Abstract
Viruses face a variety of obstacles when infecting a new host. The past few years have brought exciting new insights into the function of restriction factors, which form part of the host's innate immune system. One of the most recently identified restriction factors is bone marrow stromal antigen 2 (BST-2)/tetherin. BST-2 is an interferon-inducible gene whose expression dramatically reduces the release of viruses from infected cells. This effect of BST-2 is not specific to human immunodeficiency virus but affects a broad range of enveloped viruses. Since the identification of BST-2 as a restriction factor in 2008, much progress has been made in understanding the molecular properties and functional characteristics of this host factor. The goal of this review was to provide an update on our current understanding of the role of BST-2 in regulating virus release and to discuss its role in controlling virus spread during productive infection with special emphasis on human immunodeficiency virus-1.
Introduction
Viruses are obligate intracellular parasites whose primary goal is to infect host cells to replicate their genomes and to produce progeny virions for infection of new target cells. Some viruses cause long-lasting chronic infections, whereas others replicate in fast, lytic cycles. Most virus infections can be controlled and eliminated by the host immune system through adaptive, innate, or intrinsic immune mechanisms. Other viruses, including human immunodeficiency virus-1 (HIV-1), establish chronic life-long infections and are difficult if not impossible to eradicate. Although progress has been made in controlling the spread of HIV, the United States still reports more than 50,000 new HIV infections each year and there are currently an estimated 33 million people living with HIV worldwide.
Despite these staggering numbers, HIV infection appears to be quite inefficient. HIV is primarily transmitted through sexual contact; yet, only an estimated 1–4 of 1,000 sexual contacts result in HIV infection (Gray and others 2001). Indeed, HIV faces an uphill battle when spreading to a new host because it has to overcome several levels of host defense mechanisms. Recent years have brought rapid progress in the identification and characterization of host restriction factors affecting HIV replication. In particular, the identification of tripartite motif-containing protein 5 (Trim-5α), apolipoprotein B mRNA-editing catalytic polypeptide 3G (APOBEC3G), and bone marrow stromal antigen 2 (BST-2)/tetherin has significantly advanced our understanding of innate and intrinsic immune mechanisms involved in the defense against HIV (for a recent update, see Strebel and others 2009). Although each of these restriction factors targets a different stage of the viral replication cycle, they all have in common that their expression is regulated by type I interferons (IFNs) (Rose and others 2004; Asaoka and others 2005; Neil and others 2007; Miyagi and others 2009). It is, therefore, likely that the resulting upregulation of APOBEC3G, Trim-5α, and BST-2 contributes to the inhibition or the delay of virus spread. The present review focuses on BST-2/tetherin and aims to provide an up-to-date summary of our current knowledge of its role in controlling HIV replication.
BST-2: An IFN-Inducible Viral Restriction Factor
Viral infections can lead to the induction of IFN through the recognition of pathogen-associated molecular patterns by pattern-recognition receptors (reviewed in Randall and Goodbourn 2008). Indeed, HIV-1 was shown to induce IFN expression through engagement with Toll-like receptors, specifically TLR7 (Beignon and others 2005), and IFN has been detected in the plasma of infected individuals during acute viremia and during late-stage disease (von Sydow and others 1991; Ferbas and others 1994). Induction of IFN leads to the upregulation of other host factors. One of those IFN-induced host factors is BST-2. The upregulation of BST-2 expression by IFN is due to the presence of IFN responsive regulatory elements in the BST-2 promoter region (Ohtomo and others 1999). Interestingly, BST-2 was recently identified as a ligand for immunoglobulin-like transcript 7 (ILT7) protein (Cao and others 2009). Binding of BST-2 results in the activation of ILT7 and leads to reduced IFN expression (reviewed in Cao and Bover 2010). Thus, BST-2/ILT7 interaction may serve as an important negative feedback loop limiting INF responses to viral infections.
In addition to its potential role in limiting IFN responses to viral infections, BST-2 was recently found to play an additional role in limiting viral infections. The function was first identified for HIV-1 but appears to apply to a variety of enveloped viruses (see below). Efficient virus release from HIV-infected cells is regulated by its Vpu gene product (Strebel and others 1988; Terwilliger and others 1989). However, the dependence on Vpu for efficient virus release is cell-type dependent, leading investigators to predict the presence of a host restriction factor in Vpu-dependent cell types (Varthakavi and others 2003). Interestingly, IFN treatment of Vpu-independent cell types created a Vpu-restrictive phenotype and not only inhibited the release of HIV-1 and related retroviruses but also affected the secretion of unrelated viruses such as porcine endogenous retrovirus (PERV), Ebola, Lassa, Marburg, endogenous betaretrovirus of sheep, or Kaposi sarcoma-associated herpesvirus (KSHV) (Neil and others 2007; Jouvenet and others 2009; Kaletsky and others 2009; Sakuma and others 2009b; Arnaud and others 2010; Mattiuzzo and others 2010). These observations suggested that the Vpu-sensitive restriction factor was not specific to HIV but belonged to a family of IFN-inducible genes with general antiviral properties. A clue to the identity of the Vpu-sensitive restriction factor was first discovered during a quantitative membrane proteomics study in which Vpu expressed from an adenovirus vector was found to reduce cellular expression of BST-2 in HeLa cells (Bartee and others 2006). Subsequent reports identified BST-2 as the IFN-inducible, Vpu-sensitive factor responsible for the restriction of HIV-1 virus release (Neil and others 2008; Van Damme and others 2008). Indeed, BST-2 expression was cell-type dependent; the protein was constitutively expressed in Vpu-dependent cell types such as HeLa, Jurkat, or CD4+ T cells but was undetectable in permissive cell types such as 293T or HT1080 cells and thus corresponded to cell types known to depend on Vpu for efficient virus release (Neil and others 2008; Van Damme and others 2008). Importantly, BST-2 expression was induced by IFN treatment in 293T and HT1080 cells (Neil and others 2007, 2008) and ectopic expression of BST-2 in 293T or HT1080 cells rendered these cells Vpu dependent (Neil and others 2008; Van Damme and others 2008). Finally, small interfering RNA (siRNA) silencing of BST-2 caused virus release from HeLa cells to be Vpu independent (Neil and others 2008; Van Damme and others 2008; Rong and others 2009). Taken together these data provided strong evidence that BST-2 was indeed the host factor whose inhibitory effect on virus release was counteracted by Vpu.
