Abstract
Aims
Cardiotrophin-1 (CT-1) is a cytokine that induces hypertrophy in cardiomyocytes and is associated with left ventricular hypertrophy (LVH) in hypertensive patients. The objective of this study was to evaluate whether plasma CT-1 is associated with hypertrophic cardiomyopathy (HCM).
Methods and results
The study was performed in 124 patients with HCM. All patients underwent a full clinical evaluation and an echocardiogram. Left ventricular hypertrophy was evaluated by the measurement of the maximal LV wall thickness and the Spirito's LVH score. Plasma CT-1 was measured by an enzyme-linked immunosorbent assay. Compared with controls, patients with HCM exhibited higher (P < 0.001) plasma CT-1 levels. Significant correlations were found between CT-1 and maximal LV wall thickness (r = 0.284, P = 0.001) and the Spirito's LVH score (r = 0.287, P = 0.006) in HCM patients. In addition, the levels of CT-1 were higher (P = 0.02) in patients with severe LVH (maximal LV wall thickness ≥30 mm) than in patients with mild or moderate LVH (maximal LV wall thickness <30 mm).
Conclusions
These findings show that plasma CT-1 is associated with the severity of LVH in patients with HCM. Further studies are required to ascertain whether CT-1 is a diagnostic biomarker of this cardiomyopathy.
Keywords: Hypertrophic cardiomyopathy, Cardiotrophin, Hypertrophy
Introduction
Hypertrophic cardiomyopathy (HCM) is a heterogeneous genetic disease characterized by the development of left ventricular hypertrophy (LVH) in the absence of abnormal loading conditions that could explain it.1–4 It is one of the most common monogenic diseases, affecting 1 in 500 adults in the general population.3,5 Early diagnosis and risk stratification are mandatory in HCM because this disease is the most frequent cause of sudden death in young adults and sportsmen and women.3,6–9 Multiple sudden death risk factors have been identified, including the presence of family history of sudden death, severe LVH (maximal LV wall thickness ≥30 mm), non-sustained ventricular tachycardia, unexplained syncope, and abnormal blood pressure response on exercise.1,6,8,10–13 In addition, severe LVH is one of the main risk factors for disease progression in HCM.1,4,12,13
Cardiotrophin-1 (CT-1) is a protein member of the IL-6 family of cytokines that signals via leukaemia inhibitory factor receptor gp130-dependent pathways and was originally characterized as a factor that induces cardiomyocyte growth and survival.14,15 Recent studies have confirmed an active role for CT-1 in promoting myocardial structural changes, thereby participating in the progression of LV remodelling, which results in LV failure typical of cardiac diseases such as hypertensive heart disease, aortic stenosis, coronary artery disease, and dilated cardiomyopathy.16 In addition, CT-1 plasma levels have been reported to be elevated in patients with these conditions, and they are also correlated with the severity of the disease and heart failure.16 In particular, some recent experimental and clinical observations suggest that CT-1 may contribute to LV growth in hypertension. Experimental data show that increased myocardial expression of CT-1 is associated with cardiomyocyte hypertrophy and development of LVH in spontaneously hypertensive rats.17 Clinical data show that plasma CT-1 levels are elevated in hypertensive patients, namely in those with LVH.18,19 and that an association exists between plasma CT-1 concentration and LV mass index,18 as well as between the reduction of plasma CT-1 and the regression of LVH in treated hypertensive patients.20
We have hypothesized that CT-1 may be involved in LVH in HCM, therefore, our objectives were to analyse the potential associations between plasma CT-1 levels and the parameters assessing LVH in patients with HCM.
Methods
Subjects and clinical studies
All subjects gave written informed consent to participate in the study, and the locally appointed Ethics Committee approved the research protocol. The study conformed to the principles of the Helsinki Declaration
The population consisted of 124 consecutive patients with HCM recruited in the Complejo Hospitalario Universitario in A Coruña. All patients were followed up in a specialized clinic and were periodically evaluated with a protocol that includes personal and familial anamnesis, clinical evaluation, echocardiogram, Holter monitoring, and exercise testing. Hypertrophic cardiomyopathy was diagnosed by the presence of a non-dilated and hypertrophied left ventricle (LV maximal wall thickness ≥15 mm) in the absence of another cardiac or systemic disease capable of producing the hypertrophy observed, or on the presence of unexplained electrocardiographic abnormalities in relatives of patients with unequivocal disease.1,4 As hypertension has a high prevalence in the general population, the presence of hypertension was not considered exclusion criteria for the diagnosis of HCM when hypertension was mild and did not explain the severity of the LVH.
