IFNs themselves have been described in some studies to have probacterial effects (Rayamajhi and others 2009; Shahangian and others 2009).
A completely different line of investigation has indirectly linked the OAS/RNase L pathway to an innate immunity against bacterial infections. The nucleotide-binding and oligomerization domain-2 (NOD2) is a Nod-like receptor member that is activated by bacteria-derived muramyl dipeptide to trigger innate immunity by activating NF-kB and MAP kinases (reviewed in Ting and others) (Fig. 1). OAS2 p69 interacts with NOD2, leading to enhanced RNase L activity in poly(rI):poly(rC)-treated human acute monocytic leukemia cell line THP-1 (Dugan and others 2009). In addition, NOD2 recognizes viral ssRNA (from respiratory syncytial virus) and uses NOD2 to activate IRF3 (Sabbah and others 2009).
Cross-Regulation of HuR and RNase L Impacts mRNA Turnover
Cellular levels of RNase L are controlled in part by regulated turnover of its mRNA through sequences in its 3′-UTR and proteins that interact with these elements (Li and others 2007). The RNase L 3′-UTR contains 8 AU-rich elements (ARE), deletion of which identified both positive and negative regulatory effects. The RNase L 3′-UTR acted in cis to destabilize a heterologous mRNA for β-globin; therefore, the overall effect of the RNase L 3′-UTR was to decrease the half-life of the RNA. However, AREs 7 and 8 exerted a positive, or stabilizing effect on the RNase L mRNA. In addition, expression of the ARE-binding protein, HuR, enhanced RNase L mRNA and protein levels through 3′-UTR sequences between and including AREs 7 and 8. HuR binds RNase L mRNA during myoblast differentiation as determined in RNP immunoprecipitations. Cellular stress induced by heat shock or UVC radiation caused increases in RNase L levels that were dependent on the 3′-UTR.
In an apparent feedback loop, HuR expression increases levels of RNase L, whereas expression of RNase L decreases levels of HuR (Al-Ahmadi and others 2009). Cell growth rates and HuR levels were both elevated in RNase L-null mouse embryonic fibroblasts. The increase in HuR protein levels was correlated to enhanced stability of its mRNA in the absence of RNase L. The RNase L inhibitory effect on HuR mRNA levels was mapped to the HuR 3′-UTR, which contains U-rich/ARE-like sequences. In summary, whereas HuR stabilizes the RNase L mRNA through AREs 7 and 8, RNase L destabilizes the HuR mRNA through AREs in its 3′-UTR. Results suggest posttranscriptional cross-regulation between HuR and RNase L that determines cellular levels, and consequently cellular effects, of both proteins. RNase L effects on HuR expression was found to be most prominent in confluent cells or cells arrested in G1 phase of cell cycle, during which time both RNase L and HuR were in the cytoplasm.
RNase L and Senescence
Because of its antiproliferative and tumor suppression functions, involvement of RNase L in cell senescence and aging were explored (Andersen and others 2007). Ectopic expression of RNase L in mouse Balb-c 3T3 cells resulted in senescent morphological changes, decreased DNA synthesis, increased β-galactosidase activity, and replicative senescence. On the other hand, senescence was retarded in RNase L-null mouse fibroblasts. Further, 2-5A activation of RNase L induced senescence in primary WI38 human diploid fibroblasts. The RNase L effect was correlated with down-modulation of some cellular mRNAs, including several ribosomal protein mRNAs (Andersen and others 2009). RNase L-null mice had lifespan that was significantly prolonged (by about 20 weeks) compared with wild-type mice (Andersen and others 2007). Could polymorphisms or mutations in the human RNASEL gene affect the human lifespan?
Conclusions
Regulated turnover and processing of ssRNA by RNase L is essential for a complete IFN response against some viral infections. Studies on the structure and function of RNase L, and the related nuclease Ire1, are providing a window into how these enzymes are regulated. RNase L participates in the IFN antiviral effect directly or indirectly depending on the type of viral or cellular RNA that is cleaved. Antiviral innate immunity is triggered, in part, by processing of ssRNA into small RNA activators of retinoic acid-inducible gene-I like receptors (RLR) signaling that amplifies production of type I IFNs. RNase L is a suspected suppressor of HPC, and possibly of some other types of cancer, by inhibiting oncogenic viruses, but also through its pro-apoptotic and cell growth inhibitory properties. Determining how bacterial infections regulate RNase L activity resulting in suppression of infections will be of considerable interest. RNase L is ubiquitously expressed in a latent form, activated by 2-5A, and effective against many types of viral infections (Fig. 1). Therefore, RNase L could prove a unique target for a broad-spectrum antiviral, with possible activity against bacteria as well. What is clear after >30 years of research is that RNase L is now firmly established as an important player in overall innate immunity that controls viral pathogens.
