Abstract
Background:
Acute pulmonary embolism (PE) may be rapidly fatal if not diagnosed and treated. IV heparin reduces mortality and recurrence of PE, but the relationship between survival and timing of anticoagulation has not been extensively studied.
Methods:
We studied 400 consecutive patients in the ED diagnosed with acute PE by CT scan angiography and treated in the hospital with IV unfractionated heparin from 2002 to 2005. Patients received heparin either in the ED or after admission. Time from ED arrival to therapeutic activated partial thromboplastin time (aPTT) was calculated. Outcomes included in-hospital and 30-day mortality, hospital and ICU lengths of stay, hemorrhagic events on heparin, and recurrent venous thromboembolism within 90 days.
Results:
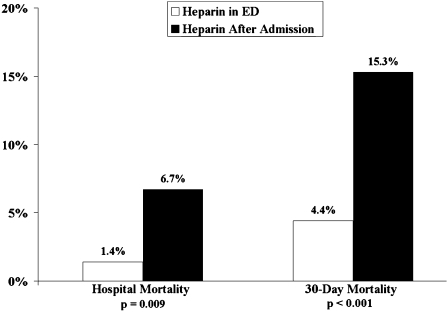
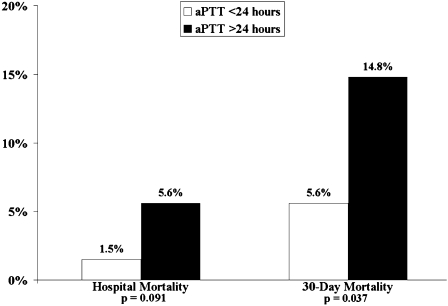
In-hospital and 30-day mortality rates were 3.0% and 7.7%, respectively. Patients who received heparin in the ED had lower in-hospital (1.4% vs 6.7%; P = .009) and 30-day (4.4% vs 15.3%; P < .001) mortality rates as compared with patients given heparin after admission. Patients who achieved a therapeutic aPTT within 24 h had lower in-hospital (1.5% vs 5.6%; P = .093) and 30-day (5.6% vs 14.8%; P = .037) mortality rates as compared with patients who achieved a therapeutic aPTT after 24 h. In multiple logistic regression models, receiving heparin in the ED remained predictive of reduced mortality, and ICU admission remained predictive of increased mortality.
Conclusions:
We report an association between early anticoagulation and reduced mortality for patients with acute PE. We advocate further study with regard to comorbidities to assess the usefulness of modifications to hospital protocols.
Acute pulmonary embolism (PE) is a common cause of death, accounting for 50,000 to 200,000 deaths annually.1‐3 As many as 95% of patients who die do so prior to diagnosis, with the majority of deaths occurring in untreated patients.3‐8 For patients who receive treatment, the 14- and 90-day mortality rates are approximately 10% and 20%, respectively.5,9‐11
Barritt and Jordan12 demonstrated that IV heparin improved overall survival for patients with PE. Others have confirmed these findings and have shown that therapeutic anticoagulation reduces rates of recurrent venous thromboembolism (VTE), which is believed to be a significant factor for mortality in patients with PE.9,13,14 Indeed, Raschke et al14 validated the weight-based heparin nomogram by demonstrating that it reduced recurrent VTE. Hull et al15 found that achieving a therapeutic activated partial thromboplastin time (aPTT) within 24 h reduced the rates of VTE following acute deep vein thrombosis (DVT). Kline et al16 reported that patients diagnosed with PE in the ED had improved outcomes as compared with those diagnosed after admission, but their study did not consider the timing of anticoagulation.
Guidelines recommend initiation of anticoagulation if clinical suspicion for PE is high, even prior to confirmatory testing.3,17,18 Data are limited, however, that examine how the timing of anticoagulation relates to mortality, because early studies did not account for when or how quickly patients were anticoagulated. We examined how the timing of initial heparinization and achieving therapeutic anticoagulation relate to mortality in a cohort of patients who presented to an ED with acute, symptomatic PE.
Materials and Methods
Patient Selection and Characterization
We conducted a retrospective review of a cohort of adult patients who presented to a single tertiary care ED with acute PE between June 17, 2002, and September 6, 2005. All PE diagnoses were confirmed by CT scan angiography. Patients were excluded if diagnosis was prior to arrival or if anticoagulation was contraindicated. All patients were initially treated with an IV weight-based heparin nomogram similar to the one described by Raschke et al14 as per our institutional practice. This study was approved by the Mayo Clinic Institutional Review Board.
Definitions and Data Collection
Acute PE was defined as a filling defect seen on CT scan angiography in patients presenting to the ED with symptoms consistent with pulmonary artery hypoperfusion or infarction (ie, dyspnea, chest pain, lightheadedness, and/or syncope). Patients were excluded if they had an asymptomatic PE found incidentally on a CT scan done for reasons other than the above symptoms.
Patients were characterized as having received heparin either in the ED or after admission. We used 24 h as a discriminating timeframe for achieving a therapeutic aPTT because this has been a validated clinical timeframe in previous studies.14,15 Primary outcomes were in-hospital and 30-day all-cause mortality. Secondary outcomes were hospital and ICU lengths-of-stay, hemorrhagic events on heparin, and recurrent VTE within 90 days.
The medical record was reviewed to determine length of follow-up and time to death. Hospital length-of-stay was determined by the earliest time documented upon ED arrival and the time when the hospital summary was signed on the discharge day. The time of heparinization was determined by review of documentation from ED or admitting physicians and the drug administration records as available. Laboratory draw times were reviewed to determine the interval between arrival and the first therapeutic aPTT, which was defined as 1.5 times the baseline aPTT or at least 50 s. A hemorrhagic event on IV heparin was defined as acute blood loss of at least 2 g of hemoglobin that required cessation of heparin and transfusion. Recurrent VTE was defined as a documented DVT or PE within 90 days of initial presentation.