BST-2: Structural Considerations
BST-2 was originally identified as a membrane protein in terminally differentiated human B cells of patients with multiple myeloma (Goto and others 1994; Ohtomo and others 1999). Subsequently, BST-2 was found on multiple types of cancer cells (Walter-Yohrling and others 2003) and BST-2 was implicated in promoting tumor invasion (Cai and others 2009). Because of that, BST-2 antibodies are under study for their potential clinical use for targeting cancer cells such as multiple myeloma (Ishikawa and others 1995), renal cell carcinoma (Kawai and others 2008), or lung cancer (Wang and others 2009a, 2009b). All studies have been quite promising and antibody treatment resulted in reduced tumor size presumably by inducing antibody-dependent cellular cytotoxicity.
BST-2 is a 30–36-kDa type II transmembrane (TM) protein, consisting of 180 amino acids (Ishikawa and others 1995). The protein has both an N-terminal TM domain and a C-terminal glycosyl-phosphatidylinositol (GPI) anchor (Kupzig and others 2003) (Fig. 1). BST-2 protein associates with lipid rafts at the cell surface and on internal membranes, presumably the trans-Golgi network (TGN) (Kupzig and others 2003; Dube and others 2009; Masuyama and others 2009). X-ray crystallography of recombinant BST-2 demonstrated that residues 47–148 of the protein's ectodomain can fold into a 90 Å parallel coil–coil structure (Hinz and others 2010). In addition, small-angle X-ray scattering analyses predicted that the complete extracellular region of BST-2 adopts a long, bent, rod-like structure that separates the TM domain and GPI anchor by ∼170 Å (Hinz and others 2010).
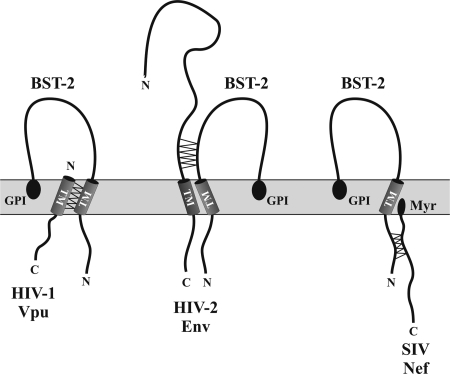
FIG. 1.
Interaction of BST-2 with HIV-1 proteins. Vpu interacts with BST-2 through its TM domain. The interaction of HIV-2 Env with BST-2 has not been mapped in detail but involves the ectodomain. Interaction of BST-2 with Nef involves a G/DDIWK in the cytoplasmic domain. This motif is missing in Nef-insensitive BST-2 variants (eg, human BST-2). Proposed interaction points are indicated by zig-zag lines. HIV, human immunodeficiency virus; SIV, simian immunodeficiency virus; TM, transmembrane; Myr, Nef myristoylation; GPI, glycosyl-phosphatidylinositol anchor; BST-2, bone marrow stromal antigen 2.
Biochemical analyses demonstrated that BST-2 forms stable cysteine-linked dimers (Goto and others 1994; Ohtomo and others 1999; Andrew and others 2009; Perez-Caballero and others 2009) and is modified by N-linked glycosylation (Ohtomo and others 1999; Kupzig and others 2003; Andrew and others 2009). The formation of cysteine-linked dimers can be catalyzed by any 1 of 3 cysteine residues in the BST-2 ectodomain. Interestingly, BST-2 dimerization was not essential for inhibition of Lassa and Marburg viruses (Sakuma and others 2009b) or for BST-2 cell-surface expression or sensitivity to Vpu but is critical for inhibition of HIV-1 release (Andrew and others 2009; Perez-Caballero and others 2009). Similarly, deletion of the coil–coil domain inhibited BST-2 function without affecting dimerization or cell surface expression (Perez-Caballero and others 2009). The functional role of BST-2 glycosylation on the other hand remains debated. Two studies found that glycosylation of BST-2 was not important for inhibition of HIV-1, Lassa, or Marburg virus release (Andrew and others 2009; Sakuma and others 2009a), whereas a third study found that lack of glycosylation almost completely inactivated BST-2 function presumably because of a trafficking defect (Perez-Caballero and others 2009). The reason for these discrepant observations is not clear. Unlike the first 2 studies, which used untagged or N-terminally tagged protein, respectively, the third study employed a BST-2 variant encoding an internal hemagglutinin (HA)-epitope tag. It is conceivable that the presence of an internal epitope tag in conjunction with the lack of carbohydrate modifications induced a conformational change in the protein, resulting in loss of activity.
BST-2, a Molecular Crosslinker?
How does BST-2 inhibit virus release? Vpu-defective particles produced in BST-2–positive cells accumulate at the cell surface. This has led to a model in which BST-2, by means of its N-terminal TM domain and its C-terminal GPI anchor, tethers otherwise fully detached virions to the producer cell (Neil and others 2008). Such a model is consistent with the observation that Vpu-defective particles can be released by protease treatment (Neil and others 2007, 2008; Kaletsky and others 2009; Miyakawa and others 2009; Fitzpatrick and others 2010) or by physical force (Klimkait and others 1990; Miyagi and others 2009). Although the tethering model is elegant and simple, it awaits formal experimental validation.
One approach to validate the tetherin model was to employ immune-electron microscopy to study the localization of BST-2 on virus-producing cells (Perez-Caballero and others 2009; Fitzpatrick and others 2010; Habermann and others 2010; Hammonds and others 2010). In these studies, tethered virions stained positive for BST-2 in support of a tethering function of BST-2. As noted above, BST-2 is capable of bridging a gap of about 17 nm because of its rod-like structure (Hinz and others 2010). Surprisingly, the measured distance between virus particles and the plasma membrane or among tethered virions was frequently significantly >17 nm (Perez-Caballero and others 2009; Fitzpatrick and others 2010; Hammonds and others 2010). Also, some of the Vpu-defective particles appeared to be connected by a stalk rather than having the appearance of being fully detached virions (Klimkait and others 1990; Habermann and others 2010; Hammonds and others 2010). Although these data do not rule out direct BST-2–mediated tethering of viral and/or cellular membranes located <17 nm apart as originally proposed by Neil and others (2008), tethering of virions across distances >17 nm cannot be explained by such a mechanism. In that case it is possible that virions are not fully detached from the plasma membrane or from each other but remain connected via a membrane stalk. Such a membrane stalk could be lined with multiple BST-2 molecules, which interact via coil–coil interactions, thereby stabilizing the membrane stalk. In support of such a model, several electron microscopic (EM) studies noted short stalks connecting virions to the plasma membrane (Klimkait and others 1990; Habermann and others 2010) or observed long filamentous structures decorated with BST-2 (Hammonds and others 2010). A stalk model would be consistent with the noted sensitivity of tethered virions to physical shearing or protease treatment because such treatments would destabilize BST-2–supported membrane stalks, resulting in the detachment of virions.
