The structure and biology of vitamin K
Vitamin K was first discovered in the early 1930s by the Danish biochemist Henrik Dam who observed – while studying cholesterol metabolism in chickens – that chicks fed with a diet free of sterols and low in fat tended to develop subcutaneous and intramuscular haemorrhages. Further studies on different foods led to the discovery of an "anti-haemorraghic factor", which was designated vitamin K (with the "K" standing for "Koagulations-Vitamin") given that it was essential for normal haemostasis1.
The term vitamin K actually denotes a group of lipophilic, hydrophobic vitamins that belong to the class of 2-methyl-1,4-naphthoquinone derivatives. All the members of the vitamin K group share a common methylated naphthoquinone ring structure, but have different aliphatic side chains attached at the 3-position. The naturally occurring compounds are vitamin K1 (also known as phylloquinone, phytomenadione or phytonadione), and vitamin K2 (also known as menaquinone or menatetrenone). The former compound is the primary source of vitamin K in humans. It is acquired through the diet and is prevalently present in leafy green vegetables such as spinach, Swiss chard, Brassica (e.g. cabbage, kale, cauliflower, turnip, and Brussels sprout), some fruits such as avocado, banana and kiwi, as well as in some vegetable oils, especially soybean oil. Interestingly, cooking does not remove significant amounts of vitamin K from these foods. Many bacteria that colonise the human intestine (especially Bacteroides) synthesise vitamin K2 or menaquinone, which is used as a redox reagent in electron transport and oxidative phosphorylation. There is, however, ongoing debate on whether bacterial synthesis of vitamin K in the intestine provides a significant supply of this vitamin in humans. The colon contains a large reservoir of bacterial vitamin K2 (~2 mg), but it is now undeniable that this pool represents only about 10% of normal human requirements and is, therefore, insufficient to satisfy these requirements. Furthermore, there is some evidence of poor bioavailability of this intestinal source of vitamin K. Bile salts are necessary for effective absorption of vitamin K, but are not present in the colon, and the intestinal synthesis of vitamin K is not sufficient to compensate for deficiency due to biliary obstruction. Moreover, intestinal menaquinones are enveloped within the bacterial membranes and are, therefore, poorly available for intestinal adsorption. Taken together, these data argue against the concept of the colon as a significant source of vitamin K for human use, so that patients at risk of deficiency remain those who cannot absorb vitamin K from the small intestine2.
Fasting vitamin K1 reference values in healthy adults range from 0.15 to 1.0 μg/L (median 0.5 μg/L)2. The average liver storage pool of phylloquinone in adults is around 9 μg, but might vary widely on an inter-individual basis. The liver, moreover, contains a larger pool of vitamin K2 (~90% of total liver vitamin K stores) which, therefore, represents a reservoir against vitamin deficiency when the more labile vitamin K1 stores are depleted. Other extra-hepatic tissues, especially the brain, kidney, and pancreas, store additional amounts of vitamin K2 (usually <2 pmol/g), which probably originates from endogenous synthesis through the metabolism of vitamin K13. Isotopic studies are consistent with a high turnover rate of vitamin K1, in that up to 70% of the oral dose is excreted in the bile and urine within a few days2.
According to the U.S. Institute of Medicine, the recommended dietary allowance (i.e., the daily intake sufficient to meet the requirements of nearly all healthy individuals) of vitamin K is 120 μg in adult men and 90 μg in adult women4, but it is much lower in other countries and Europe (including Italy), where, for example, a daily average of ~1 μg per kg of body weight is recommended5. The Third National Health and Nutrition Examination Survey set the thresholds of adequate vitamin K intake as 2 μg/die for infants in the first 6 months of life and 2.5 μg/die for infants aged 7–12 months6. After this age, the adequate intake progressively increases from 30 μg/die in children aged 1–3 years, up to 75 μg/die in adolescents (up to 18 years old).
While vitamin K1 is commercially manufactured for medicinal use under several brand names (Phylloquinone®, Phytonadione®, AquaMEPHYTON®, Mephyton®, Konakion® ), there are three additional synthetic forms of vitamin K (i.e., vitamin K3, K4, and K5), which are used in many areas including the pet food industry (vitamin K3) and to inhibit fungal growth (vitamin K5). A water-soluble preparation of vitamin K3 (menadione) is also available for adults.