Demographic data, presenting vital signs, and laboratory values were cataloged, and a Wells score for PE was calculated for each patient in the standard fashion.19,20 In order to calculate the Wells score, we reviewed documentation to determine if a diagnosis other than PE was considered more or less likely. Comorbidities considered were history of VTE or coagulopathy, active malignancy, COPD, coronary artery disease (CAD), congestive heart failure (CHF), or active nicotine use. Tachycardia was defined as a heart rate > 100 beats per minute, hypotension as a systolic BP (SBP) < 100 mm Hg, leukocytosis as a WBC count > 10 × 109 cells/mL, a positive troponin T as > 0.01 ng/mL, and a positive d-dimer as > 500 ng/mL.
Data Analysis
Median values are reported with interquartile ranges (IQR) because data were not normally distributed. Fisher exact and Student t tests were used to compare the frequency of baseline demographics and comorbidities. Odds ratios (OR) were calculated for categorical variables based on χ2 analyses, and OR for a one-unit change in continuous variables were estimated using logistic regression. Ninety-five percent CIs are reported. The type 1 error rate was set at 0.05 (two-side) a priori, and no correction factor has been applied to reported P values to account for multiple comparison issues.
Because patients were not randomized, propensity scores for the likelihood of receiving heparin in the ED or achieving a therapeutic aPTT within 24 h were calculated. Propensity scores were calculated in a multiple logistic regression model including the following 17 factors: age, gender, heart rate, SBP nicotine use, CAD, CHF, COPD, oral contraceptive use, coagulation disorder, history of previous VTE, malignancy, hemoptysis, recent surgery or immobility (within 30 days prior to presentation), Wells score, leukocyte count, and whether an alternative diagnosis was less likely. The estimated propensity score was used as a covariate in multiple logistic regression models.
Kaplan-Meier curves were created to compare the times from arrival to therapeutic aPTT for the survivor and nonsurvivor populations. Given the limited number of in-hospital deaths, multiple logistic regression modeling was performed only for 30-day mortality in order to avoid an over-fit in-hospital model. Data were analyzed with JMP 8.0 (2008; SAS Institute Inc.; Cary, NC). Statistical analyses were aided by the Center for Translational Science Activities at our institution.
Results
Search of the Mayo Clinic electronic medical record yielded 400 patients seen in the ED between 2002 and 2005 who met the aforementioned criteria for acute PE (Tables 1, 2). Median age was 68.0 years (IQR, 54.0-76.0), with 48.8% men. Patients were hospitalized for a median 4.6 days (IQR, 2.1-6.9). Seventy-seven patients (19.3%) required ICU admission, and the median ICU length-of-stay was 2.0 days (IQR, 1.0-3.0). The median follow-up time was 1,411.9 days (IQR 294.9-1,777.8), and 392 patients (98.0%) were accounted for by 30 days. Acute PE was the primary cause of death listed on the medical records or, if available, the autopsy reports for all patients who died in the hospital.
Table 1.
—Baseline Characteristics of Patients Based on In-Hospital and 30-d Mortality
| Characteristics | All Patients (N = 400) | Hospital Survivors (n = 388) | Hospital Nonsurvivors (n = 12) | 30-d Survivors (n = 362) | 30-d Nonsurvivors (n = 30) |
| Age, y (IQR) | 68.0 (54.0-76.0) | 68.0 (54.3-76.8) | 72.5 (48.3-75.8) | 68.0 (54.0-76.0) | 73.0 (60.8-79.0) |
| Male sex | 195 (48.8) | 191 (49.2) | 4 (33.3) | 177 (48.9) | 14 (46.7) |
| Heparin in ED | 280 (70.0) | 276 (71.1) | 4 (33.3)a | 262 (72.4) | 12 (40.0)b |
| Hours to aPTT (IQR) | 10.8 (7.7-17.3) | 10.7 (7.7-16.6) | 20.9 (8.4-28.8)b | 10.8 (7.8-16.5) | 16.3 (5.3-28.6)a |
| aPTT within 24 h | 325 (85.8) | 320/371 (86.3) | 5/8 (62.5) | 301/347 (86.7) | 18/26 (69.2)c |
| Temperature, °C (IQR) | 36.8 (36.2-37.3) | 36.8 (36.2-37.3) | 36.5 (35.7-37.3) | 36.9 (36.2-37.3) | 36.6 (36.2-37.0) |
| Heart rate, bpm (IQR) | 92 (77-107) | 92 (77-107) | 102 (90-120) | 91 (77-106) | 102 (84-119) |
| Tachycardia | 152 (38.4) | 144 (37.1) | 8 (66.7)c | 130 (35.9) | 18 (60.0)a |
| SBP, mm Hg (IQR) | 135 (118-155) | 135 (120-156) | 105 (101-119)b | 135 (120-155) | 122 (104-145) |
| Hypotension | 24 (6.9) | 23 (5.9) | 1 (8.3) | 20 (5.5) | 3 (10.0) |
| Respiratory rate (IQR) | 20 (18-24) | 20 (18-24) | 24 (20-30) | 20 (18-24) | 22 (20-28) |