Virion Association of BST-2
BST-2 is a cell-surface marker and associates with membrane lipid rafts (Kupzig and others 2003; Masuyama and others 2009), which are also critical for budding of HIV-1 (Ono and Freed 2001). Irrespective of whether BST-2 directly tethers virions to the plasma membrane or stabilizes a membrane stalk, both models predict that Vpu-deficient particles released from the cell surface by physical force will contain BST-2. However, we and others previously failed to detect BST-2 in Vpu-deficient virions derived from cells expressing endogenous BST-2 (Miyagi and others 2009; Perez-Caballero and others 2009). On the other hand, nonfunctional BST-2 variants were readily detected in cell-free virus preparations (Perez-Caballero and others 2009; Habermann and others 2010), suggesting that BST-2 is not actively excluded from virions. Finally, wild type (wt) HA-tagged BST-2 expressed in stable 293T cells was also readily identified in virus-like particles when particle production was boosted using a codon-optimized expression vector (Perez-Caballero and others 2009). The authors argue that high levels of Gag expression and modest levels of tetherin expression might dilute the available cell-surface BST-2 among a large number of nascent particles, thereby reducing its potency and perhaps enabling its detection in released virions (Perez-Caballero and others 2009). On the other hand, if surface BST-2 were rate limiting and increased Gag expression could lead to loss of virus tethering, the accumulation of Vpu-deficient budding particles on a cell surface as observed in many EM studies should eventually result in depletion of BST-2, which would in turn lead to gradually increasing release of Vpu-deficient particles. However, this has never been observed. The fact remains that virus-associated BST-2 was thus far only observed under conditions where BST-2 was either defective or otherwise unable to control virus release.
Another interesting observation that came from the use of immune-EM is that BST-2 is present not only in Vpu-deficient virions but in wt virions as well (Fitzpatrick and others 2010; Habermann and others 2010). In fact, the relative density of BST-2 in the membranes of viral particles appeared to be higher than in the adjacent plasma membranes and Vpu had surprisingly little effect on the relative density of BST-2 in virions (Fitzpatrick and others 2010; Habermann and others 2010). This suggests that BST-2 incorporation into HIV-1 may not be directly correlated with Vpu-mediated downregulation from the plasma membrane in HeLa cells (Habermann and others 2010). It should be noted that the particles analyzed by immune-EM are not derived from cell-free virus preparations but are located adjacent to virus-producing cells and may or may not be physically attached to the cells. It is possible that under normal conditions even some wt virions are tethered to the plasma membrane. This would explain why siRNA silencing of BST-2 or functional inactivation of endogenous BST-2 can enhance release of Vpu-deficient as well as wt virus (Strebel, manuscript in preparation).
How Does Vpu Counteract the BST-2–Imposed Restriction of Virus Release?
It is generally accepted that Vpu regulates the detachment of otherwise complete and infectious virions from the cell surface (Klimkait and others 1990; Neil and others 2006). This effect of Vpu is not limited to HIV-1 but was shown to affect other retroviruses such as HIV-2, visna virus, Moloney murine leukemia virus, and xenotropic murine leukemia virus-related virus, as well as unrelated enveloped viruses such as Ebola (Gottlinger and others 1993; Bour and others 1996; Neil and others 2007; Jouvenet and others 2009; Kaletsky and others 2009; Groom and others 2010). A number of models have been proposed over the years to explain how Vpu enhances virus release. These include an ion channel model as well as the inactivation of or interference with other host factors such as Task-1 and UBP (reviewed in Strebel 2007). The identification of BST-2 has, at least temporarily, sidelined earlier models of Vpu function and focused the Vpu field on BST-2.
Vpu and BST-2 are both integral membrane proteins albeit with different membrane topologies. BST-2 has a short N-terminal cytoplasmic domain, with the bulk of the protein comprising the C-terminal ectodomain. Vpu, on the other hand, has virtually no ectodomain and essentially consists of an N-terminal TM domain and a C-terminal cytoplasmic domain (Fig. 1). Recent data suggest that the BST-2 TM domain is critical for interference by Vpu (Douglas and others 2009; Gupta and others 2009a; McNatt and others 2009; Mitchell and others 2009; Perez-Caballero and others 2009; Rong and others 2009), consistent with our previous observation of the importance of the Vpu TM domain for the regulation of virus release (Schubert and others 1996). Indeed, physical interaction of Vpu and BST-2 and the critical importance of the BST-2 TM domain for this interaction was demonstrated in the course of co-immunoprecipitation studies (Douglas and others 2009; Gupta and others 2009a; Iwabu and others 2009; Jia and others 2009; McNatt and others 2009; Rong and others 2009; Dube and others 2010). The exact cellular site at which Vpu targets BST-2 remains unknown. Infection of cells by wt virus results in the redistribution of BST-2 from the plasma membrane to early endosomes (Neil and others 2006; Habermann and others 2010). This involves internalization of BST-2 through clathrin-dependent endocytosis, although there is an ongoing debate whether this involves the AP-2 adapter complex (Rollason and others 2007; Mitchell and others 2009) or a direct interaction with α-adaptin (Masuyama and others 2009). As for Vpu, the protein is predominantly localized to membranes of the Golgi and TGN (Klimkait and others 1990; Schubert and others 1996; Varthakavi and others 2006) and was found to colocalize with BST-2 in endosomes and the TGN (Rollason and others 2007; Neil and others 2008; Van Damme and others 2008; Douglas and others 2009; Dube and others 2009). Further, mutations affecting TGN localization of Vpu were unable to antagonize BST-2, suggesting that Vpu targets BST-2 in this compartment (Dube and others 2009). However, Vpu was also identified at the cell surface (Bour and others 1999) and a direct effect of Vpu on cell-surface BST-2 cannot be ruled out. In fact, 1 recent study proposed that Vpu targets BST-2 at the plasma membrane (Iwabu and others 2009).