Acknowledgments
The authors wish to gratefully acknowledge financial support for their research studies from the NIH (NCI), grant CA044059 (to R.H.S.) and to David Schumick, B.S., C.M.I., Center for Medical Art and Photography, Cleveland Clinic for outstanding artwork.
Author Disclosure Statement
R.H.S.: Abbott Laboratories, Inc. (patents and consulting) and Alios BioPharma, Inc. (patents and consulting).
References
- Al-Ahmadi W. Al-Haj L. Al-Mohanna FA. Silverman RH. Khabar KS. RNase L downmodulation of the RNA-binding protein, HuR, and cellular growth. Oncogene. 2009;28(15):1782–1791. doi: 10.1038/onc.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MD. Buchberger A. Bycroft M. The PUB domain functions as a p97 binding module in human peptide N-glycanase. J Biol Chem. 2006;281(35):25502–25508. doi: 10.1074/jbc.M601173200. [DOI] [PubMed] [Google Scholar]
- Andersen JB. Li XL. Judge CS. Zhou A. Jha BK. Shelby S. Zhou L. Silverman RH. Hassel BA. Role of 2-5A-dependent RNase-L in senescence and longevity. Oncogene. 2007;26(21):3081–3088. doi: 10.1038/sj.onc.1210111. [DOI] [PubMed] [Google Scholar]
- Andersen JB. Mazan-Mamczarz K. Zhan M. Gorospe M. Hassel BA. Ribosomal protein mRNAs are primary targets of regulation in RNase-L-induced senescence. RNA Biol. 2009;6(3):305–315. doi: 10.4161/rna.6.3.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold RS. Makarova N. Osunkoya AO. Suppiah S. Scott TA. Johnson NA. Bhosle SM. Liotta D. Hunter E. Marshall FF. Ly H. Molinaro RJ. Blackwell JL. Petros JA. XMRV infection in prostate cancer patients: novel serologic assay and correlation with PCR and FISH. Urology. 2010;75:755–761. doi: 10.1016/j.urology.2010.01.038. [DOI] [PubMed] [Google Scholar]
- Barrick D. Ferreiro DU. Komives EA. Folding landscapes of ankyrin repeat proteins: experiments meet theory. Curr Opin Struct Biol. 2008;18(1):27–34. doi: 10.1016/j.sbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch DK. Fendrich V. Slater EP. Sina-Frey M. Rieder H. Greenhalf W. Chaloupka B. Hahn SA. Neoptolemos JP. Kress R. RNASEL germline variants are associated with pancreatic cancer. Int J Cancer. 2005;117(5):718–722. doi: 10.1002/ijc.21254. [DOI] [PubMed] [Google Scholar]
- Bialik S. Bresnick AR. Kimchi A. DAP-kinase-mediated morphological changes are localization dependent and involve myosin-II phosphorylation. Cell Death Differ. 2004;11(6):631–644. doi: 10.1038/sj.cdd.4401386. [DOI] [PubMed] [Google Scholar]
- Bisbal C. Martinand C. Silhol M. Lebleu B. Salehzada T. Cloning and characterization of a RNAse L inhibitor. A new component of the interferon-regulated 2-5A pathway. J Biol Chem. 1995;270(22):13308–13317. doi: 10.1074/jbc.270.22.13308. [DOI] [PubMed] [Google Scholar]
- Carpten J. Nupponen N. Isaacs S. Sood R. Robbins C. Xu J. Faruque M. Moses T. Ewing C. Gillanders E. Hu P. Bujnovszky P. Makalowska I. Baffoe-Bonnie A. Faith D. Smith J. Stephan D. Wiley K. Brownstein M. Gildea D. Kelly B. Jenkins R. Hostetter G. Matikainen M. Schleutker J. Klinger K. Connors T. Xiang Y. Wang Z. De Marzo A. Papadopoulos N. Kallioniemi OP. Burk R. Meyers D. Gronberg H. Meltzer P. Silverman R. Bailey-Wilson J. Walsh P. Isaacs W. Trent J. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat Genet. 2002;30(2):181–184. doi: 10.1038/ng823. [DOI] [PubMed] [Google Scholar]
- Carroll SS. Cole JL. Viscount T. Geib J. Gehman J. Kuo LC. Activation of RNase L by 2′,5′-oligoadenylates. Kinetic characterization. J Biol Chem. 1997;272(31):19193–19198. doi: 10.1074/jbc.272.31.19193. [DOI] [PubMed] [Google Scholar]
- Casey G. Neville PJ. Plummer SJ. Xiang Y. Krumroy LM. Klein EA. Catalona WJ. Nupponen N. Carpten JD. Trent JM. Silverman RH. Witte JS. RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. Nat Genet. 2002;32(4):581–583. doi: 10.1038/ng1021. [DOI] [PubMed] [Google Scholar]