Physiological functions of vitamin K
The vitamins belonging to the K group are involved in the carboxylation of glutamate residues in proteins, to form gamma (γ)-carboxyglutamate residues (Gla-residues), typically located within Gla domains. These Gla-residues, which are usually involved in calcium binding, are essential for the function of most – if not all - known Gla-proteins 7. Vitamin K is essential for the function of several proteins involved in blood coagulation (prothrombin, also know as factor II, factors VII, IX, and X, protein C, protein S, and protein Z)8, bone metabolism (osteocalcin, periostin and matrix Gla protein), as well as vascular biology, cell growth, and apoptosis (growth-arrest-specific gene 6 protein)2,9. A poor vitamin K status is, therefore, currently regarded as a risk factor not only for bleeding, but also for increased postmenopausal bone loss and arterial calcification, especially in diabetics and in patients with chronic renal disease.
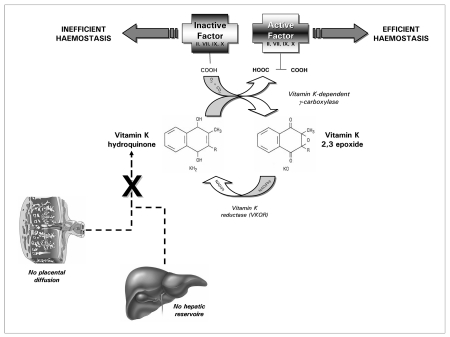
The pivotal importance of vitamin K in haemostasis arises from the fact that all vitamin K-dependent coagulation factors require γ-carboxylation of glutamic acid residues at their Gla domains to enable binding of calcium and attachment to phospholipid membranes. This enzymatic reaction is catalysed by a microsomal, vitamin K-dependent enzyme, γ-glutamyl carboxylase, which in turn is linked to a cyclic salvage pathway known as the vitamin K epoxide cycle. This carboxylation process necessarily requires a functional vitamin K cycle to produce the active vitamin K co-factor (vitamin K quinole) for the γ-carboxylase which post-translationally modifies the precursors of the vitamin K-dependent proteins (Figure 1). When the cyclic inter-conversion of vitamin K to its 2,3 epoxide is blocked, as in the case of oral anticoagulant therapy with coumarin derivatives, the net effect is the appearance in plasma of coagulation factors called PIVKA (Protein Induced by Vitamin K Absence), which are virtually non-functional for the clotting process10.
Figure 1.
Functions of vitamin K
Vitamin K deficiency
As previously described, only a very small amount of vitamin K is necessary for blood coagulation in humans. Dietary deficiency of vitamin K is, therefore, extremely rare in adults, and, when it does occur, it is usually associated with profoundly inadequate dietary intake, intestinal disorders (e.g., regional enteritis, cystic fibrosis, intestinal resection), malabsorption and, to a lesser extent, decreased production by normal flora (e.g., during the use of a broad spectrum antibiotic) and renal failure2. Vitamin K deficiency is, however, much more frequent in neonates, due to both endogenous and exogenous deficiency. The former case, which is probably less clinically significant, has been attributed to insufficient intestinal colonisation by bacteria, whereas the latter case arises from poor placental transport of the vitamin and its low concentration in breast milk. The main exogenous source of vitamin K in neonates, which is almost exclusively milk, cannot adequately compensate for deficient endogenous production, since human breast milk contains between 1 and 4 μg/L of vitamin K1 (and a much lower concentration of vitamin K2).
As in other circumstances in science and medicine, there is an apparent paradox in haemostasis in neonates in that prolonged global coagulation tests (i.e., activated partial thromboplastin time and prothrombin time) do not translate into a particular bleeding phenotype. In fact, it is now clear that the physiology of haemostasis in childhood differs considerably from that in adults11,12. Studies in humans and animals clearly indicate that coagulation factors in neonates are qualitatively similar, in terms of molecular weights and degree of glycosylation, to those in adults. The greatest difference between the two age periods is quantitative, with plasma levels of many coagulation factors being different throughout childhood from those found in adults, with some of the deficits being attributed to vitamin K deficiency.