A group of 29 subjects with the same age and sex distribution of the study group recruited in the University Clinic in Pamplona were used as control subjects for plasma CT-1 studies, comparing the CT-1 levels of the 124 HCM patients with those of the 29 controls. The presence of arterial hypertension, diabetes, coronary artery disease, aortic stenosis, and HCM was excluded after complete clinical and cardiac examination of these control subjects.
Echocardiographic study
Standard measurements, including Doppler parameters, were obtained using an ultrasound system equipped with second harmonic imaging (either Acuson-Siemens Sequoia C512, Mountain View, CA, USA, or Sonos 5500, Philips Medical Systems, Andover, MA, USA). Echocardiographic images were recorded on a magneto-optical disk. All measurements were analysed offline by the same reader (L.M.) to minimize the variability of the measurements, following the guidelines of the American Society of Echocardiography.21 Left ventricle wall thickness was measured at 14 LV segments using paraesternal short views at mitral, papillary, and apical levels, paraesternal long-axis and apical 4, 3, and 2 chambers views. At the mitral and papillary muscles level, LV thickness was measured at anterior, anterior septum, posterior septum, posterior, and lateral walls. At the apical level LV wall thickness was measured at anterior, posterior, septal, and lateral walls. The LV hypertrophy score proposed by Spirito and Maron12 was calculated as the sum of the following measurements: maximal wall thickness of anterior septum (either basal or mid-ventricular level), maximal wall thickness of posterior septum (basal or mid-ventricular), maximal wall thickness of the posterior wall (basal or mid-ventricular), and maximal wall thickness of the anterolateral wall (basal or mid-ventricular). LV ejection fraction (EF) was calculated with the Simpson biplane method and systolic dysfunction was defined by the presence of an EF < 50%. Left ventricle outflow tract velocities were measured using continuous wave Doppler, and LV outflow tract gradients were calculated using the modified Bernoulli equation [LV outflow tract gradient = 4 (LV outflow tract velocity)2].
Holter monitoring
Two-channel ambulatory electrocardiogram monitoring was performed in 118 patients. Non-sustained ventricular tachycardia was defined as three or more consecutive ventricular beats at a rate of ≥120beats/min, lasting for <30 s.
Exercise testing
Symptom-limited exercise was performed on a treadmill using the Bruce protocol. A physician was present during all studies to encourage maximal exertion. Exercise capacity was defined as achieved metabolic equivalents. Blood pressure was measured at rest, every minute during exercise and for the first 5min of recovery.
Blood pressure response to exercise was considered abnormal when systolic blood pressure failed to increase by more than 25 mmHg from baseline, or when there was a decrease of more than 10 mmHg from the maximum blood pressure during exercise.6
Determination of plasma cardiotrophin-1
Venous blood samples were taken at 08:30 h with all the subjects being in fasting conditions. Plasma CT-1 was measured by an enzyme-linked immunosorbent assay (ELISA) as previously reported.18 The inter-assay and intra-assay coefficients of variation were 6.9 and 7.4%, respectively. The lower limit of detection was 2.9fmol CT-1/mL. The upper limit of normality for plasma CT-1 values measured in the control population was of 41 fmol/mL.
Statistic analysis
The statistical analysis was done using the statistical package SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Categorical variables are presented as percentages and continuous variables are presented as mean value ± standard deviation. Cardiotrophin-1 levels were compared between two groups using the Student t-test for unpaired data once normality was demonstrated (Shapiro–Wilk test); otherwise, a non-parametric test (Mann–Whitney's U test) was used. The correlation between continuous variables was tested calculating Pearson correlation coefficient and, when applicable, Spearman correlation coefficient. Partial correlation coefficients were calculated to exclude the influence of potential confounding factors. Categorical variables were analysed by the chi-square (χ2) or Fisher exact test. For all the analyses a bilateral P-value of <0.05 was considered statistically significant.
Results
Clinical characteristics
We studied 78 males (63%) and 46 females (37%) from 101 different families. Age at diagnosis of HCM was 51 ± 16 years (range 15–83) and at the first evaluation in our clinic it was 53 ± 15 years (range 16–83). The main clinical and echocardiographic variables of the study group, including sudden death risk factors, are summarized in Tables 1 and 2.