- Castelli JC. Hassel BA. Wood KA. Li XL. Amemiya K. Dalakas MC. Torrence PF. Youle RJ. A study of the interferon antiviral mechanism: apoptosis activation by the 2-5A system. J Exp Med. 1997;186(6):967–972. doi: 10.1084/jem.186.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens MJ. Williams BR. Inhibition of cell-free protein synthesis by pppA2′p5′A2′p5′A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Cockman ME. Webb JD. Kramer HB. Kessler BM. Ratcliffe PJ. Proteomics-based identification of novel factor inhibiting hypoxia-inducible factor (FIH) substrates indicates widespread asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Mol Cell Proteomics. 2009;8(3):535–546. doi: 10.1074/mcp.M800340-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demettre E. Bastide L. D'Haese A. De Smet K. De Meirleir K. Tiev KP. Englebienne P. Lebleu B. Ribonuclease L proteolysis in peripheral blood mononuclear cells of chronic fatigue syndrome patients. J Biol Chem. 2002;277(38):35746–35751. doi: 10.1074/jbc.M201263200. [DOI] [PubMed] [Google Scholar]
- Dong B. Kim S. Hong S. Das Gupta J. Malathi K. Klein EA. Ganem D. Derisi JL. Chow SA. Silverman RH. An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc Natl Acad Sci U S A. 2007;104(5):1655–1660. doi: 10.1073/pnas.0610291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B. Niwa M. Walter P. Silverman RH. Basis for regulated RNA cleavage by functional analysis of RNase L and Ire1p. RNA. 2001;7(3):361–373. doi: 10.1017/s1355838201002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B. Silverman RH. 2-5A-dependent RNase molecules dimerize during activation by 2-5A. J Biol Chem. 1995;270(8):4133–4137. doi: 10.1074/jbc.270.8.4133. [DOI] [PubMed] [Google Scholar]
- Dong B. Silverman RH. A bipartite model of 2-5A-dependent RNase L. J Biol Chem. 1997;272(35):22236–22242. doi: 10.1074/jbc.272.35.22236. [DOI] [PubMed] [Google Scholar]
- Dong B. Silverman RH. Alternative function of a protein kinase homology domain in 2′, 5′-oligoadenylate dependent RNase L. Nucleic Acids Res. 1999;27(2):439–445. doi: 10.1093/nar/27.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B. Xu L. Zhou A. Hassel BA. Lee X. Torrence PF. Silverman RH. Intrinsic molecular activities of the interferon-induced 2-5A-dependent RNase. J Biol Chem. 1994;269(19):14153–14158. [PubMed] [Google Scholar]
- Dugan JW. Albor A. David L. Fowlkes J. Blackledge MT. Martin TM. Planck SR. Rosenzweig HL. Rosenbaum JT. Davey MP. Nucleotide oligomerization domain-2 interacts with 2′-5′-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cells. Mol Immunol. 2009;47(2–3):560–566. doi: 10.1016/j.molimm.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlwein O. Kaye S. McClure MO. Weber J. Wills G. Collier D. Wessely S. Cleare A. Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS ONE. 2010;5(1):e8519. doi: 10.1371/journal.pone.0008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd-Smith G. Slattery E. Lengyel P. Interferon action: RNA cleavage pattern of a (2′-5′)oligoadenylate—dependent endonuclease. Science. 1981;212(4498):1030–1032. doi: 10.1126/science.6165080. [DOI] [PubMed] [Google Scholar]
- Gorina S. Pavletich NP. Structure of the p53 tumor suppressor bound to the ankyrin and SH3 domains of 53BP2. Science. 1996;274(5289):1001–1005. doi: 10.1126/science.274.5289.1001. [DOI] [PubMed] [Google Scholar]