It is widely accepted that Vpu downregulates BST-2 from the cell surface and that the reduced surface expression of BST-2 accounts for the increased virus release (Van Damme and others 2008; Douglas and others 2009; Le Tortorec and Neil 2009; Mitchell and others 2009; Miyagi and others 2009; Sato and others 2009; Habermann and others 2010; Pardieu and others 2010; Schindler and others 2010). Interestingly, Vpu does not seem to increase the rate of BST-2 endocytosis (Mitchell and others 2009; Dube and others 2010). This suggests that Vpu may affect resupply or surface delivery of BST-2, a function that could be exerted from an intracellular location such as the trans-Golgi compartment. Vpu was also found to reduce total cellular levels of endogenous as well as exogenously expressed BST-2 (Bartee and others 2006; Douglas and others 2009; Goffinet and others 2009; Mangeat and others 2009; Mitchell and others 2009; Miyagi and others 2009). How this is accomplished remains under debate and it is not clear yet whether the reduced BST-2 levels are a cause or consequence of BST-2 surface downmodulation. Several studies reported the involvement of a proteasomal degradation pathway (Goffinet and others 2009) and suggested β-transducin repeat-containing protein (β-TrCP) dependence (Mangeat and others 2009). In contrast, other studies reported a β-TrCP–dependent endolysosomal pathway to be important for degradation (Douglas and others 2009; Iwabu and others 2009; Mitchell and others 2009). The involvement of β-TrCP in the virus release activity of Vpu necessitates conservation of Vpu's TrCP-binding motif. However, mutation of this motif was previously found to only partially affect Vpu's virus release activity (Schubert and Strebel 1994; Schubert and others 1995; Van Damme and others 2008), and in more recent studies, expression of a TrCP-binding mutant of Vpu (Vpu26) supported HIV replication with wild-type virus kinetics in a variety of cell types including peripheral blood mononuclear cells (PBMC) (Miyagi and others 2009). Expression of the TrCP binding–deficient Vpu26 mutant had no effect on total cellular BST-2 levels and appeared to stabilize or even increase BST-2 surface expression (Miyagi and others 2009). Importantly, degradation of BST-2 was not essential for Vpu to enhance virus release (Miyagi and others 2009; Goffinet and others 2010), suggesting it may be a downstream consequence of the Vpu-induced BST-2 surface downmodulation. Clearly, more work needs to be done to sort out which of the effects of Vpu on BST-2, ie, surface expression, degradation, or intracellular sequestration, are critical for the enhancement of virus release.
Other Viruses Encode Vpu-Like Factors
As noted above, the ability to enhance virus release is not unique to Vpu. In fact, some HIV-2 isolates have been known for many years to encode a Vpu-like activity in their Env glycoproteins (GPs) (Bour and others 1996; Bour and Strebel 1996; Ritter and others 1996; Abada and others 2005; Douglas and others 2009; Le Tortorec and Neil 2009). Interestingly, the Env GP of SIVtan also encodes a Vpu-like activity. In fact, SIVtan Env has broad specificity and can target BST-2 from Tantalus monkeys, rhesus monkeys, sooty mangabeys, and humans, but not from pigs (Gupta and others 2009b). Both HIV-2 and SIVtan Env proteins cause downregulation of BST-2 from the cell surface (Douglas and others 2009; Gupta and others 2009b; Le Tortorec and Neil 2009; Lopez and others 2010). Unlike Vpu, however, these Env proteins do not cause degradation of BST-2 (Douglas and others 2009; Gupta and others 2009b; Le Tortorec and Neil 2009; Lopez and others 2010). Also, Vpu interacts with BST-2 through its TM domain, whereas HIV-2 and SIVtan Env presumably interact with BST-2 through their ectodomains (Fig. 1) (Gupta and others 2009b; Le Tortorec and Neil 2009; Lopez and others 2010). This is consistent with our previous finding that the Vpu-like activity of HIV-2 Env can be controlled by a single amino acid change in its ectodomain (Bour and others 2003).
Intriguingly, a similar Vpu-like activity was now also shown for the Nef protein of several simian immunodeficiency viruses, including SIVmac and SIVagm (Jia and others 2009; Sauter and others 2009; Zhang and others 2009; Yang and others 2010). Like Vpu, SIV Nef functions species specific and does not target human BST-2 but can antagonize rhesus macaque, pig-tail macaque, or African green monkey BST-2 (Jia and others 2009; Sauter and others 2009; Zhang and others 2009; Yang and others 2010). As with HIV-2 Env, SIV Nef induces cell-surface downmodulation without affecting the stability of BST-2 (Douglas and others 2009; Jia and others 2009). Vpu targets the TM domain and HIV-2 Env the ectodomain, whereas Nef targets the cytoplasmic domain of BST-2 (Fig. 1) (Jia and others 2009; Zhang and others 2009; Yang and others 2010). In particular, a G/DDIWK motif that is missing in human BST-2 appears to be critical for Nef antagonism (Jia and others 2009; Zhang and others 2009). Transfer of this motif to human BST-2 rendered the protein sensitive to SIV Nef (Sauter and others 2009; Yang and others 2010), suggesting that this motif is necessary and sufficient for sensitivity to SIV Nef.
Aside from these retroviral Vpu-like factors, the K5 protein of Kaposi sarcoma–associated herpesvirus was shown to reduce levels of endogenous BST-2 in HeLa cells (Bartee and others 2006). In the absence of K5 or when BST-2 is overexpressed, KSHV release is reduced (Mansouri and others 2009; Pardieu and others 2010). K5 targets BST-2 for lysosomal destruction via the MVB pathway (Mansouri and others 2009). K5-mediated reduction of BST-2 levels involves ubiquitination of lysine 18 in the BST-2 cytoplasmic domain followed by endosomal degradation (Mansouri and others 2009; Pardieu and others 2010). The ability of K5 to replace Vpu in HIV-1 virus release is dependent on lysine 18 (Pardieu and others 2010). Vpu also induces ubiquitination of lysines in the BST-2 cytoplasmic tail. However, mutation of these residues does not affect the ability of Vpu to downregulate BST-2 (Pardieu and others 2010). This suggests that K5 functions mechanistically different from Vpu, Nef, or Env.