Table 1.
Clinical and echocardiographic characteristics of the study group
| Variable | Number (%) |
|---|---|
| Males/females | 78 (63)/46 (37) |
| Family history of sudden death | 20 (16) |
| Family history of HCM | 51 (41) |
| Severe hypertrophy (≥30 mm) | 13 (11) |
| Previous syncope | 18 (15) |
| Non-sustained ventricular tachycardia | 29 (23) |
| Abnormal blood pressure response on exercise test | 31 (25) |
| Previous angina | 56 (45) |
| Obstructive (dynamic gradient ≥30 mmHg) | 33 (27) |
| Systolic dysfunction | 7 (6) |
| Previous atrial fibrillation | 42 (34) |
| NYHA functional class | |
| NYHA I | 41 (33) |
| NYHA II | 69 (56) |
| NYHA III | 13 (11) |
| NYHA IV | 1 (1) |
| Number of sudden death risk factorsa | |
| 0 risk factors | 48 (38) |
| 1 risk factors | 51 (41) |
| 2 risk factors | 16 (13) |
| 3 risk factors | 8 (7) |
| 4 risk factors | 1 (1) |
aSudden death risk factors included: family history of sudden death, severe hypertrophy, previous syncope, abnormal blood pressure response on exercise test, and non-sustained ventricular tachycardia on Holter monitoring.
Table 2.
Correlations between cardiotrophin-1 levels and echocardiographic measurements
| Variable | Mean value ± SD | Correlation r | P-value |
|---|---|---|---|
| Basal anterior wall | 15 ± 5 | 0.329 | 0.007 |
| Basal anterior septum | 19 ± 6 | 0.269 | 0.003 |
| Basal posterior septum | 17 ± 6 | 0.278 | 0.005 |
| Basal posterior wall | 11 ± 2 | 0.229 | 0.011 |
| Basal lateral wall | 13 ± 4 mm | 0.371 | 0.001 |
| Maximum basal wall thickness | 20 ± 6 mm | 0.250 | 0.005 |
| Mid-ventricular anterior wall | 15 ± 4 mm | 0.159 | 0.217 |
| Mid-ventricular anterior septum | 18 ± 6 mm | 0.168 | 0.107 |
| Mid-ventricular posterior septum | 18 ± 7 mm | 0.216 | 0.056 |
| Mid-ventricular posterior wall | 13 ± 3 mm | 0.286 | 0.009 |
| Mid-ventricular lateral wall | 13 ± 3 mm | 0.292 | 0.017 |
| Maximum mid-ventricular wall thickness | 19 ± 7 mm | 0.241 | 0.018 |
| Apical anterior wall | 15 ± 5 mm | 0.223 | 0.054 |
| Apical septal wall | 14 ± 5 mm | 0.256 | 0.060 |
| Apical posterior wall | 13 ± 4 mm | 0.055 | 0.644 |
| Apical lateral wall | 14 ± 5 mm | 0.443 | 0.001 |
| Maximum apical wall thickness | 16 ± 5 mm | 0.325 | 0.002 |
| LV end-diastolic diameter | 44 ± 6 mm | 0.288 | 0.001 |
| LV end-diastolic diameter/body surface area | 24 ± 4 mm/m2 | 0.310 | <0.001 |
| LV end-systolic diameter | 26 ± 7 mm | 0.184 | 0.043 |
| LV end-systolic diameter/body surface area | 14 ± 4 mm/m2 | 0.219 | 0.015 |
| LV ejection fraction | 70 ± 12% | -0.028 | 0.760 |
| Left atrial diameter | 45 ± 9 mm | 0.205 | 0.023 |
| Left atrial diameter/body surface area | 25 ± 5 mm/m2 | 0.255 | 0.004 |
| Aortic root diameter | 34 ± 4 mm | 0.056 | 0.541 |
| LV outflow tract gradient | 28 ± 37 mmHg | 0.029 | 0.754 |
LV, left ventricular.
Values in bold represent statistical significance.