| Wells score (IQR) | 4.5 (2.5-6.0) | 4.5 (2.5-6.0) | 3.5 (1.5-6.8) | 4.5 (2.5-5.6) | 5.5 (2.5-7.0)c |
| Leukocytosis | 171 (42.9) | 161 (41.5) | 10 (83.3)a | 148 (40.9) | 19 (63.3)c |
| d-dimer > 500 ng/mL | 160/192 (83.3) | 153/185 (82.7) | 7/7 (100) | 143/175 (81.7) | 15/15 (100) |
| Troponin > 0.01 ng/mL | 87/312 (27.9) | 81/301 (26.9) | 6/11 (54.6) | 75/284 (26.4) | 11/23 (47.8)c |
| Active nicotine use | 40 (10.0) | 40 (10.3) | 0 (0) | 36 (9.9) | 3 (10.0) |
| CAD | 81 (20.3) | 80 (20.6) | 1 (8.3) | 78 (21.6) | 3 (10.0) |
| CHF | 36 (9.0) | 34 (8.8) | 2 (16.7) | 31 (8.6) | 5 (16.7) |
| CAD or CHF | 101 (25.3) | 98 (25.3) | 3 (25.0) | 94 (26.0) | 7 (23.3) |
| COPD | 42 (10.5) | 38 (9.8)c | 4 (33.3)c | 36 (9.9)c | 6 (20.0) |
| Malignancy | 129 (32.3) | 124 (32.0) | 5 (41.7) | 110 (30.4) | 16 (53.3)c |
| History of VTE | 71 (17.8) | 69 (17.8) | 2 (16.7) | 64 (17.7) | 5 (16.7) |
| Current DVT | 62/214 (29.0) | 61/211 (28.9) | 1/3 (33.3) | 55/199 (27.6) | 6/12 (50.0) |
| Immobile/recent surgery | 169 (42.3) | 162 (41.8) | 7 (58.3) | 146 (40.3) | 19 (63.3)c |
| Coagulation disorder | 22 (5.5) | 22 (5.7) | 0 (0) | 22 (6.1) | 0 (0) |
| Oral contraceptive use | 23 (5.7) | 22 (5.7) | 1 (8.3) | 22 (6.1) | 1 (3.3) |
| Hospital days (IQR) | 4.6 (2.1-6.9) | 4.6 (2.1-7.0) | 5.4 (1.4-6.6) | 4.4 (2.1-6.9) | 5.7 (3.5-7.9) |
| ICU admission | 77 (19.3) | 69 (17.8) | 8 (66.7)b | 63 (17.4) | 12 (40.0)a |
| ICU days (IQR) | 2.0 (1.0-3.0) | 2.0 (1.0-3.0) | 1.5 (1.0-4.5) | 2.0 (1.0-3.0) | 1.5 (1.0-4.5) |
| Hemorrhagic events | 21 (5.3) | 18 (4.6) | 3 (25.0) | 16 (4.4)c | 4 (13.3) |
| Intubation | 11 (2.8) | 9 (2.3) | 2 (16.7)c | 9 (2.5) | 2 (6.7) |
| Recurrent VTE in 90 d | 6/391 (1.5) | 6/379 (1.6) | 0/12 (0.0) | 6/357 (1.7) | 0 (0) |
Data are presented as No. (%) unless otherwise indicated. aPTT = activated partial thromboplastin time; bpm = beats per minute; CAD = coronary artery disease; CHF = congestive heart failure; DVT = deep vein thrombosis; IQR = interquartile range; SBP = systolic blood pressure; VTE = venous thromboembolism.
P < .01.
P < .001.
P < .05.
Table 2.
—Odds Ratios for In-Hospital and 30-d Mortality Based on Baseline Characteristics
| Hospital Mortality |
30-d Mortality |
|||||
| Characteristics | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Age, y | 1.00 | 0.97-1.04 | .422 | 0.28 | 0.04-1.65 | .910 |
| Male sex | 0.52 | 0.15-1.74 | .382 | 0.91 | 0.43-1.93 | .851 |
| Heparin in ED | 0.20 | 0.06-0.69 | .009 | 0.25 | 0.12-0.55 | <.001 |
| aPTT within 24 h | 0.27 | 0.06-1.15 | .091 | 0.34 | 0.14-0.84 | .037 |
| Temperature, °C | 1.50 | 0.56-3.70 | .203 | 1.29 | 0.73-2.25 | .183 |
| Tachycardia | 4.46 | 1.17-17.09 | .025 | 2.88 | 1.32-6.29 | .009 |
| Hypotension | 1.72 | 0.21-14.33 | .478 | 2.13 | 0.58-7.75 | .212 |
| Respiratory rate | 0.95 | 0.88-1.07 | .837 | 0.96 | 0.91-1.03 | .873 |
| Wells score | 0.84 | 0.06-12.25 | .553 | 0.17 | 0.03-0.96 | .044 |
| Leukocytosis | 7.02 | 1.52-32.46 | .006 | 2.49 | 1.15-5.38 | .021 |
| d-dimer > 500 ng/mL | 3.18 | 0.18-57.01 | .603 | 7.02 | 0.41-120.38 | .079 |
| Troponin > 0.01 ng/mL | 3.26 | 0.97-10.97 | .079 | 2.55 | 1.08-6.03 | .029 |
| Active nicotine use | 0.25 | 0.01-4.23 | .619 | 1.01 | 0.29-3.48 | 1.000 |
| CAD | 0.35 | 0.04-2.75 | .473 | 0.40 | 0.12-1.37 | .163 |
| CHF | 2.08 | 0.44-9.89 | .295 | 2.14 | 0.76-5.97 | .177 |
| CAD or CHF | 0.99 | 0.26-3.72 | 1.000 | 0.87 | 0.36-2.09 | .832 |
| COPD | 4.61 | 1.32-16.01 | .028 | 2.26 | 0.87-5.90 | .116 |
| Malignancy | 1.52 | 0.47-4.89 | .535 | 2.62 | 1.23-5.55 | .010 |
| History of VTE | 0.92 | 0.20-4.31 | 1.000 | 0.93 | 0.34-2.52 | 1.000 |
| Current DVT | 1.23 | 0.11-13.81 | 1.000 | 2.62 | 0.81-8.46 | .110 |
| Immobile/recent surgery | 1.95 | 0.61-6.26 | .374 | 2.56 | 1.12-5.53 | .012 |
| Alternative diagnosis | 0.56 | 0.18-1.78 | .367 | 0.86 | 0.40-1.84 | .698 |
| Coagulation disorder | 0.65 | 0.04-11.36 | 1.000 | 0.25 | 0.01-4.19 | .397 |
| Oral contraceptive use | 1.51 | 0.19-12.25 | .514 | 0.53 | 0.07-4.10 | 1.000 |
| Hospital days | 1.02 | 0.97-1.20 | .390 | 1.00 | 0.97-1.05 | .574 |
| ICU admission | 9.25 | 2.71-31.60 | <.001 | 3.16 | 1.45-6.90 | .006 |
| Hemorrhagic events | 6.85 | 1.71-27.50 | .020 | 3.33 | 1.04-10.68 | .057 |
| Intubation | 8.42 | 1.61-44.11 | .039 | 2.80 | 0.58-13.60 | .203 |
Unit ORs are recorded for continuous variables. OR = odds ratio. See Table 1 for expansion of other abbreviations.