Finally, the Ebola GP also inhibits virus release (Neil and others 2007; Kaletsky and others 2009). Functionally, the Ebola GP has much in common with the other BST-2 antagonists already discussed. For instance, Ebola GP physically interacts with BST-2 but, like HIV-2 and SIVtan Env, does not affect steady-state levels of BST-2 (Kaletsky and others 2009). Unlike Vpu, Ebola GP is broadly active and able to overcome the inhibitory effects of multiple tetherins, including an artificial tetherin molecule (Lopez and others 2010). Interestingly, Ebola GP can overcome BST-2 restriction without significant effect on cell surface expression (Lopez and others 2010). This is reminiscent of our own finding that efficient HIV-1 release from BST-2–expressing cells is Vpu dependent but can occur in the absence of measurable BST-2 cell-surface downmodulation (Miyagi and others 2009). Therefore, it remains to be shown whether Vpu, Env, Nef, K5, and GP employ a common strategy to antagonize BST-2 or whether these proteins evolved independent modes of action.
Is BST-2 a Viral Restriction Factor?
A final point concerns the question of whether or not BST-2 deserves the label of viral restriction factor. It is true that BST-2 can potently inhibit the release of viral particles, especially if the protein is overexpressed. Also, the fact that multiple viral proteins such as HIV-1 Vpu, HIV-2 Env, SIV Nef, or KSHV K5 have evolved to target BST-2 could suggest that this protein presents a problem that these viruses need to control. On the other hand, one could argue that BST-2 does not so much represent a threat to virus spread as it offers an opportunity for the virus to control its mode of transmission. There are several arguments to support such a view. First of all, BST-2 does not actually restrict replication of Vpu-deficient HIV-1 but simply shifts virus spread from a cell-free to a cell-to-cell mode (Schubert and others 1995) (Fig. 2). Indeed, Vpu-deficient HIV-1 replicates in tissue culture with the same kinetics as wild-type virus except that the levels of cell-free virus are lower (Strebel and others 1988; Terwilliger and others 1989; Klimkait and others 1990; Friborg and others 1995). It is also noteworthy that the effect of Vpu on virus replication in human PBMC is quite modest, consistent with the low BST-2 levels in these cells (Schubert and others 1995; Miyagi and others 2009; Schindler and others 2010). The effect of BST-2 on virus secretion from monocyte-derived macrophages is more pronounced (Schubert and others 1995; Schindler and others 2010). However, even in these cultures, Vpu-deficient HIV-1 was able to spread via cell–cell mode (Schubert and others 1995). Another reason to believe that viruses can use BST-2 to their advantage is that some HIV-1 isolates, including the ADA clone AD8 (Theodore and others 1996) or the LAV Mal (GenBank accession No. A07116) and Yu2 isolates (GenBank accession No. HIVYU2X), carry a point mutation in the vpu initiation codon that disables expression of Vpu. Vpu expression in these isolates can easily be restored by a single-nucleotide change. Similarly, the HIV-2 Env protein has the ability to turn its Vpu-like activity on or off through a single point mutation in the TM subunit (Bour and others 2003; Abada and others 2005). This allows the virus to adapt to changes in the host milieu by switching between cell-free and cell-to-cell modes of transmission. Based on current knowledge we argue that BST-2 is not a viral restriction factor in the sense that it limits virus spread. Rather, BST-2 is a modulator that affects the mode of viral transmission and may, in fact, help the virus avoid the host's immune system.
FIG. 2.
Cell-free versus cell-to-cell transmission of HIV-1 is modulated by BST-2. In the top half of the figure, the unrestricted transmission of virions by cell-free virions is shown. This situation applies to viruses expressing Vpu and/or viruses replicating in BST-2–negative cells. In the absence of Vpu, virions produced from BST-2–expressing cells remain tethered to the surface of the donor cell (bottom half of the figure). These particles are fusion competent and fully infectious (Klimkait and others 1990) and can fuse with an adjacent target cell to establish a cell-to-cell virus transmission.
Acknowledgments
This work was supported in part by a grant from the NIH Intramural AIDS Targeted Antiviral Program (to K.S.) and by the Intramural Research Program of the NIH, NIAID.
Author Disclosure Statement
No competing financial interests exist.