- Groom HC. Boucherit VC. Makinson K. Randal E. Baptista S. Hagan S. Gow JW. Mattes FM. Breuer J. Kerr JR. Stoye JP. Bishop KN. Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology. 2010;7:10. doi: 10.1186/1742-4690-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JQ. Townsend HL. Jha BK. Paranjape JM. Silverman RH. Barton DJ. A phylogenetically conserved RNA structure in the poliovirus open reading frame inhibits the antiviral endoribonuclease RNase L. J Virol. 2007;81(11):5561–5572. doi: 10.1128/JVI.01857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan GE. Leung-Hagesteijn C. Fitz-Gibbon L. Coppolino MG. Radeva G. Filmus J. Bell JC. Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature. 1996;379(6560):91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- Hassel BA. Zhou A. Sotomayor C. Maran A. Silverman RH. A dominant negative mutant of 2-5A-dependent RNase suppresses antiproliferative and antiviral effects of interferon. EMBO J. 1993;12(8):3297–3304. doi: 10.1002/j.1460-2075.1993.tb05999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J. Lin JH. Li H. Stevens N. Walter P. Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian AG. Brown RE. Kerr IM. Synthesis of low molecular weight inhibitor of protein synthesis with enzyme from interferon-treated cells. Nature. 1977;268(5620):537–540. doi: 10.1038/268537a0. [DOI] [PubMed] [Google Scholar]
- Ireland DD. Stohlman SA. Hinton DR. Kapil P. Silverman RH. Atkinson RA. Bergmann CC. RNase L mediated protection from virus induced demyelination. PLoS Pathog. 2009;5(10):e1000602. doi: 10.1371/journal.ppat.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen H. Krause D. Friedman RM. Silverman RH. Induction of ppp(A2′p)nA-dependent RNase in murine JLS-V9R cells during growth inhibition. Proc Natl Acad Sci U S A. 1983;80(16):4954–4958. doi: 10.1073/pnas.80.16.4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuta S. Shibata S. Iwakura Y. Genomic structure of the mouse 2′,5′-oligoadenylate synthetase gene family. J Interferon Cytokine Res. 2002;22(9):981–993. doi: 10.1089/10799900260286696. [DOI] [PubMed] [Google Scholar]
- Kerr IM. Brown RE. pppA2′p5′A2′p5′A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korennykh AV. Egea PF. Korostelev AA. Finer-Moore J. Zhang C. Shokat KM. Stroud RM. Walter P. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457(7230):687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen H. Henrik Gad H. Eskildsen-Larsen S. Despres P. Hartmann R. The Oligoadenylate Synthetase family: An ancient protein family with multiple antiviral activities. J Interferon Cytokine Res. 2010;31(1):41–47. doi: 10.1089/jir.2010.0107. [DOI] [PubMed] [Google Scholar]
- Kruger S. Silber AS. Engel C. Gorgens H. Mangold E. Pagenstecher C. Holinski-Feder E. von Knebel Doeberitz M. Moeslein G. Dietmaier W. Stemmler S. Friedl W. Ruschoff J. Schackert HK. Arg462Gln sequence variation in the prostate-cancer-susceptibility gene RNASEL and age of onset of hereditary non-polyposis colorectal cancer: a case-control study. Lancet Oncol. 2005;6(8):566–572. doi: 10.1016/S1470-2045(05)70253-9. [DOI] [PubMed] [Google Scholar]
- Kubota K. Nakahara K. Ohtsuka T. Yoshida S. Kawaguchi J. Fujita Y. Ozeki Y. Hara A. Yoshimura C. Furukawa H. Haruyama H. Ichikawa K. Yamashita M. Matsuoka T. Iijima Y. Identification of 2′-phosphodiesterase, which plays a role in the 2-5A system regulated by interferon. J Biol Chem. 2004;279(36):37832–37841. doi: 10.1074/jbc.M400089200. [DOI] [PubMed] [Google Scholar]
- Kumar H. Kawai T. Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420(1):1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- Lee KP. Dey M. Neculai D. Cao C. Dever TE. Sicheri F. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell. 2008;132(1):89–100. doi: 10.1016/j.cell.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Tumor-suppressor genes: news about the interferon connection. Proc Natl Acad Sci U S A. 1993;90(13):5893–5895. doi: 10.1073/pnas.90.13.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL. Andersen JB. Ezelle HJ. Wilson GM. Hassel BA. Post-transcriptional regulation of RNase-L expression is mediated by the 3′-untranslated region of its mRNA. J Biol Chem. 2007;282(11):7950–7960. doi: 10.1074/jbc.M607939200. [DOI] [PubMed] [Google Scholar]