As previously highlighted, neonates are prone to vitamin K deficiency due to the limited stores at birth and insufficient intake13. Vitamin K deficiency-related bleeding (VKDB) is defined as a bleeding disorder in which the coagulation is rapidly corrected by vitamin K supplementation. The diagnosis is suggested by an international normalised ratio =4 or a prothrombin time =4 times the control value in the presence of a normal platelet count and normal fibrinogen level. Confirmation of the diagnosis requires measurement of the specific vitamin K-dependent factors (II, VII, IX, X) whose levels are rapidly corrected by the parenteral administration of 1 mg vitamin K14. VKDB is usually classified by aetiology (idiopathic and secondary) and by the age of onset (early, classical and late)14. In idiopathic VKDB no cause other than breast-feeding can be demonstrated. In secondary VKDB there is usually an underlying cause, such as the effect of drugs that have been given to the mother or infant or a hereditary hepatobiliary/malabsorption disease (e.g., biliary atresia, a-1-antitrypsin deficiency, cystic fibrosis)15. In addition, autosomal recessive vitamin K-dependent coagulation factor deficiencies (VKCFD), due to mutations in the gene encoding for g-glutamyl carboxylase (VKCFD type I) and in the gene encoding for vitamin K epoxide reductase (VKCFD type II), have been reported8.
According to the age of onset, early VKDB presents within 24 hours of birth and is almost exclusively seen in infants of mothers taking drugs which inhibit vitamin K. These drugs include anticonvulsants (carbamazepine, phenytoin and barbiturates), antituberculosis drugs (isoniazid, rifampicin), some antibiotics (cephalosporins) and vitamin K antagonists (coumarin, warfarin). The clinical presentation is often severe with cephalic haematoma and intracranial and intra-abdominal haemorrhages16. The incidence of early VKDB in neonates of mothers taking these drugs without vitamin K supplementation varies from 6% to 12%17,18.
Classical VKDB occurs between 24 hours and 7 days of life and is associated with delayed or insufficient feeding. The clinical presentation is often mild, with bruises, gastrointestinal blood loss or bleeding from the umbilicus and puncture sites. Blood loss can, however, be significant, and intracranial haemorrhage, although rare, has been described15. Estimates of the frequency vary from 0.25% to 1.5% in older reviews19 and 0–0.44% in more recent reviews20.
Late VKDB is associated with exclusive breast-feeding. It occurs between the ages of 2 and 12 weeks. The clinical presentation is severe, with a mortality rate of 20% and intracranial haemorrhage occurring in 50%. Persistent neurological damage is frequent in survivors. In fully breast-fed infants who did not receive vitamin K at birth, the incidence is between 1/15,000 and 1/20,000. Babies with cholestasis or malabsorption syndromes are at particular risk21.
Discussion
Once the diagnosis of VKDB has been confirmed, intravenous vitamin K should be administered to correct the existing deficiency. In the presence of major bleeding, factor replacement therapy may also be required with fresh-frozen plasma or prothrombin complex concentrates15. As regards the prevention of VKDB, the best prophylactic method has been the subject of considerable debate in recent years and still remains to be completely resolved22. The dispute originated from the publication of two retrospective studies in the early 1990s, in which a possible association between vitamin K injections in neonates and the development of childhood leukaemia and other forms of cancer was suspected23,24. This alarming suspicion was, however, confuted by two large retrospective studies in the USA and Sweden, which failed to find any evidence of a relationship between childhood cancers and vitamin K injections at birth25,26. A further pooled analysis of six case-control studies, including 2,431 children diagnosed with childhood cancer and 6,338 cancer-free children, also confirmed the lack on any epidemiological association between vitamin K injections in neonates and an increased risk of leukaemia27. Although it might be concluded that there is no definitive evidence, the confirmed benefits of vitamin K prophylaxis seem to largely outweigh the hypothetical association with childhood cancer28,29.