References
- Abada P. Noble B. Cannon PM. Functional domains within the human immunodeficiency virus type 2 envelope protein required to enhance virus production. J Virol. 2005;79(6):3627–3638. doi: 10.1128/JVI.79.6.3627-3638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew AJ. Miyagi E. Kao S. Strebel K. The formation of cysteine-linked dimers of BST-2/tetherin is important for inhibition of HIV-1 virus release but not for sensitivity to Vpu. Retrovirology. 2009;6(1):80. doi: 10.1186/1742-4690-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud F. Black SG. Murphy L. Griffiths DJ. Neil SJ. Spencer TE. Palmarini M. Interplay between ovine bone marrow stromal cell antigen 2/tetherin and endogenous retroviruses. J Virol. 2010;84(9):4415–4425. doi: 10.1128/JVI.00029-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaoka K. Ikeda K. Hishinuma T. Horie-Inoue K. Takeda S. Inoue S. A retrovirus restriction factor TRIM5alpha is transcriptionally regulated by interferons. Biochem Biophys Res Commun. 2005;338(4):1950–1956. doi: 10.1016/j.bbrc.2005.10.173. [DOI] [PubMed] [Google Scholar]
- Bartee E. McCormack A. Fruh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathog. 2006;2(10):e107. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beignon AS. McKenna K. Skoberne M. Manches O. DaSilva I. Kavanagh DG. Larsson M. Gorelick RJ. Lifson JD. Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115(11):3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour S. Akari H. Miyagi E. Strebel K. Naturally occurring amino acid substitutions in the HIV-2 ROD envelope glycoprotein regulate its ability to augment viral particle release. Virology. 2003;309(1):85–98. doi: 10.1016/s0042-6822(02)00128-9. [DOI] [PubMed] [Google Scholar]
- Bour S. Perrin C. Strebel K. Cell surface CD4 inhibits HIV-1 particle release by interfering with Vpu activity. J Biol Chem. 1999;274(47):33800–33806. doi: 10.1074/jbc.274.47.33800. [DOI] [PubMed] [Google Scholar]
- Bour S. Schubert U. Peden K. Strebel K. The envelope glycoprotein of human immunodeficiency virus type 2 enhances viral particle release: a Vpu-like factor? J Virol. 1996;70(2):820–829. doi: 10.1128/jvi.70.2.820-829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour S. Strebel K. The human immunodeficiency virus (HIV) type 2 envelope protein is a functional complement to HIV type 1 Vpu that enhances particle release of heterologous retroviruses. J Virol. 1996;70(12):8285–8300. doi: 10.1128/jvi.70.12.8285-8300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D. Cao J. Li Z. Zheng X. Yao Y. Li W. Yuan Z. Up-regulation of bone marrow stromal protein 2 (BST2) in breast cancer with bone metastasis. BMC Cancer. 2009;9:102. doi: 10.1186/1471-2407-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W. Bover L. Signaling and ligand interaction of ILT7: receptor-mediated regulatory mechanisms for plasmacytoid dendritic cells. Immunol Rev. 2010;234(1):163–176. doi: 10.1111/j.0105-2896.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W. Bover L. Cho M. Wen X. Hanabuchi S. Bao M. Rosen DB. Wang YH. Shaw JL. Du Q. Li C. Arai N. Yao Z. Lanier LL. Liu YJ. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206(7):1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JL. Viswanathan K. McCarroll MN. Gustin JK. Fruh K. Moses AV. Vpu directs the degradation of the HIV restriction factor BST-2/tetherin via a {beta}TrCP-dependent mechanism. J Virol. 2009;83(16):7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M. Roy BB. Guiot-Guillain P. Binette J. Mercier J. Chiasson A. Cohen EA. Antagonism of tetherin restriction of HIV-1 release by Vpu involves binding and sequestration of the restriction factor in a perinuclear compartment. PLoS Pathog. 2010;6(4):e1000856. doi: 10.1371/journal.ppat.1000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube M. Roy BB. Guiot-Guillain P. Mercier J. Binette J. Leung G. Cohen EA. Suppression of Tetherin-restricting activity upon human immunodeficiency virus type 1 particle release correlates with localization of Vpu in the trans-Golgi network. J Virol. 2009;83(9):4574–4590. doi: 10.1128/JVI.01800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbas JJ. Toso JF. Logar AJ. Navratil JS. Rinaldo CR., Jr. CD4+ blood dendritic cells are potent producers of IFN-alpha in response to in vitro HIV-1 infection. J Immunol. 1994;152(9):4649–4662. [PubMed] [Google Scholar]
- Fitzpatrick K. Skasko M. Deerinck TJ. Crum J. Ellisman MH. Guatelli J. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 2010;6(3):e1000701. doi: 10.1371/journal.ppat.1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friborg J. Ladha A. Gottlinger H. Haseltine WA. Cohen EA. Functional analysis of the phosphorylation sites on the human immunodeficiency virus type 1 Vpu protein. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(1):10–22. [PubMed] [Google Scholar]
- Goffinet C. Allespach I. Homann S. Tervo HM. Habermann A. Rupp D. Oberbremer L. Kern C. Tibroni N. Welsch S. Krijnse-Locker J. Banting G. Krausslich HG. Fackler OT. Keppler OT. HIV-1 antagonism of CD317 is species specific and involves Vpu-mediated proteasomal degradation of the restriction factor. Cell Host Microbe. 2009;5(3):285–297. doi: 10.1016/j.chom.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Goffinet C. Homann S. Ambiel I. Tibroni N. Rupp D. Keppler OT. Fackler OT. Antagonism of CD317 restriction of HIV-1 particle release and depletion of CD317 are separable activities of HIV-1 Vpu. J Virol. 2010;84(8):4089–4094. doi: 10.1128/JVI.01549-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T. Kennel SJ. Abe M. Takishita M. Kosaka M. Solomon A. Saito S. A novel membrane antigen selectively expressed on terminally differentiated human B cells. Blood. 1994;84(6):1922–1930. [PubMed] [Google Scholar]