- Li XL. Ezelle HJ. Kang TJ. Zhang L. Shirey KA. Harro J. Hasday JD. Mohapatra SK. Crasta OR. Vogel SN. Cross AS. Hassel BA. An essential role for the antiviral endoribonuclease, RNase-L, in antibacterial immunity. Proc Natl Acad Sci U S A. 2008;105(52):20816–20821. doi: 10.1073/pnas.0807265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linding R. Russell RB. Neduva V. Gibson TJ. GlobPlot: exploring protein sequences for globularity and disorder. Nucleic Acids Res. 2003;31(13):3701–3708. doi: 10.1093/nar/gkg519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV. Procko E. Jin X. Phelps CB. Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54(6):905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Lombardi VC. Ruscetti FW. Das Gupta J. Pfost MA. Hagen KS. Peterson DL. Ruscetti SK. Bagni RK. Petrow-Sadowski C. Gold B. Dean M. Silverman RH. Mikovits JA. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326(5952):585–589. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- Madsen BE. Ramos EM. Boulard M. Duda K. Overgaard J. Nordsmark M. Wiuf C. Hansen LL. Germline mutation in RNASEL predicts increased risk of head and neck, uterine cervix and breast cancer. PLoS ONE. 2008;3(6):e2492. doi: 10.1371/journal.pone.0002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K. Dong B. Gale M., Jr. Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448(7155):816–819. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K. Paranjape JM. Ganapathi R. Silverman RH. HPC1/RNASEL mediates apoptosis of prostate cancer cells treated with 2′,5′-oligoadenylates, topoisomerase I inhibitors, and tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2004;64(24):9144–9151. doi: 10.1158/0008-5472.CAN-04-2226. [DOI] [PubMed] [Google Scholar]
- Malathi K. Saito T. Crochet N. Barton DJ. Gale M., Jr. Silverman RH. RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. Rna. 2010 doi: 10.1261/rna.2244210. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G. Whyte DB. Martinez R. Hunter T. Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- Mashimo T. Lucas M. Simon-Chazottes D. Frenkiel MP. Montagutelli X. Ceccaldi PE. Deubel V. Guenet JL. Despres P. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc Natl Acad Sci U S A. 2002;99(17):11311–11316. doi: 10.1073/pnas.172195399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MS. Penney KL. Stark JR. Schumacher F. Sesso H. Loda M. Fiorentino M. Finn S. Flavin R. Kurth T. Price A. Giovannucci E. Fall K. Stampfer MJ. Ma J. Mucci LA. Genetic variation in RNASEL associated with prostate cancer risk and progression. Carcinogenesis. 2010;31(9):1597–1603. doi: 10.1093/carcin/bgq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M. Tanaka N. Mizutani Y. Mochizuki M. Ueno Y. Nakamura KT. Kitade Y. Functional characterization of 2′,5′-linked oligoadenylate binding determinant of human RNase L. J Biol Chem. 2005;280(50):41694–41699. doi: 10.1074/jbc.M507424200. [DOI] [PubMed] [Google Scholar]
- Perelygin AA. Scherbik SV. Zhulin IB. Stockman BM. Li Y. Brinton MA. Positional cloning of the murine flavivirus resistance gene. Proc Natl Acad Sci U S A. 2002;99(14):9322–9327. doi: 10.1073/pnas.142287799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps CB. Wang RR. Choo SS. Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem. 2010;285(1):731–740. doi: 10.1074/jbc.M109.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SB. Jensen SB. Nielsen C. Quartin E. Kato H. Chen ZJ. Silverman RH. Akira S. Paludan SR. Herpes simplex virus infection is sensed by both toll-like receptors and retinoic acid-inducible gene- like receptors, which synergize to induce type I interferon production. J Gen Virol. 2009;90(Pt 1):74–78. doi: 10.1099/vir.0.005389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner L. Wiegand RC. Farrell PJ. Sen GC. Cabrer B. Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Rayamajhi M. Humann J. Penheiter K. Andreasen K. Lenz LL. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med. 2010;207(2):327–337. doi: 10.1084/jem.20091746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch L. Zhou A. Silverman RH. Caspase-dependent apoptosis by 2′,5′-oligoadenylate activation of RNase L is enhanced by IFN-beta. J Interferon Cytokine Res. 2000;20(12):1091–1100. doi: 10.1089/107999000750053762. [DOI] [PubMed] [Google Scholar]