The median time from ED arrival to CT diagnosis was 2.4 h (IQR, 1.4-7.6). Two hundred sixty patients (65.0%) were diagnosed in the ED, and 280 patients (70.0%) received heparin in the ED; thus at least 20 patients (5.0%) were given heparin prior to diagnosis. The median time from ED arrival to therapeutic aPTT was 10.8 h (IQR, 7.7-17.3), and 325 patients (85.8%) had a therapeutic aPTT within 24 h of ED arrival. Twenty-one patients (5.3%) were excluded because they did not achieve a therapeutic aPTT: Four died before they achieved a therapeutic aPTT, and 17 were changed from unfractionated to low-molecular-weight heparin (LMWH) before a therapeutic aPTT was achieved.
Primary Outcomes
In-hospital and 30-day mortality rates were 3.0% and 7.7%, respectively. Patients who received heparin in the ED had lower in-hospital mortality (1.4% vs 6.7%; OR, 0.20; 95% CI, 0.06-0.69; P = .009) and 30-day mortality (4.4% vs 15.3%; OR, 0.25; 95% CI, 0.12-0.55; P < .001) (Fig 1). Patients who achieved a therapeutic aPTT within 24 h had lower 30-day mortality (5.6% vs 14.8%; OR, 0.34; 95% CI, 0.14-0.84; P = .037) and tended to have lower in-hospital mortality (1.5% vs 5.6%; OR, 0.27; 95% CI, 0.06-1.15; P = .091) (Fig 2).
Figure 1.
Hospital and 30-day mortality rates for patients who received heparin in the ED compared with those who received heparin after admission.
Figure 2.
Hospital and 30-day mortality rates for patients who achieved a therapeutic aPTT prior to 24 h from ED arrival compared with those who achieved a therapeutic aPTT after 24 hours. aPTT = activated partial thromboplastin time.
Patients who died in the hospital required longer times to achieve a therapeutic aPTT (median 10.7 h [IQR, 7.7-16.6] vs 20.9 h [IQR, 8.4-28.8]; P < .001). Similarly, patients who died by 30 days also required longer times to achieve a therapeutic aPTT (median 10.8 h [IQR, 7.8-16.5] vs 16.3 h [IQR, 5.3-28.6]; P = .002). Kaplan-Meier curves supported these differences between survivor and nonsurvivor populations (Fig 3).
Figure 3.
Kaplan-Meier plots of when survivors and nonsurvivors achieved a therapeutic aPTT. See Figure 2 legend for expansion of abbreviation.
Patients who required ICU admission had higher in-hospital and 30-day mortality (Tables 1, 2). Patients with COPD had higher in-hospital mortality, whereas patients with malignancies had higher 30-day mortality (Tables 1 and 2). Patients with tachycardia had higher in-hospital and 30-day mortality. Patients who died in the hospital presented with lower SBP, although hypotension was not associated with in-hospital or 30-day mortality. Patients with a positive troponin, higher Wells score, or who had recent immobilization or surgery had higher 30-day mortality. Eleven patients (2.8%) were intubated, which was associated with higher in-hospital mortality.
Patients who received heparin in the ED were younger with a higher Wells score and less likely to have CAD, CHF, COPD, positive D-dimer, or positive troponin (Table 3). Patients who achieved a therapeutic aPTT within 24 h were also younger and less likely to have CAD (Table 4). Patients for whom it was believed that an alternative diagnosis was less likely were more likely to receive heparin in the ED and to achieve a therapeutic aPTT within 24 h. The baseline demographics, presenting vital signs, laboratory data, and comorbidities were otherwise not significantly different between patients who received heparin in the ED or after admission; the same is true for patients who achieved a therapeutic aPTT within 24 h or later.
Table 3.