Echocardiographic characteristics
Mean maximal LV wall thickness was 21.3 ± 6.1 mm (median 20, range 9–42 mm, interquartile range 7). Table 2 summarizes the wall thickness measurements in the different LV segments. Spirito's score was 66 ± 17 mm (median 64, range 36–118 mm, intercuartile range 17.5). Left atrial diameter was 45 ± 9 mm (median 45, range 25–96 mm, interquartile range 11). Thirty-three patients (27%) had an LV outflow tract gradient ≥30 mmHg (obstructive HCM) and 12 (10%) had severe obstruction with a gradient ≥90 mmHg. Mean EF was 70 ± 12% (median 71, range 32–94%, interquartile range 17) and systolic dysfunction was present in six cases (5%).
Cardiotrophin-1 levels
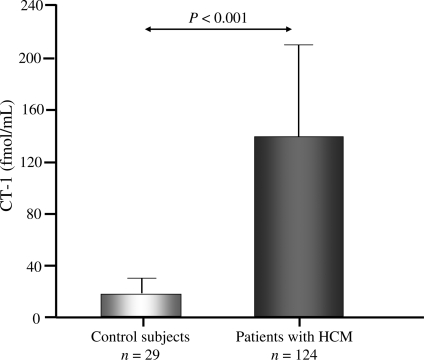
As shown in Figure 1, plasma CT-1 was increased (P < 0.001) in patients with HCM (136.28 ± 72.01 fmol/mL) compared with healthy controls (17.92 ± 12.03 fmol/mL). The range of CT-1 values in patients was from 43 to 362 fmol/mL, with all patients exhibiting values above the upper limit of normality of the control population (mean + 1.96 SD). Plasma CT-1 levels were not influenced by the presence of hypertension or antihypertensive therapy.
Figure 1.
Plasma concentration of cardiotrophin-1 (CT-1) measured in 29 control subjects and in 124 patients with hypertrophic cardiomyopathy (HCM).
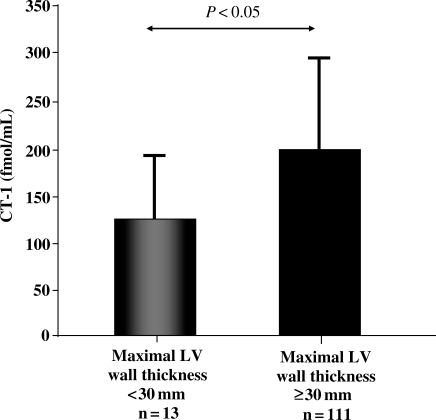
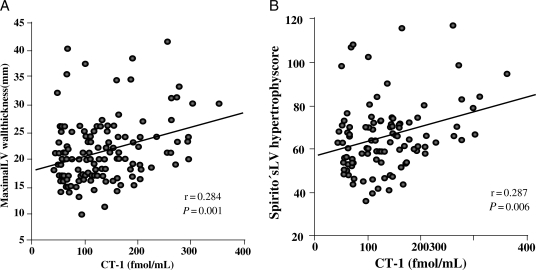
Cardiotrophin-1 was higher (P = 0.02) in patients with severe LVH (maximal LV wall thickness ≥30 mm) than in patients with mild o moderate LVH (maximal LV wall thickness <30 mm; Figure 2). Direct correlations were found between CT-1 and maximal LV wall thickness (r = 0.284, P = 0.001; Figure 3A), and Spirito's score (r = 0.287, P = 0.006; Figure 3B). In addition, CT-1 was directly correlated with wall thickness in 9 out of the 14 LV segments evaluated and with the maximal thickness at basal, mid-ventricular, and apical levels, as well as with LV and left atrial dimensions (Table 2). The associations of CT-1 with maximal LV wall thickness and Spirito's score remained significant when we adjusted for the influence of these echocardiographic confounding factors. No significant correlations were found between CT-1 levels and sex, presence of non-sustained ventricular tachycardia, left ventricular outflow tract obstruction, previous syncope, abnormal blood pressure response on exercise test, or previous atrial fibrillation. Cardiotrophin-1 values were non-significantly higher in patients with more sudden death risk factors, which could be explained by the association of CT-1 values with the presence of severe hypertrophy. The group of six patients with left ventricular systolic dysfunction showed also higher CT-1 values than patients without systolic dysfunction, but this difference was not statistically significant (Table 3).
Figure 2.
Plasma concentration of cardiotrophin-1 (CT-1) in patients with hypertrophic cardiomyopathy classified according to maximal left ventricular (LV) wall thickness.
Figure 3.