- Sabbah A. Chang TH. Harnack R. Frohlich V. Tominaga K. Dube PH. Xiang Y. Bose S. Activation of innate immune antiviral responses by Nod2. Nat Immunol. 2009;10(10):1073–1080. doi: 10.1038/ni.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T. Owen DM. Jiang F. Marcotrigiano J. Gale M., Jr. Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454(7203):523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbik SV. Paranjape JM. Stockman BM. Silverman RH. Brinton MA. RNase L plays a role in the antiviral response to West Nile virus. J Virol. 2006;80(6):2987–2999. doi: 10.1128/JVI.80.6.2987-2999.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaberg R. Choe DJ. Brown KR. Thaker HM. Singh IR. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci U S A. 2009;106(38):16351–16356. doi: 10.1073/pnas.0906922106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shahangian A. Chow EK. Tian X. Kang JR. Ghaffari A. Liu SY. Belperio JA. Cheng G. Deng JC. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119(7):1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C. Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90(6):1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- Silverman RH. Implications for RNase L in prostate cancer biology. Biochemistry. 2003;42(7):1805–1812. doi: 10.1021/bi027147i. [DOI] [PubMed] [Google Scholar]
- Silverman RH. Viral encounters with OAS and RNase L during the IFN antiviral response. J Virol. 2007;81(23):12720–12729. doi: 10.1128/JVI.01471-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH. Nguyen C. Weight CJ. Klein EA. The human retrovirus XMRV in prostate cancer and chronic fatigue syndrome. Nat Rev Urol. 2010;7(7):392–402. doi: 10.1038/nrurol.2010.77. [DOI] [PubMed] [Google Scholar]
- Silverman RH. Skehel JJ. James TC. Wreschner DH. Kerr IM. rRNA cleavage as an index of ppp(A2′p)nA activity in interferon-treated encephalomyocarditis virus-infected cells. J Virol. 1983;46(3):1051–1055. doi: 10.1128/jvi.46.3.1051-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman RH. Williams BR. Translational control perks up. Nature. 1999;397(6716):208–209. doi: 10.1038/16586. 211. [DOI] [PubMed] [Google Scholar]
- Slattery E. Ghosh N. Samanta H. Lengyel P. Interferon, double-stranded RNA, and RNA degradation: activation of an endonuclease by (2′-5′)An. Proc Natl Acad Sci U S A. 1979;76(10):4778–4782. doi: 10.1073/pnas.76.10.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J. Zhou A. Hassel BA. Nie H. Silverman RH. Localization of the interferon-induced, 2-5A-dependent RNase gene (RNS4) to human chromosome 1q25. Genomics. 1994;19(1):174–175. doi: 10.1006/geno.1994.1033. [DOI] [PubMed] [Google Scholar]
- Suhadolnik RJ. Peterson DL. O'Brien K. Cheney PR. Herst CV. Reichenbach NL. Kon N. Horvath SE. Iacono KT. Adelson ME. De Meirleir K. De Becker P. Charubala R. Pfleiderer W. Biochemical evidence for a novel low molecular weight 2-5A-dependent RNase L in chronic fatigue syndrome. J Interferon Cytokine Res. 1997;17(7):377–385. doi: 10.1089/jir.1997.17.377. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Park H. Till EA. Lennarz WJ. The PUB domain: a putative protein-protein interaction domain implicated in the ubiquitin-proteasome pathway. Biochem Biophys Res Commun. 2001;287(5):1083–1087. doi: 10.1006/bbrc.2001.5688. [DOI] [PubMed] [Google Scholar]