—Baseline Characteristics Associated With the Timing of Heparin Administration
| Characteristic | Heparin in ED (n = 280) | Heparin After Admission (n = 120) | OR | 95% CI | P Value |
| Age, y (IQR) | 62.5 (48.3-73.0) | 75.0 (68.0-81.0) | 0.94 | 0.92-0.96 | <.001 |
| Wells score (IQR) | 4.5 (3.0-6.0) | 3.0 (1.5-5.5) | 1.22 | 1.11-1.34 | <.001 |
| CAD | 43 (15.4%) | 38 (31.7%) | 0.39 | 0.24-0.65 | <.001 |
| CHF | 13 (4.6%) | 23 (19.2%) | 0.21 | 0.10-0.42 | <.001 |
| COPD | 20 (7.1%) | 22 (18.3%) | 0.34 | 0.18-0.66 | .002 |
| Troponin > 0.01 ng/mL | 47 (16.8%) | 40 (33.3%) | 0.48 | 0.29-0.80 | .005 |
| D-dimer > 500 ng/mL | 105/133(78.9%) | 55/59 (93.2%) | 0.27 | 0.09-0.82 | .020 |
| Alternative diagnosis less likely | 201 (71.8%) | 53 (44.2%) | 3.21 | 2.06-5.01 | <.001 |
Table 4.
—Baseline Characteristics Associated With the Timing of Therapeutic aPTT
| Characteristic | aPTT <24 h (n = 325) | aPTT >24 h (n = 54) | OR | 95% CI | P Value |
| Age, y (IQR) | 67.0 (53.0-76.0) | 74.0 (63.0-81.0) | 1.03 | 1.01-1.06 | .002 |
| CAD | 59 (18.2%) | 20 (37.0%) | 0.38 | 0.20-0.70 | .003 |
| Alternative diagnosis less likely | 215 (66.2%) | 26 (48.1%) | 2.10 | 1.18-3.76 | .014 |
To account for comorbidities that would confound the relationship between the timing of anticoagulation and mortality, we calculated propensity scores and did subgroup analyses. The propensity scores for hospital and 30-day survivors and nonsurvivors were compared in standard box plot fashion and found to be similar (data not shown). This suggests that adjusting for propensity scores would allow for appropriate comparisons of survivor and nonsurvivor populations with regard to early vs delayed anticoagulation. We then cross-referenced comorbidities associated with mortality (Table 2) with those associated with delayed anticoagulation (Table 3) and identified COPD and a positive troponin as comorbidities that were associated with both delayed anticoagulation and increased mortality. The Breslow Day test of the common OR (pooled estimate across strata) for COPD found that the subgroup results did not contradict the propensity score, so propensity scores remained valid for multiple variate analyses to account for COPD. Troponin data were not incorporated into the propensity score model because data were available for only 312 patients (78%). Furthermore, for patients with and without COPD, receiving heparin in the ED remained predictive of reduced 30-day mortality. For patients with and without a positive troponin, receiving heparin in the ED remained predictive of reduced in-hospital mortality. Early anticoagulation did not remain predictive of reduced mortality in the other COPD and troponin subgroup analyses.
Because of the limited number of in-hospital deaths (n = 12), a multiple logistic regression model for in-hospital mortality was not constructed, because it would be over-fit. Because there were 30 deaths by 30 days, multiple logistic regression models with three variables were considered to be most appropriate to avoid over-fitting. The first variable in these models was the timing of anticoagulation. We created two separate models: one for whether patients received heparin in the ED and another for whether they achieved a therapeutic aPTT within 24 h. Indeed, these two timing variables had inherent covariance (χ2 = 60.1; P < .001), so including them in one model would have confounded results. Admission to the ICU was used as the second variable in both models because it was a significant clinical factor associated with mortality but not included in the propensity score. The propensity score was used as the third variable in both models because it accounted for many demographic, laboratory, and comorbidity baseline characteristics. Each model used a propensity score that had been calculated for the respective timing variable (ie, either the score for receiving heparin in the ED or for achieving a therapeutic aPTT within 24 h).
In the first model, receiving heparin in the ED remained an independent predictor of reduced 30-day mortality (OR, 0.22; 95% CI, 0.08-0.60; P = .003), and the point estimate of the association remained comparable to the unadjusted value (Table 5). In the second model, no variables remained independent predictors of 30-day mortality. There was more attenuation in the association (unadjusted OR, 0.34; adjusted OR, 0.49), which suggests that some of the baseline differences observed above may have introduced some confounding into the unadjusted results.
Table 5.
—Multiple Logistic Regression Models of 30-d Mortality
| Model 1 |
Model 2 |
|||||
| Variable | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Heparin in ED | 0.22 | 0.08-0.60 | .003 | … | … | … |
| Therapeutic aPTT <24h | … | … | … | 0.49 | 0.18-1.53 | .207 |
| ICU admission | 3.65 | 1.47-8.84 | .006 | 2.36 | 0.84-6.13 | .098 |
| Propensity scorea | 0.43 | 0.04-3.75 | .457 | 20.42 | 0.23-1368 | .182 |
Receiving heparin in the ED and achieving a therapeutic aPTT within 24 h of arrival had significant covariance, so two models were created. See Tables 1 and 2 for expansion of abbreviations.
Each model used the propensity score for the respective first variable (ie, either the score for receiving heparin in the ED or achieving a therapeutic aPTT within 24 h).
Secondary Outcomes
Patients who received heparin in the ED had reduced hospital lengths-of-stay (3.9 days [IQR, 1.8-6.0] vs 6.6 days [IQR, 4.0-8.9]; P < .001) and ICU lengths-of-stay (2.0 days [IQR, 1.0-3.0] vs 2.0 days [IQR, 1.0-5.0]; P = .032). Twenty-one patients (5.3%) had a hemorrhagic event that required heparin cessation. These patients had higher in-hospital but not 30-day mortality (Tables 1, 2). Patients who received heparin in the ED had similar rates of hemorrhagic events compared with patients who received heparin after admission. Patients who had hemorrhagic events had similar initial aPTT values as compared with those who did not have events. However, achieving a therapeutic aPTT within 24 h was associated with a reduced risk of hemorrhagic events (OR, 0.28; 95% CI, 0.11-0.74; P = .014). Six patients (1.5%) had a recurrent VTE within 90 days, although none of these VTEs were fatal.