(A) Direct correlation between plasma CT-1 and maximal left ventricular (LV) wall thickness (y = 0.024x + 18.05) in patients with hypertrophic cardiomyopathy. (B) Direct correlation between plasma cardiotrophin-1 (CT-1) and Spirito's LV hypertrophy score (y = 0.067x + 56.91) in patients with HCM.
Table 3.
Relation between cardiotrophin-1 levels and categorical clinical variables
| Variable | Number (%) of patients with/without the variable | Mean ± SD CT-1 levels in patients with the variable | Mean ± SD CT-1 levels in patients without the variable | P-value |
|---|---|---|---|---|
| Male sex | 78 (63)/46 (37) | 136 ± 70 | 137 ± 76 | 0.853 |
| Non-sustained ventricular tachycardia | 29 (23)/95 (77) | 153 ± 83 | 129 ± 68 | 0.119 |
| Family history of HCM | 51 (41)/73 (59) | 129 ± 66 | 141 ± 76 | 0.357 |
| Maximum wall thickness ≥30 mm | 13 (10)/111 (90) | 200 ± 103 | 129 ± 64 | 0.031 |
| Obstruction (gradient ≥30 mmHg) | 33 (27)/91 (73) | 137 ± 74 | 136 ± 72 | 0.928 |
| Systolic dysfunction | 6 (5)/118 (95) | 162 ± 49 | 134 ± 72 | 0.342 |
| Previous syncope | 18 (15)/106 (85) | 133 ± 56 | 137 ± 75 | 0.817 |
| Family history of sudden death | 20 (16)/104 (84) | 124 ± 68 | 139 ± 73 | 0.398 |
| Abnormal blood pressures response | 31 (25)/93 (75) | 142 ± 77 | 135 ± 75 | 0.660 |
| Previous angina | 56 (45)/68 (55) | 133 ± 74 | 139 ± 71 | 0.648 |
| Atrial fibrillation ever | 42 (34)/82 (66) | 143 ± 69 | 133 ± 74 | 0.446 |
| Number of sudden death risk factors | 0.07* | |||
| 0 risk factors | 48 (38) | 123 ± 65 | ||
| 1 risk factor | 51 (41) | 140 ± 70 | ||
| 2 risk factors | 16 (13) | 178 ± 83 | ||
| 3 or more risk factors | 9 (8) | 126 ± 84 |
*P-value for linear association.
Values in bold represent statistical significance.
Discussion
The main findings of this study are as follows: (i) plasma CT-1 levels are increased in patients with HCM; and (ii) plasma CT-1 is associated with the severity of LVH in patients with HCM.
The observation that plasma CT-1 was abnormally increased in patients with HCM expands previous findings in patients with cardiac pathologies who present LVH due to pressure overload such as hypertensive patients18,22 and patients with aortic stenosis.23 Since it has been reported that the human heart secretes CT-1 via the coronary sinus into the peripheral circulation24 and that plasma CT-1 is directly correlated with myocardial expression of CT-1,25 increased plasma levels of CT-1 seen in patients with HCM may reflect cardiac over-spilling of this cytokine. The question arises about the factors determining the exaggerated cardiac production of CT-1 in patients with HCM. Since the dynamic outflow gradient represents a pressure load for the left ventricle in patients with obstructive HCM, a mechanical factor may be involved in up-regulation of CT-1 in some of these patients (those with obstructive forms of the disease). In support of this possibility, it has been reported that in patients with aortic valve stenosis plasma CT-1 levels correlate with trans-valvular aortic pressure gradients.23 Furthermore, ventricular stretch of Langendorff perfused rat hearts resulted in significant release of CT-1.19 However, only 27% of our patients had LV obstruction, and no difference was found in the CT-1 levels between patients with and without obstruction. This means that CT-1 levels are more likely associated with the presence of LVH than with the presence of obstruction.