- Gottlinger HG. Dorfman T. Cohen EA. Haseltine WA. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc Natl Acad Sci USA. 1993;90(15):7381–7385. doi: 10.1073/pnas.90.15.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH. Wawer MJ. Brookmeyer R. Sewankambo NK. Serwadda D. Wabwire-Mangen F. Lutalo T. Li X. vanCott T. Quinn TC. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- Groom HC. Yap MW. Galao RP. Neil SJ. Bishop KN. Susceptibility of xenotropic murine leukemia virus-related virus (XMRV) to retroviral restriction factors. Proc Natl Acad Sci USA. 2010;107(11):5166–5171. doi: 10.1073/pnas.0913650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK. Hue S. Schaller T. Verschoor E. Pillay D. Towers GJ. Mutation of a single residue renders human tetherin resistant to HIV-1 Vpu-mediated depletion. PLoS Pathog. 2009a;5(5):e1000443. doi: 10.1371/journal.ppat.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RK. Mlcochova P. Pelchen-Matthews A. Petit SJ. Mattiuzzo G. Pillay D. Takeuchi Y. Marsh M. Towers GJ. Simian immunodeficiency virus envelope glycoprotein counteracts tetherin/BST-2/CD317 by intracellular sequestration. Proc Natl Acad Sci USA. 2009b;106(49):20889–20894. doi: 10.1073/pnas.0907075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann A. Krijnse-Locker J. Oberwinkler H. Eckhardt M. Homann S. Andrew A. Strebel K. Krausslich HG. CD317/tetherin is enriched in the HIV-1 envelope and downregulated from the plasma membrane upon virus infection. J Virol. 2010;84(9):4646–4658. doi: 10.1128/JVI.02421-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammonds J. Wang JJ. Yi H. Spearman P. Immunoelectron microscopic evidence for tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 2010;6(2):e1000749. doi: 10.1371/journal.ppat.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz A. Miguet N. Natrajan G. Usami Y. Yamanaka H. Renesto P. Hartlieb B. McCarthy AA. Simorre JP. Gottlinger H. Weissenhorn W. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell Host Microbe. 2010;7:314–323. doi: 10.1016/j.chom.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa J. Kaisho T. Tomizawa H. Lee BO. Kobune Y. Inazawa J. Oritani K. Itoh M. Ochi T. Ishihara K. Molecular cloning and chromosomal mapping of a bone marrow stromal cell surface gene, BST2, that may be involved in pre-B-cell growth. Genomics. 1995;26(3):527–534. doi: 10.1016/0888-7543(95)80171-h. and others. [DOI] [PubMed] [Google Scholar]
- Iwabu Y. Fujita H. Kinomoto M. Kaneko K. Ishizaka Y. Tanaka Y. Sata T. Tokunaga K. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J Biol Chem. 2009;284(50):35060–35072. doi: 10.1074/jbc.M109.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B. Serra-Moreno R. Neidermyer W. Rahmberg A. Mackey J. Fofana IB. Johnson WE. Westmoreland S. Evans DT. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvenet N. Neil SJ. Zhadina M. Zang T. Kratovac Z. Lee Y. McNatt M. Hatziioannou T. Bieniasz PD. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83(4):1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletsky RL. Francica JR. Agrawal-Gamse C. Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc Natl Acad Sci USA. 2009;106(8):2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S. Azuma Y. Fujii E. Furugaki K. Ozaki S. Matsumoto T. Kosaka M. Yamada-Okabe H. Interferon-alpha enhances CD317 expression and the antitumor activity of anti-CD317 monoclonal antibody in renal cell carcinoma xenograft models. Cancer Sci. 2008;99(12):2461–2466. doi: 10.1111/j.1349-7006.2008.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimkait T. Strebel K. Hoggan MD. Martin MA. Orenstein JM. The human immunodeficiency virus type 1-specific protein vpu is required for efficient virus maturation and release. J Virol. 1990;64(2):621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S. Korolchuk V. Rollason R. Sugden A. Wilde A. Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4(10):694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- Le Tortorec A. Neil SJ. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J Virol. 2009;83(22):11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LA. Yang SJ. Hauser H. Exline CM. Haworth KG. Oldenburg J. Cannon PM. Ebola glycoprotein counteracts BST-2/tetherin restriction in a sequence independent manner that does not require tetherin surface removal. J Virol. 2010;84(14):7243–7255. doi: 10.1128/JVI.02636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B. Gers-Huber G. Lehmann M. Zufferey M. Luban J. Piguet V. HIV-1 Vpu neutralizes the antiviral factor tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 2009;5(9):e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M. Viswanathan K. Douglas JL. Hines J. Gustin J. Moses AV. Fruh K. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J Virol. 2009;83(19):9672–9681. doi: 10.1128/JVI.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama N. Kuronita T. Tanaka R. Muto T. Hirota Y. Takigawa A. Fujita H. Aso Y. Amano J. Tanaka Y. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with {alpha}-adaptin. J Biol Chem. 2009;284(23):15927–15941. doi: 10.1074/jbc.M109.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzo G. Ivol S. Takeuchi Y. Regulation of porcine endogenous retrovirus release by porcine and human tetherins. J Virol. 2010;84(5):2618–2622. doi: 10.1128/JVI.01928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatt MW. Zang T. Hatziioannou T. Bartlett M. Fofana IB. Johnson WE. Neil SJ. Bieniasz PD. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog. 2009;5(2):e1000300. doi: 10.1371/journal.ppat.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RS. Katsura C. Skasko MA. Fitzpatrick K. Lau D. Ruiz A. Stephens EB. Margottin-Goguet F. Benarous R. Guatelli JC. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009;5(5):e1000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagi E. Andrew AJ. Kao S. Strebel K. Vpu enhances HIV-1 virus release in the absence of Bst-2 cell surface down-modulation and intracellular depletion. Proc Natl Acad Sci USA. 2009;106(8):2868–2873. doi: 10.1073/pnas.0813223106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa K. Ryo A. Murakami T. Ohba K. Yamaoka S. Fukuda M. Guatelli J. Yamamoto N. BCA2/Rabring7 promotes tetherin-dependent HIV-1 restriction. PLoS Pathog. 2009;5(12):e1000700. doi: 10.1371/journal.ppat.1000700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ. Eastman SW. Jouvenet N. Bieniasz PD. HIV-1 Vpu promotes release and prevents endocytosis of nascent retrovirus particles from the plasma membrane. PLoS Pathog. 2006;2(5):e39. doi: 10.1371/journal.ppat.0020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ. Sandrin V. Sundquist WI. Bieniasz PD. An interferon-alpha-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2(3):193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ. Zang T. Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Ohtomo T. Sugamata Y. Ozaki Y. Ono K. Yoshimura Y. Kawai S. Koishihara Y. Ozaki S. Kosaka M. Hirano T. Tsuchiya M. Molecular cloning and characterization of a surface antigen preferentially overexpressed on multiple myeloma cells. Biochem Biophys Res Commun. 1999;258(3):583–591. doi: 10.1006/bbrc.1999.0683. [DOI] [PubMed] [Google Scholar]