Discussion
Guidelines recommend early anticoagulation for patients with acute PE.3,17 These guidelines come from data showing that anticoagulation reduces overall mortality and VTE recurrence.6,9,11‐13,21‐27 However, prior studies have not evaluated how the timing of anticoagulation relates to mortality. This study is the first to consider how the timing of anticoagulation is associated with mortality for acute PE. Our data demonstrate reduced in-hospital and 30-day mortality in patients who received heparin in the ED. The findings associated with therapeutic aPTT within 24 h were similar but less significant.
Quality improvement is directed toward expediting management in critical care.28‐30 Despite guidelines, some studies contest the need for expedited anticoagulation for acute PE. It has been proposed that PE is overdiagnosed and overtreated, citing the lack of correlation between increased diagnosis and decreased mortality.1,6,26,31‐33 Kline et al16 reported that delayed diagnosis increases the risks of in-hospital adverse events, including shock, intubation, and death. Their study was similar to this investigation in that both evaluated the correlation of early care and clinical outcomes, but this study was not limited to ICU care and focused on the timing of anticoagulation rather than diagnosis.
Our data imply benefit to early anticoagulation, raising the mechanistic question of why receiving heparin in the ED or achieving a therapeutic aPTT within 24 h would improve mortality. Heparin is not thrombolytic, but rather it prevents clot propagation and recurrent VTE.34,35 Our data did not find a difference in the rates of recurrent VTE, but data regarding recurrent VTE and timing of anticoagulation are mixed. Anand et al36 found that patients who were subtherapeutic at 48 h did not have increased recurrent VTE. In contrast, Hull et al15,37,38 report in multiple publications that patients who were not therapeutic within 24 h had an increased rate of recurrent VTE. Given our demonstration of an association between early anticoagulation and survival, we hypothesize that by reducing the rate of clot propagation and its associated deleterious hemodynamic effects, heparin provides survival benefit. An alternate mechanism would be that heparin reduces the rate of acute secondary thromboembolic phenomena, although this is not directly supported by our results. Regardless, it is important to note that our retrospective results do not prove direct causation between early anticoagulation and survival, and so these discussions regarding mechanism must remain in the realm of speculation.
We identified several important demographic and comorbidity differences between patients who had early vs delayed anticoagulation (Tables 3,4). Our findings suggest that older patients with more comorbid conditions may have presented a diagnostic challenge, because the cardiopulmonary symptoms of acute PE may be mistaken for symptoms of CAD, CHF, or COPD. To account further for baseline differences, we used propensity score modeling to account for variables that may have increased the likelihood that patients received heparin in the ED or achieved a therapeutic aPTT within 24 h. Although there were several differences in the populations, the regression modeling of the propensity scores suggested that the survivor and nonsurvivor populations were not inherently different with regard to conditions that might have predisposed to early anticoagulation. Nevertheless, this was a retrospective study that did not have the advantage of randomization of patients to early vs late anticoagulation. Therefore, even with propensity score modeling, we can only demonstrate associations rather than prove cause and effect.
Mortality has been associated in previous studies with malignancy, COPD, older age, male sex, cardiovascular disease, and hemodynamic instability.5,39‐42 In our cohort, malignancy and COPD were indeed univariate predictors of mortality, and there was a trend toward older age in nonsurvivors. Sex and rate of cardiovascular disease were not significantly different between survivors and nonsurvivors. COPD and a positive troponin were potential confounding comorbidities that were associated with both delayed anticoagulation and increased mortality. Subgroup analyses found that receiving heparin in the ED remained predictive of reduced mortality in patients with and without either COPD or a positive troponin. Furthermore, COPD was accounted for by the propensity scores, but the population with a positive troponin remains an area of limitation and further investigation.
We found an association between decreased SBP and in-hospital mortality (Table 1). However, SBPs and the rates of hypotension did not differ between the patients who received heparin in the ED or after admission. Further investigation regarding the timing of anticoagulation could be directed toward the subgroup of patients with hemodynamic instability, especially because guidelines recommend anticoagulation before diagnosis in these patients.3 In our study, only a minority of patients (5.0%) were started on IV heparin prior to diagnosis. Although this study does not have the power to address this issue, this number is strikingly low and represents an area of potential quality improvement.
We initially hypothesized that more rapid anticoagulation would be associated with hemorrhagic events, but this was not the case. The heparin nomogram has been shown to be effective without increasing the risk of hemorrhagic events.14 Patients who had hemorrhagic complications were taken off IV heparin, so their mortality data are difficult to compare with those who remained on anticoagulation. Furthermore, our data did not include aPTT values after the initial therapeutic value, so perhaps patients who required longer times to achieve a therapeutic level were also more difficult to maintain in a therapeutic range. Despite use of a standard nomogram, it is theoretically possible that patients with higher bleeding risks were anticoagulated more cautiously and therefore required longer to achieve a therapeutic aPTT. These issues require further investigation.
We considered only IV unfractionated heparin rather than LMWH, given our institution’s practice for treating acute PE. Guidelines now support LMWH for acute PE, and LMWH has been shown to achieve therapeutic anticoagulation more rapidly than IV unfractionated heparin.3,18 We are initiating protocols and further study to determine how the timing of LMWH administration affects outcomes.
Precise times of initial IV infusion were not available for all patients, and thus we chose to characterize patients as having received heparin in the ED or after admission. This is an artificial discriminatory timeframe, and different institutions have varying practices of ED management. For example, some institutions manage patients in their ED for significant lengths of time prior to admission because of bed availability. Therefore, although we feel that our data present important temporal findings, we recognize that initiating heparin in the ED is an artificial timeframe that may not readily translate for all institutions.