Our finding that plasma CT-1 correlates with parameters assessing LV growth is in accordance with previous findings showing similar correlations in hypertensive patients17 and in patients with dilated cardiomyopathy,26 thus suggesting that this cytokine may also contribute to the development of LVH in HCM. Furthermore, we also found that CT-1 levels are associated with the severity of LVH, suggesting that up-regulation of this cytokine may be involved in the progression of LVH in patients with HCM. Moreover, our results also suggest a potential association between CT-1 levels and the development of systolic dysfunction in HCM, even thought the number of patients with systolic dysfunction included in this study was not sufficient to validate this hypothesis. Up to 10% of the patients with HCM evolve to a so-called burn-out phase of the disease, characterized by the development of progressive wall thinning, LV dilatation and systolic dysfunction.1,3,27 This evolution is more frequent in patients with early expression of the disease and higher degrees of LVH. It is associated with a higher incidence of ventricular arrhythmias, atrial fibrillation, symptomatic heart failure and sudden death, and requires therapeutic adjustments.1,7,27 It would be interesting to evaluate in prospective studies whether CT-1 levels could be early predictors of this evolution.
The stratification of the severity of LVH in patients with HCM is highly relevant because morbidity, mortality, and sudden death risk in these patients are associated with the severity of hypertrophy.6,8,12,13 However, the echocardiographic quantification of LV mass has multiple limitations. In addition, the same wall thickness or LV mass correspond to different severity of hypertrophy in patients with different body surface area, sex or height and this is not usually considered when analysing the prognostic significance of LVH in individual patients. Even thought the associations found between CT-1 levels and maximal wall thickness and Spirito's score were statistically significant, they presented modest correlation coefficients (r = 0.284 and r = 0.287). This means that CT-1 levels do not provide the same information as the other parameters. The associations here reported between plasma CT-1 and maximal LV wall thickness and the Spirito's LVH score, as well as with the different LV segments and with the LV end-diastolic diameter suggest that the plasma level of this cytokine may be of potential usefulness as indicators of global left ventricular remodelling in patients with HCM.
Risk prediction is still an unsolved issue in patients with HCM. In this regard, it has been reported that increased plasma CT-1 behaves as a prognostic marker of mortality in patients with chronic HF.28 The potential mechanisms linking CT-1 with poor prognosis are unclear, however, it has been reported that CT-1 induces cardiomyocyte dysfunction in reconstituted cardiac tissue29 and an association has been found between plasma CT-1 and the impairment of systolic function in hypertensive patients with stage congestive heart failure.22 Additionally, CT-1 may be also involved in the development of myocardial fibrosis, an important component of myocardial remodelling, since CT-1 induces extracellular matrix protein synthesis in cardiac fibroblasts.30 Both the severity of LVH and the presence of systolic dysfunction are associated with an increased risk of sudden death in HCM and appear to be associated with CT-1 levels. Therefore, the possibility that CT-1 may be related to prognosis in HCM deserves further investigation.
In our study CT-1 levels did not show association with other well-established sudden death risk factors, such as family history of sudden death, presence of non-sustained ventricular tachycardia, abnormal blood pressure response on exercise, or previous syncope. The explanation for this lack of associations may be the limited power of the study to detect modest associations. Cardiotrophin-1 levels could also have a prognostic significance independent of these factors; however, this aspect has not been evaluated in the present study. Further work is necessary to assess whether this cytokine may add prognostic information in patients with HCM.
Some limitations of the current study must be acknowledged. First of all, due to the limitations of echocardiography for an accurate assessment of LV mass in HCM, magnetic resonance imaging would have provided a better estimate of the LV mass, but it was not available in a high number of cases. Second, there was no one to one matching between healthy controls and patients with HCM, and this imbalance precluded paired analysis. Third, although it has been shown that the human heart secretes CT-1 via the coronary sinus into the peripheral circulation,24,25 given the earlier evidence showing that CT-1 mRNA is expressed in other organs,31 potential additional sources of circulating CT-1 can not be excluded in patients with HCM.
Conclusions
For the first time, we show that CT-1 plasma levels are increased in patients with HCM. In addition, we report that plasma levels of this cytokine are associated with the severity of LVH in patients with HCM. Thus, the measurement of plasma CT-1 could provide incremental value in the assessment of LVH severity in these patients. Nevertheless, further large-scale prospective studies are necessary to definitively validate this approach and to evaluate the potential prognostic implications of CT-1 levels in patients with HCM.
Funding
This work was supported by the agreement between the Foundation for Applied Medical Research (FIMA) and UTE project CIMA, the red Temática de Investigación Cooperativa en Enfermedades Cardiovasculares (RECAVA) from the Instituto de Salud Carlos III, Ministry of Health, Spain (grant RD06/0014/0008), and the Instituto de Salud Carlos III, Ministry of Science and Innovation (grant PS09/02234). M.O. was supported by a grant from Fundacion Carolina-BBVA. Funding to pay the Open Access publication charges for this article was provided by Fundación del Complejo Hospitalario Universitario de A Coruña.