- Tanaka N. Nakanishi M. Kusakabe Y. Goto Y. Kitade Y. Nakamura KT. Structural basis for recognition of 2′,5′-linked oligoadenylates by human ribonuclease L. EMBO J. 2004;23(20):3929–3938. doi: 10.1038/sj.emboj.7600420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif KD. Mori K. Kaufman RJ. Siddiqui A. Hepatitis C virus suppresses the IRE1-XBP1 pathway of the unfolded protein response. J Biol Chem. 2004;279(17):17158–17164. doi: 10.1074/jbc.M312144200. [DOI] [PubMed] [Google Scholar]
- Ting JP. Duncan JA. Lei Y. How the noninflammasome NLRs function in the innate immune system. Science. 2001;327(5963):286–290. doi: 10.1126/science.1184004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend HL. Jha BK. Han JQ. Maluf NK. Silverman RH. Barton DJ. A viral RNA competitively inhibits the antiviral endoribonuclease domain of RNase L. Rna. 2008a;14(6):1026–1036. doi: 10.1261/rna.958908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend HL. Jha BK. Silverman RH. Barton DJ. A putative loop E motif and an H-H kissing loop interaction are conserved and functional features in a group C enterovirus RNA that inhibits ribonuclease L. RNA Biol. 2008b;5(4):263–272. doi: 10.4161/rna.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urisman A. Molinaro RJ. Fischer N. Plummer SJ. Casey G. Klein EA. Malathi K. Magi-Galluzzi C. Tubbs RR. Ganem D. Silverman RH. Derisi JL. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2(3):e25. doi: 10.1371/journal.ppat.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- van Kuppeveld FJ. de Jong AS. Lanke KH. Verhaegh GW. Melchers WJ. Swanink CM. Bleijenberg G. Netea MG. Galama JM. van der Meer JW. Prevalence of xenotropic murine leukaemia virus-related virus in patients with chronic fatigue syndrome in the Netherlands: retrospective analysis of samples from an established cohort. BMJ. 2010;340:c1018. doi: 10.1136/bmj.c1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Coscoy L. Zylberberg M. Avila PC. Boushey HA. Ganem D. DeRisi JL. Microarray-based detection and genotyping of viral pathogens. Proc Natl Acad Sci U S A. 2002;99(24):15687–15692. doi: 10.1073/pnas.242579699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner DH. James TC. Silverman RH. Kerr IM. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2′p)nA) in interferon-treated cells. Nucleic Acids Res. 1981a;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner DH. McCauley JW. Skehel JJ. Kerr IM. Interferon action—sequence specificity of the ppp(A2′p)nA-dependent ribonuclease. Nature. 1981b;289(5796):414–417. doi: 10.1038/289414a0. [DOI] [PubMed] [Google Scholar]
- Wreschner DH. Silverman RH. James TC. Gilbert CS. Kerr IM. Affinity labelling and characterization of the ppp(A2′p)nA-dependent endoribonuclease from different mammalian sources. Eur J Biochem. 1982;124(2):261–268. doi: 10.1111/j.1432-1033.1982.tb06586.x. [DOI] [PubMed] [Google Scholar]
- Xiang Y. Wang Z. Murakami J. Plummer S. Klein EA. Carpten JD. Trent JM. Isaacs WB. Casey G. Silverman RH. Effects of RNase L mutations associated with prostate cancer on apoptosis induced by 2′,5′-oligoadenylates. Cancer Res. 2003;63(20):6795–6801. [PubMed] [Google Scholar]
- Zhou A. Hassel BA. Silverman RH. Expression cloning of 2-5A-dependent RNAase: a uniquely regulated mediator of interferon action. Cell. 1993;72(5):753–765. doi: 10.1016/0092-8674(93)90403-d. [DOI] [PubMed] [Google Scholar]
- Zhou A. Molinaro RJ. Malathi K. Silverman RH. Mapping of the human RNASEL promoter and expression in cancer and normal cells. J Interferon Cytokine Res. 2005;25(10):595–603. doi: 10.1089/jir.2005.25.595. [DOI] [PubMed] [Google Scholar]
- Zhou A. Paranjape J. Brown TL. Nie H. Naik S. Dong B. Chang A. Trapp B. Fairchild R. Colmenares C. Silverman RH. Interferon action and apoptosis are defective in mice devoid of 2′,5′-oligoadenylate-dependent RNase L. EMBO J. 1997;16(21):6355–6363. doi: 10.1093/emboj/16.21.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]