Abstract
Major metabolic effects of human growth hormone (HGH) were assessed in the African Babinga pygmy. Plasma free fatty acid (FFA) and glucose concentrations were measured in pygmies, HGH-deficient dwarfs, Bantu tribesmen, and Caucasian controls after each received 4 mg of HGH intravenously over a 20 min period. Pygmies had an early decrease of plasma FFA and glucose concentration, but did not exhibit a later lipolytic response.
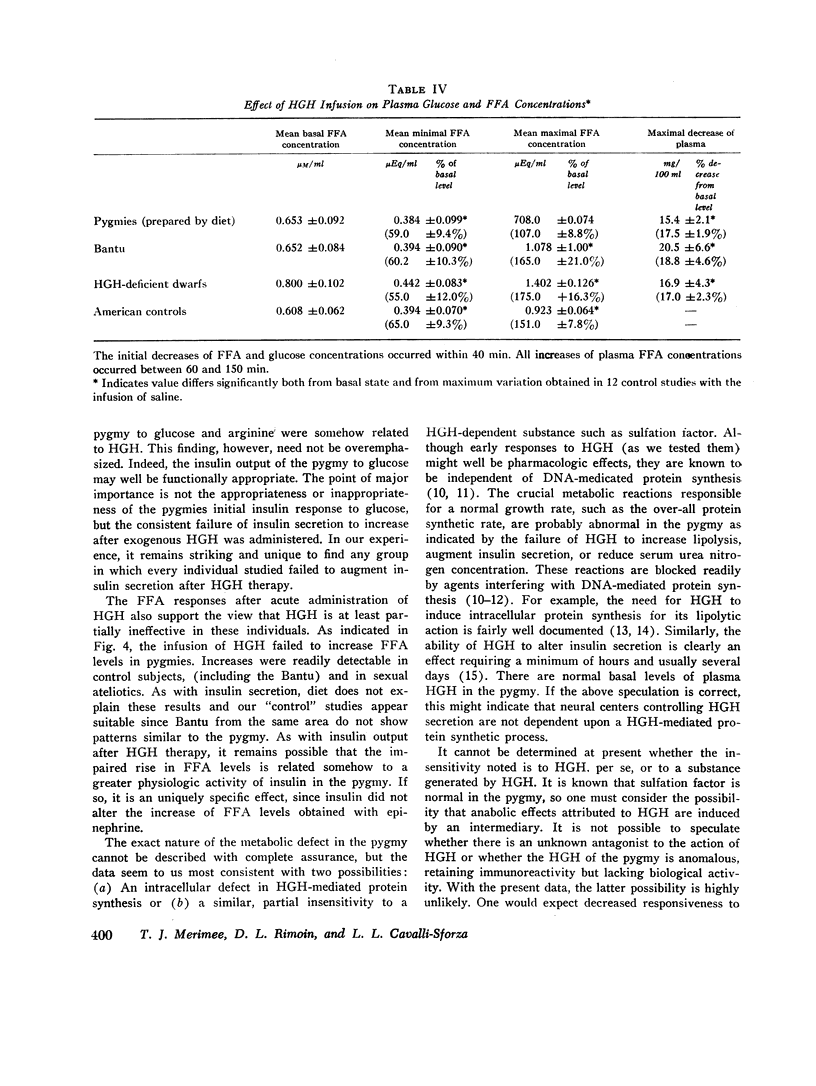
In neighboring Bantu tribesmen, American controls, and HGH-deficient dwarfs, both the early and late responses to intravenous HGH were present. The failure of plasma FFA concentration to increase in the pygmy after intravenous HGH was not due to a generalized defect in lipolysis since a normal lipolytic response was obtained with epinephrine (2 μg/min for 20 min).
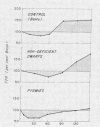
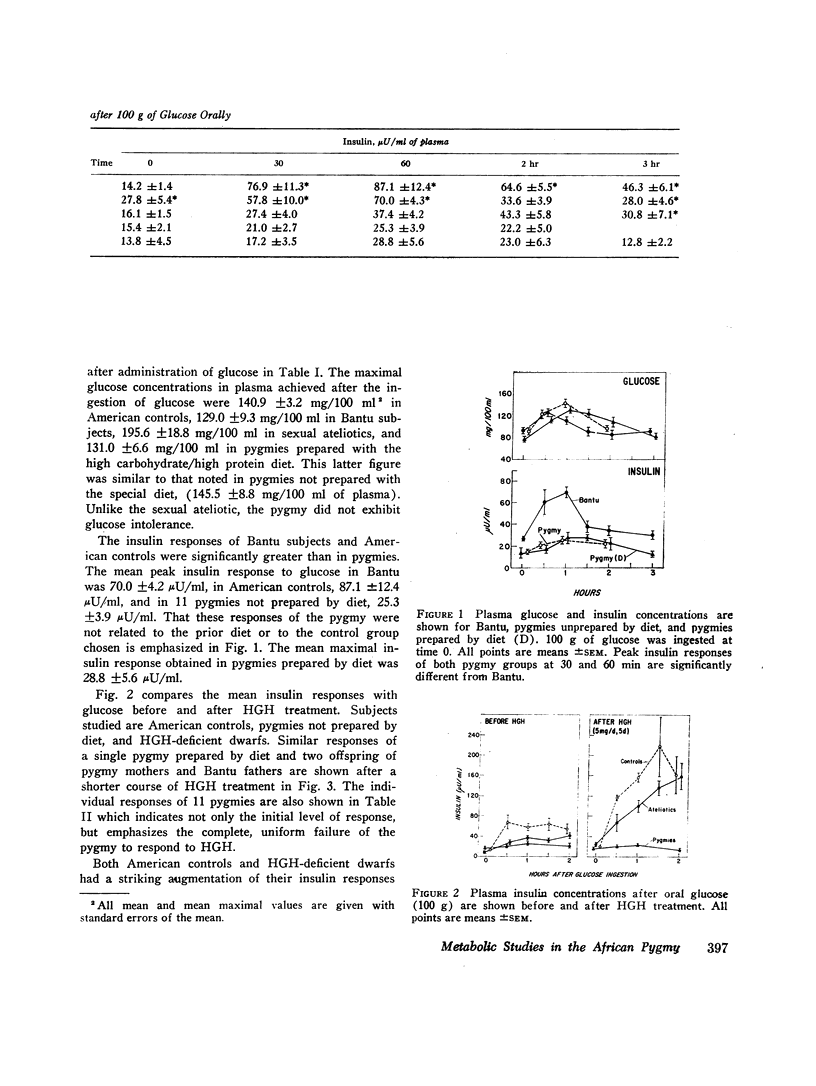
Pygmies, like HGH-deficient dwarfs, had significantly reduced insulin responses to both oral glucose and arginine. Insulin secretion was significantly reduced when compared with either Bantu tribesmen or American controls and was not altered by 2 wk of a high carbohydrate/high protein diet. HGH treatment in pygmies (5 mg b.i.d. for 5 days) failed to augment either glucose or arginine-induced insulin secretion. Glucagon consistently caused normal insulin secretion in HGH-deficient dwarfs and was, likewise, effective in each pygmy studied. In two offspring from different pygmy mothers and Bantu fathers, insulin responses to glucose were initially normal and increased in a normal manner after HGH treatment.
In previous studies, HGH failed to reduce serum urea nitrogen concentration in pygmies. Sulfation factor was found to be normal. A consideration of the data in toto is consistent with a hypothesis that the metabolic findings in the pygmy may result from partial nonresponsiveness to either HGH or to a factor generated by HGH. This defect is not transmitted as either an autosomal or sex-linked dominant trait.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOLE V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J Clin Invest. 1956 Feb;35(2):150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Dodd A., Novak L. Enzyme regulation in gluconeogenesis and lipogenesis. Relationship of protein synthesis and cyclic AMP to lipolytic action of growth hormone and glucocorticoids. Metabolism. 1971 Feb;20(2):109–118. doi: 10.1016/0026-0495(71)90086-2. [DOI] [PubMed] [Google Scholar]