Conflicts of interest: none declared.
Acknowledgements
The authors thank Sonia Martínez and Elena Veira for their valuable technical assistance.
References
- 1.Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH, 3rd, Spirito P, Ten Cate FJ, Wigle ED Task Force on Clinical Expert Consensus Documents.: American College of Cardiology; Committee for Practice Guidelines. European Society of Cardiology. ACC/ESC clinical expert consensus document on hypertrophic cardiomyopathy: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines (Committee to Develop an Expert Consensus Document on Hypertrophic Cardiomyopathy) Eur Heart J. 2003;24:1965–1991. doi: 10.1016/s0195-668x(03)00479-2. [DOI] [PubMed] [Google Scholar]
- 2.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 3.Elliott PM, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- 4.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation. 1995;92:785–789. doi: 10.1161/01.cir.92.4.785. [DOI] [PubMed] [Google Scholar]
- 6.Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. doi: 10.1016/s0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 7.McKenna WJ, Monserrat Iglesias L. Sudden death (V). Identification and treatment of patients with hypertrophic cardiomyopathy at risk of sudden death. Rev Esp Cardiol. 2000;53:123–130. doi: 10.1016/s0300-8932(00)75069-x. [DOI] [PubMed] [Google Scholar]
- 8.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 9.Maron BJ, Roberts WC, Epstein SE. Sudden death in hypertrophic cardiomyopathy: a profile of 78 patients. Circulation. 1982;65:1388–1394. doi: 10.1161/01.cir.65.7.1388. [DOI] [PubMed] [Google Scholar]
- 10.Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ. Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients. J Am Coll Cardiol. 2003;42:873–879. doi: 10.1016/s0735-1097(03)00827-1. [DOI] [PubMed] [Google Scholar]
- 11.Sadoul N, Prasad K, Elliott PM, Bannerjee S, Frenneaux MP, McKenna WJ. Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy. Circulation. 1997;96:2987–2991. doi: 10.1161/01.cir.96.9.2987. [DOI] [PubMed] [Google Scholar]
- 12.Spirito P, Maron BJ. Relation between extent of left ventricular hypertrophy and occurrence of sudden cardiac death in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1990;15:1521–1526. doi: 10.1016/0735-1097(90)92820-r. [DOI] [PubMed] [Google Scholar]
- 13.Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–4. doi: 10.1016/S0140-6736(00)04005-8. [DOI] [PubMed] [Google Scholar]
- 14.Pennica D, King KL, Shaw KJ, Luis E, Rullamas J, Luoh SM, Darbonne WC, Knutzon DS, Yen R, Chien KR, Baker JB, Wood WI. Expression cloning of cardiotrophin-1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci USA. 1995;92:1142–1146. doi: 10.1073/pnas.92.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng Z, Pennica D, Wood WI, Chien KR. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development. 1996;122:419–428. doi: 10.1242/dev.122.2.419. [DOI] [PubMed] [Google Scholar]
- 16.Calabro P, Limongelli G, Riegler L, Maddaloni V, Palmieri R, Golia E, Roselli T, Masarone D, Pacileo G, Golino P, Calabro R. Novel insights into the role of cardiotrophin-1 in cardiovascular diseases. J Mol Cell Cardiol. 2009;46:142–148. doi: 10.1016/j.yjmcc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.López N, Díez J, Fortuño MA. Differential hypertrophic effects of cardiotrophin-1 on adult cardiomyocytes from normotensive and spontaneously hypertensive rats. J Mol Cell Cardio. 2006;41:902–913. doi: 10.1016/j.yjmcc.2006.03.433. [DOI] [PubMed] [Google Scholar]
- 18.López B, González A, Lasarte JJ, Sarobe P, Borrás F, Díaz A, Barba J, Tomás L, Lozano E, Serrano M, Varo N, Beloqui O, Fortuño MA, Díez J. Is plasma cardiotrophin-1 a marker of hypertensive heart disease? J Hypertens. 2005;23:625–632. doi: 10.1097/01.hjh.0000160221.09468.d3. [DOI] [PubMed] [Google Scholar]