This was a retrospective study rather than a randomized trial that manipulated when patients were given heparin. However, a randomized study manipulating the timing of heparinization would be unethical given current guidelines. Our data are therefore correlative, and we describe a relationship between early anticoagulation and mortality rather than proving cause and effect. Given some of the inconsistencies observed with respect to aPTT after adjustment, further research is warranted with respect to the timing variable. The attenuation of the results may suggest confounding, or it may mean that the target time of 24 h may not be universally appropriate. There could be patient-specific profiles that could be developed to increase the reliability of the prediction of mortality. Future research is warranted on these matters.
Conclusions
We provide novel data regarding how the timing of anticoagulation relates to mortality for patients with acute PE. Delayed anticoagulation in our cohort was a risk factor associated with increased mortality. Further investigations are warranted to elucidate the influence of certain demographics and comorbidities, but we nevertheless advocate that quality improvement measures be considered to expedite management of acute PE.
Acknowledgments
Author Contributions: Dr Smith: contributed to collecting and analyzing the data.
Dr Geske: contributed to collecting and analyzing the data.
Dr Maguire: contributed to collecting and analyzing the data.
Mr Zane: contributed to collecting and analyzing the data.
Dr Carter: contributed to providing statistical support.
Dr Morgenthaler: contributed to providing review, guidance, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources or NIH. Information on the National Center for Research Resources is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/.
Abbreviations
- aPTT
activated partial thromboplastin time
- CAD
coronary artery disease
- CHF
congestive heart failure
- DVT
deep vein thrombosis
- IQR
interquartile range
- LMWH
low-molecular-weight heparin
- OR
odds ratio
- PE
pulmonary embolism
- SBP
systolic BP
- VTE
venous thromboembolism
Funding/Support: This study was supported by the National Center for Research Resources, a component of the National Institutes of Health (NIH) [Grant 1 UL1 RR024150-01] and the NIH Roadmap for Medical Research. The Center for Translation Science Activities at Mayo Clinic has NIH funding.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Burge AJ, Freeman KD, Klapper PJ, Haramati LB. Increased diagnosis of pulmonary embolism without a corresponding decline in mortality during the CT era. Clin Radiol. 2008;63(4):381–386. doi: 10.1016/j.crad.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Becattini C, Agnelli G. Acute pulmonary embolism: risk stratification in the emergency department. Intern Emerg Med. 2007;2(2):119–129. doi: 10.1007/s11739-007-0033-y. [DOI] [PubMed] [Google Scholar]
- 3.Torbicki A, Perrier A, Konstantinides S, et al. Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Eur Heart J. 2008;29(18):2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- 4.Dalen JE. Pulmonary embolism: what have we learned since Virchow?: treatment and prevention. Chest. 2002;122(5):1801–1817. doi: 10.1378/chest.122.5.1801. [DOI] [PubMed] [Google Scholar]
- 5.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999;353(9162):1386–1389. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 6.Calder KK, Herbert M, Henderson SO. The mortality of untreated pulmonary embolism in emergency department patients. Ann Emerg Med. 2005;45(3):302–310. doi: 10.1016/j.annemergmed.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Jiménez Castro D, Sueiro A, Díaz G, et al. Prognostic significance of delays in diagnosis of pulmonary embolism. Thromb Res. 2007;121(2):153–158. doi: 10.1016/j.thromres.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Ryu JH, Olson EJ, Pellikka PA. Clinical recognition of pulmonary embolism: problem of unrecognized and asymptomatic cases. Mayo Clin Proc. 1998;73(9):873–879. doi: 10.4065/73.9.873. [DOI] [PubMed] [Google Scholar]
- 9.Alpert JS, Smith R, Carlson J, Ockene IS, Dexter L, Dalen JE. Mortality in patients treated for pulmonary embolism. JAMA. 1976;236(13):1477–1480. [PubMed] [Google Scholar]
- 10.Ota M, Nakamura M, Yamada N, et al. Prognostic significance of early diagnosis in acute pulmonary thromboembolism with circulatory failure. Heart Vessels. 2002;17(1):7–11. doi: 10.1007/s003800200036. [DOI] [PubMed] [Google Scholar]
- 11.Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975;17(4):259–270. doi: 10.1016/s0033-0620(75)80017-x. [DOI] [PubMed] [Google Scholar]
- 12.Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet. 1960;1(7138):1309–1312. doi: 10.1016/s0140-6736(60)92299-6. [DOI] [PubMed] [Google Scholar]
- 13.Bauer G. The introduction of heparin therapy in cases of early thrombosis. Circulation. 1959;19(1):108–109. doi: 10.1161/01.cir.19.1.108. [DOI] [PubMed] [Google Scholar]
- 14.Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993;119(9):874–881. doi: 10.7326/0003-4819-119-9-199311010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hull RD, Raskob GE, Brant RF, Pineo GF, Valentine KA. Relation between the time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis. Arch Intern Med. 1997;157(22):2562–2568. [PubMed] [Google Scholar]
- 16.Kline JA, Hernandez-Nino J, Jones AE, Rose GA, Norton HJ, Camargo CA., Jr Prospective study of the clinical features and outcomes of emergency department patients with delayed diagnosis of pulmonary embolism. Acad Emerg Med. 2007;14(7):592–598. doi: 10.1197/j.aem.2007.03.1356. [DOI] [PubMed] [Google Scholar]