- Ono A. Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci USA. 2001;98(24):13925–13930. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardieu C. Vigan R. Wilson SJ. Calvi A. Zang T. Bieniasz P. Kellam P. Towers GJ. Neil SJ. The RING-CH Ligase K5 antagonizes restriction of KSHV and HIV-1 particle release by mediating ubiquitin-dependent endosomal degradation of tetherin. PLoS Pathog. 2010;6(4):e1000843. doi: 10.1371/journal.ppat.1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Caballero D. Zang T. Ebrahimi A. McNatt MW. Gregory DA. Johnson MC. Bieniasz PD. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139(3):499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall RE. Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- Ritter GD. Yamshchikov G. Cohen SJ. Mulligan MJ. Human immunodeficiency virus type 2 glycoprotein enhancement of particle budding: role of the cytoplasmic domain. J. Virol. 1996;70:2669–2673. doi: 10.1128/jvi.70.4.2669-2673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollason R. Korolchuk V. Hamilton C. Schu P. Banting G. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J Cell Sci. 2007;120(Pt 21):3850–3858. doi: 10.1242/jcs.003343. [DOI] [PubMed] [Google Scholar]
- Rong L. Zhang J. Lu J. Pan Q. Lorgeoux RP. Aloysius C. Guo F. Liu SL. Wainberg MA. Liang C. The transmembrane domain of BST-2 determines its sensitivity to down-modulation by HIV-1 Vpu. J Virol. 2009;83(15):7536–7546. doi: 10.1128/JVI.00620-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KM. Marin M. Kozak SL. Kabat D. Transcriptional regulation of APOBEC3G, a cytidine deaminase that hypermutates human immunodeficiency virus. J Biol Chem. 2004;279(40):41744–41749. doi: 10.1074/jbc.M406760200. [DOI] [PubMed] [Google Scholar]
- Sakuma T. Noda T. Urata S. Kawaoka Y. Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J Virol. 2009a;83(5):2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma T. Sakurai A. Yasuda J. Dimerization of tetherin is not essential for its antiviral activity against Lassa and Marburg viruses. PLoS One. 2009b;4(9):e6934. doi: 10.1371/journal.pone.0006934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. Yamamoto SP. Misawa N. Yoshida T. Miyazawa T. Koyanagi Y. Comparative study on the effect of human BST-2/tetherin on HIV-1 release in cells of various species. Retrovirology. 2009;6(1):53. doi: 10.1186/1742-4690-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D. Schindler M. Specht A. Landford WN. Munch J. Kim KA. Votteler J. Schubert U. Bibollet-Ruche F. Keele BF. Takehisa J. Ogando Y. Ochsenbauer C. Kappes JC. Ayouba A. Peeters M. Learn GH. Shaw G. Sharp PM. Bieniasz P. Hahn BH. Hatziioannou T. Kirchhoff F. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6(5):409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M. Rajan D. Banning C. Wimmer P. Koppensteiner H. Iwanski A. Specht A. Sauter D. Dobner T. Kirchhoff F. Vpu serine 52 dependent counteraction of tetherin is required for HIV-1 replication in macrophages, but not in ex vivo human lymphoid tissue. Retrovirology. 2010;7(1):1. doi: 10.1186/1742-4690-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U. Bour S. Ferrer-Montiel AV. Montal M. Maldarell F. Strebel K. The two biological activities of human immunodeficiency virus type 1 Vpu protein involve two separable structural domains. J Virol. 1996;70(2):809–819. doi: 10.1128/jvi.70.2.809-819.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U. Clouse KA. Strebel K. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J Virol. 1995;69(12):7699–7711. doi: 10.1128/jvi.69.12.7699-7711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert U. Strebel K. Differential activities of the human immunodeficiency virus type 1-encoded Vpu protein are regulated by phosphorylation and occur in different cellular compartments. J Virol. 1994;68(4):2260–2271. doi: 10.1128/jvi.68.4.2260-2271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K. HIV Accessory genes Vif and Vpu. Adv Pharmacol. 2007;55:199–232. doi: 10.1016/S1054-3589(07)55006-4. [DOI] [PubMed] [Google Scholar]
- Strebel K. Klimkait T. Martin MA. A novel gene of HIV-1, vpu, and its 16-kilodalton product. Science. 1988;241(4870):1221–1223. doi: 10.1126/science.3261888. [DOI] [PubMed] [Google Scholar]
- Strebel K. Luban J. Jeang KT. Human cellular restriction factors that target HIV-1 replication. BMC Med. 2009;7(1):48. doi: 10.1186/1741-7015-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger EF. Cohen EA. Lu YC. Sodroski JG. Haseltine WA. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci USA. 1989;86(13):5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore TS. Englund G. Buckler-White A. Buckler CE. Martin MA. Peden KW. Construction and characterization of a stable full-length macrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res Hum Retroviruses. 1996;12(3):191–194. doi: 10.1089/aid.1996.12.191. [DOI] [PubMed] [Google Scholar]
- Van Damme N. Goff D. Katsura C. Jorgenson RL. Mitchell R. Johnson MC. Stephens EB. Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3(4):245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varthakavi V. Smith RM. Bour SP. Strebel K. Spearman P. Viral protein U counteracts a human host cell restriction that inhibits HIV-1 particle production. Proc Natl Acad Sci USA. 2003;100(25):15154–15159. doi: 10.1073/pnas.2433165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varthakavi V. Smith RM. Martin KL. Derdowski A. Lapierre LA. Goldenring JR. Spearman P. The pericentriolar recycling endosome plays a key role in Vpu-mediated enhancement of HIV-1 particle release. Traffic. 2006;7(3):298–307. doi: 10.1111/j.1600-0854.2005.00380.x. [DOI] [PubMed] [Google Scholar]
- von Sydow M. Sonnerborg A. Gaines H. Strannegard O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7(4):375–380. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]
- Walter-Yohrling J. Cao X. Callahan M. Weber W. Morgenbesser S. Madden SL. Wang C. Teicher BA. Identification of genes expressed in malignant cells that promote invasion. Cancer Res. 2003;63(24):8939–8947. [PubMed] [Google Scholar]
- Wang W. Nishioka Y. Ozaki S. Jalili A. Abe S. Kakiuchi S. Kishuku M. Minakuchi K. Matsumoto T. Sone S. HM1.24 (CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody. Cancer Immunol Immunother. 2009a;58(6):967–976. doi: 10.1007/s00262-008-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Nishioka Y. Ozaki S. Jalili A. Verma VK. Hanibuchi M. Abe S. Minakuchi K. Matsumoto T. Sone S. Chimeric and humanized anti-HM1.24 antibodies mediate antibody-dependent cellular cytotoxicity against lung cancer cells. Lung Cancer. 2009b;63(1):23–31. doi: 10.1016/j.lungcan.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Yang SJ. Lopez LA. Hauser H. Exline CM. Haworth KG. Cannon PM. Anti-tetherin activities in Vpu-expressing primate lentiviruses. Retrovirology. 2010;7:13. doi: 10.1186/1742-4690-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. Wilson SJ. Landford WC. Virgen B. Gregory D. Johnson MC. Munch J. Kirchhoff F. Bieniasz PD. Hatziioannou T. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6(1):54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]