- GLICK S. M., ROTH J., YALOW R. S., BERSON S. A. IMMUNOASSAY OF HUMAN GROWTH HORMONE IN PLASMA. Nature. 1963 Aug 24;199:784–787. doi: 10.1038/199784a0. [DOI] [PubMed] [Google Scholar]
- Goodman H. M. Growth hormone and the metabolism of carbohydrate and lipid in adipose tissue. Ann N Y Acad Sci. 1968 Feb 5;148(2):419–440. doi: 10.1111/j.1749-6632.1968.tb20367.x. [DOI] [PubMed] [Google Scholar]
- Laron Z., Pertzelan A., Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone--a new inborn error of metabolism? Isr J Med Sci. 1966 Mar-Apr;2(2):152–155. [PubMed] [Google Scholar]
- Merimee T. J., Burgess J. A., Rabinowitz D. Influence of growth hormone on insulin secretion. Studies of growth-hormone deficient subjects. Diabetes. 1967 Jul;16(7):478–482. doi: 10.2337/diab.16.7.478. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Hall J. D., Rimoin D. L., McKusick V. A. A metabolic and hormonal basis for classifying ateliotic dwarfs. Lancet. 1969 May 10;1(7602):963–965. doi: 10.1016/s0140-6736(69)91861-3. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Rabinowitz D., Hall J., Rimoin D. L., McKusick V. A. Isolated human growth hormone deficiency. IV. The response of sexual ateliotic dwards to exogenous growth hormone. Metabolism. 1968 Nov;17(11):1012–1018. doi: 10.1016/0026-0495(68)90007-3. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Rimoin D. L., Cavalli-Sforza L. C., Rabinowitz D., McKusick V. A. Metabolic effects of human growth hormone in the African pygmy. Lancet. 1968 Jul 27;2(7561):194–195. doi: 10.1016/s0140-6736(68)92624-x. [DOI] [PubMed] [Google Scholar]
- Merimee T. J., Rimoin D. L., Rabinowitz D., Cavalli-Sforza L. L., McKusick V. A. Metabolic studies in the African pygmy. Trans Assoc Am Physicians. 1968;81:221–230. [PubMed] [Google Scholar]
- Rimoin D. L., Merimee T. J., Rabinowitz D., McKusick V. A. Genetic aspects of clinical endocrinology. Recent Prog Horm Res. 1968;24:365–437. doi: 10.1016/b978-1-4831-9827-9.50014-9. [DOI] [PubMed] [Google Scholar]
- SWISLOCKI N. I., SZEGO C. M. ACUTE REDUCTION OF PLASMA NONESTERIFIED FATTY ACID BY GROWTH HORMONE IN HYPOPHYSECTOMIZED AND HOUSSAY RATS. Endocrinology. 1965 Apr;76:665–672. doi: 10.1210/endo-76-4-665. [DOI] [PubMed] [Google Scholar]