- 17.Charlebois D. Early recognition of pulmonary embolism: the key to lowering mortality. J Cardiovasc Nurs. 2005;20(4):254–259. doi: 10.1097/00005082-200507000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. American College of Chest Physicians Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 19.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416–420. [PubMed] [Google Scholar]
- 20.Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135(2):98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 21.Duner H, Pernow B, Rigner KG. The prognosis of pulmonary embolism. A medical and physiological follow-up examination of patients treated at the Department of Internal Medicine and Surgery, Karolinska Sjukhuset, in 1952-1958. Acta Med Scand. 1960;168:381–395. [PubMed] [Google Scholar]
- 22.Phear D. Pulmonary embolism. A study of late prognosis. Lancet. 1960;2(7155):832–835. doi: 10.1016/s0140-6736(60)91903-6. [DOI] [PubMed] [Google Scholar]
- 23.Freiman DG, Suyemoto J, Wessler S. Frequency of pulmonary thromboembolism in man. N Engl J Med. 1965;272:1278–1280. doi: 10.1056/NEJM196506172722406. [DOI] [PubMed] [Google Scholar]
- 24.Hryniuk WH, Stiver HG, Driscoll J. Mortality of pulmonary embolism. N Engl J Med. 1971;284(13):731. doi: 10.1056/NEJM197104012841324. [DOI] [PubMed] [Google Scholar]
- 25.Paraskos JA, Adelstein SJ, Smith RE, et al. Late prognosis of acute pulmonary embolism. N Engl J Med. 1973;289(2):55–58. doi: 10.1056/NEJM197307122890201. [DOI] [PubMed] [Google Scholar]
- 26.Egermayer P. Value of anticoagulants in the treatment of pulmonary embolism: a discussion paper. J R Soc Med. 1981;74(9):675–681. doi: 10.1177/014107688107400911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogg KE, Brown MD, Kline JA. Estimating the pretest probability threshold to justify empiric administration of heparin prior to pulmonary vascular imaging for pulmonary embolism. Thromb Res. 2006;118(5):547–553. doi: 10.1016/j.thromres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343(10):710–722. doi: 10.1056/NEJM200009073431007. [DOI] [PubMed] [Google Scholar]
- 29.Dellinger RP, Levy MM, Carlet JM, et al. International Surviving Sepsis Campaign Guidelines Committee. American Association of Critical-Care Nurses. American College of Chest Physicians. American College of Emergency Physicians. Canadian Critical Care Society. European Society of Clinical Microbiology and Infectious Diseases. European Society of Intensive Care Medicine. European Respiratory Society. International Sepsis Forum. Japanese Association for Acute Medicine. Japanese Society of Intensive Care Medicine. Society of Critical Care Medicine. Society of Hospital Medicine. Surgical Infection Society. World Federation of Societies of Intensive and Critical Care Medicine Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. [Google Scholar]
- 30.Masoudi FA, Bonow RO, Brindis RG, et al. ACC/AHA Task Force on Performance Measures ACC/AHA 2008 Statement on Performance Measurement and Reperfusion Therapy: a report of the ACC/AHA Task Force on Performance Measures (work group to address the challenges of performance measurement and reperfusion therapy) J Am Coll Cardiol. 2008;52(24):2100–2112. doi: 10.1016/j.jacc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Egermayer P, Town GI. The clinical significance of pulmonary embolism: uncertainties and implications for treatment—a debate. J Intern Med. 1997;241(1):5–10. doi: 10.1046/j.1365-2796.1997.74880000.x. [DOI] [PubMed] [Google Scholar]
- 32.Egermayer P, Town GI. The mortality of untreated pulmonary embolism in patients with intermediate probability lung scans. Chest. 1998;114(5):1497. doi: 10.1378/chest.114.5.1497. [DOI] [PubMed] [Google Scholar]
- 33.Cundiff DK. Does anticoagulant treatment reduce the mortality of acute pulmonary embolism? Arch Intern Med. 2001;161(17):2148. doi: 10.1001/archinte.161.17.2148. [DOI] [PubMed] [Google Scholar]
- 34.Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. Chest. 2001;119(1 suppl):176S–193S. doi: 10.1378/chest.119.1_suppl.176s. [DOI] [PubMed] [Google Scholar]
- 35.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 36.Anand SS, Bates S, Ginsberg JS, et al. Recurrent venous thrombosis and heparin therapy: an evaluation of the importance of early activated partial thromboplastin times. Arch Intern Med. 1999;159(17):2029–2032. doi: 10.1001/archinte.159.17.2029. [DOI] [PubMed] [Google Scholar]
- 37.Hull RD, Raskob GE, Brant RF, Pineo GF, Valentine KA. The importance of initial heparin treatment on long-term clinical outcomes of antithrombotic therapy. The emerging theme of delayed recurrence. Arch Intern Med. 1997;157(20):2317–2321. [PubMed] [Google Scholar]
- 38.Hull RD, Raskob GE, Hirsh J, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986;315(18):1109–1114. doi: 10.1056/NEJM198610303151801. [DOI] [PubMed] [Google Scholar]
- 39.Carson JL, Terrin ML, Duff A, Kelley MA. Pulmonary embolism and mortality in patients with COPD. Chest. 1996;110(5):1212–1219. doi: 10.1378/chest.110.5.1212. [DOI] [PubMed] [Google Scholar]
- 40.Carson JL, Kelley MA, Duff A, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 41.Borrero S, Aujesky D, Stone RA, Geng M, Fine MJ, Ibrahim SA. Gender differences in 30-day mortality for patients hospitalized with acute pulmonary embolism. J Womens Health (Larchmt) 2007;16(8):1165–1170. doi: 10.1089/jwh.2006.0236. [DOI] [PubMed] [Google Scholar]
- 42.Wicki J, Perrier A, Perneger TV, Bounameaux H, Junod AF. Predicting adverse outcome in patients with acute pulmonary embolism: a risk score. Thromb Haemost. 2000;84(4):548–552. [PubMed] [Google Scholar]