- 19.Pemberton CJ, Raudsepp SD, Yandle TG, Cameron VA, Richards AM. Plasma cardiotrophin-1 is elevated in human hypertension and stimulated by ventricular stretch. Cardiovasc Res. 2005;68:109–117. doi: 10.1016/j.cardiores.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 20.González A, López B, Martín-Raymondi D, Lozano E, Varo N, Barba J, Serrano M, Díez J. Usefulness of plasma cardiotrophin-1 in assessment of left ventricular hypertrophy regression in hypertensive patients. J Hypertens. 2005;23:2297–2304. doi: 10.1097/01.hjh.0000184406.12634.f9. [DOI] [PubMed] [Google Scholar]
- 21.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 22.López B, González A, Querejeta R, Barba J, Díez J. Association of plasma cardiotrophin-1 with stage C heart failure in hypertensive patients: potential diagnostic implications. J Hypertens. 2009;27:418–424. doi: 10.1097/HJH.0b013e32831ac981. [DOI] [PubMed] [Google Scholar]
- 23.Talwar S, Downie PF, Squire IB, Davies JE, Barnett DB, Ng LL. Plasma N-terminal pro BNP and cardiotrophin-1 are elevated in aortic stenosis. Eur J Heart Fail. 2001;3:15–19. doi: 10.1016/s1388-9842(00)00074-x. [DOI] [PubMed] [Google Scholar]
- 24.Asai S, Saito Y, Kuwahara K, Mizuno Y, Yoshimura M, Higashikubo C, Tsuji T, Kishimoto I, Harada M, Hamanaka I, Takahashi N, Yasue H, Nakao K. The heart is a source of circulating cardiotrophin-1 in humans. Biochem Biophys Res Commun. 2000;279:320–323. doi: 10.1006/bbrc.2000.3932. [DOI] [PubMed] [Google Scholar]
- 25.González A, Ravassa S, Loperena I, López B, Beaumont J, Querejeta R, Larman M, Díez J. Association of depressed cardiac gp130-mediated antiapoptotic pathways with stimulated cardiomyocyte apoptosis in hypertensive patients with heart failure. J Hypertens. 2007;25:2148–2157. doi: 10.1097/HJH.0b013e32828626e2. [DOI] [PubMed] [Google Scholar]
- 26.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Fujii M, Matsumoto T, Yamamoto T, Wang X, Asai S, Tsuji T, Tanaka H, Saito Y, Kuwahara K, Nakao K, Kinoshita M. Relationship between plasma level of cardiotrophin-1 and left ventricular mass index in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;8:1485–1490. doi: 10.1016/s0735-1097(01)01576-5. [DOI] [PubMed] [Google Scholar]
- 27.Thaman R, Gimeno JR, Murphy RT, Kubo T, Sachdev B, Mogensen J, Elliott PM, McKenna WJ. Prevalence and clinical significance of systolic impairment in hypertrophic cardiomyopathy. Heart. 2005;91:920–925. doi: 10.1136/hrt.2003.031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsutamoto T, Asai S, Tanaka T, Sakai H, Nishiyama K, Fujii M, Yamamoto T, Ohnishi M, Wada A, Saito Y, Horie M. Plasma level of cardiotrophin-1 as a prognostic predictor in patients with chronic heart failure. Eur J Heart Fail. 2007;10:1032–1037. doi: 10.1016/j.ejheart.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Zolk O, Engmann S, Münzel F, Krajcik R. Chronic cardiotrophin-1 stimulation impairs contractile function in reconstituted heart tissue. Am J Physiol Endocrinol Metab. 2005;288:E1214–E1221. doi: 10.1152/ajpendo.00261.2004. [DOI] [PubMed] [Google Scholar]
- 30.Freed DH, Borowiec AM, Angelovska T, Dixon IM. Induction of protein synthesis in cardiac fibroblasts by cardiotrophin-1: integration of multiple signaling pathways. Cardiovasc Res. 2003;60:365–375. doi: 10.1016/s0008-6363(03)00534-0. [DOI] [PubMed] [Google Scholar]
- 31.Pennica D, Swanson TA, Shaw KJ, Kuang WJ, Gray CL, Beatty BG, Wood WI. Human cardiotrophin-1: protein and gene structure, biological and binding activities, and chromosomal localization. Cytokine. 1996;8:183–189. doi: 10.1006/cyto.1996.0026. [DOI] [PubMed] [Google Scholar]