In 2003 the American Academy of Pediatrics recommended that vitamin K1 should be given to all neonates as a single, intramuscular dose of 0.5 to 1 mg29, and this recommendation was recently reaffirmed in 200930. A similar recommendation was issued and reaffirmed in 2009 by the Canadian Paediatric Society and the Committee on Child and Adolescent Health, College of Family Physicians of Canada. Accordingly, it is recommended that vitamin K1 should be given as a single intramuscular dose of 0.5 mg (for babies weighing 1,500 g or less at birth) or 1.0 mg (for babies weighing more than 1,500 g at birth) to all neonates within the first 6 hours after birth following initial stabilisation of the baby and an appropriate opportunity for maternal (family)-baby interaction31. Several European countries are increasingly moving towards a uniform policy. Prophylaxis with 1 mg vitamin K was endorsed by the UK Department of Health in 1998, while no preference was stated for either administration route (i.e., intramuscular or oral), concluding that this is a matter for professionals and services to agree locally32. In the 2008 guidelines of the UK National Health System, it is, however, recommended that babies weighing less than 2.5 kg should be administered 400 μg/kg, whereas the dose for babies weighing more than 2.5 kg is 1 mg. It is especially important that babies at extra risk receive vitamin K via the intramuscular route. When the intramuscular route is declined by the parent, two oral doses of 2 mg should be offered instead (the first dose within 6 hours of birth, and the second between 4 – 7 days of age)33. A consensus conference of the Italian Society of Neonatology held in 2004 established that 0.5 mg of vitamin K should be administered intramuscularly at birth, followed by 25 μg/die orally from the second to the fourteenth week of life. An alternative strategy accepted by the consensus conference is the administration of 2 mg of vitamin K at birth, followed by 25 μg/die from the seventh day to the fourteenth week of life34.
It should, however, be noted that Kumar et al. found extremely high plasma K levels on day 14 of life in premature infants (<28th gestational week) who received 1 mg of vitamin K intramuscularly shortly after birth35. In another study, by Costakos et al., preterm neonates who were given 0.5 to 1 mg vitamin K prophylaxis also showed vitamin K levels that were 1,900 to 2,600 times higher (2 days afterwards) and 550 to 600 times higher (10 days afterwards) than normal adult plasma values (0.5 ng/mL)36.
Conclusion
The haemostatic system is not fully mature until 3 to 6 months of age. It is, therefore, essential to acknowledge that the differences observed between adults and infants are probably physiological and do not always reflect an underlying pathological condition. Several clinical observations support the hypothesis that children have natural protective mechanisms that justify the existence of these broad variations, since they have both an increased capacity to inhibit thrombin and a decreased capacity to generate it11,12. Despite the presence of specific homeostatic mechanisms equilibrating the haemostatic balance in neonates and infants, the concentration and activity of vitamin K-dependent procoagulant factors might be dramatically reduced due to insufficient storage and poor transfer of vitamin K across the placental barrier. Although haemostasis might still be "appropriate" and the vast majority of neonates would not bleed, it is now universally accepted that all infants should be given prophylaxis with vitamin K at birth in order to prevent classical and late VKDB37,38. Although both intramuscular and oral administration of 1 mg of vitamin K protect against classical VKDB, a single oral dose does not protect all infants against late VKDB. The intramuscular route of administration of vitamin K prophylaxis has, therefore, been universally adopted, but oral administration should be continued subsequently, according to one of the available guidelines (Table I). This approach seems to effective at preventing VKDB, but also has some drawbacks, the foremost being the fact that available commercial products cost a hundred times more than the basic cost of their one active ingredient, so that broadening this policy to developing countries might be challenging22.
Table I.
Summary of the available recommendations about vitamin K administration in neonates.
| Organisation | Vitamin K dosage |
|---|---|
| American Academy of Pediatrics | Single intramuscular dose of 0.5 to 1 mg |
| Canadian Paediatric Society, Committee on Child and Adolescent Health, College of Family Physicians of Canada. | Single intramuscular dose of 0.5 mg (birthweight =1,500 g) or 1.0 mg (birthweight >1,500 g) within the first 6 h after birth |
| UK Department of Health in 1998 | Single intramuscular or oral dose of 400 μg/kg (babies <2.5 kg) or 1 mg (babies >2.5 kg) |
| Italian Society of Neonatology (two alternatives) | - Single intramuscular dose of 0.5 mg at birth, followed by 25 μg/die orally from the 2nd to the 14th week. |
| - Single intramuscular dose of 2 mg at birth, followed by 25 μg/die from the 7th day to the 14th week |
References
- 1.Henrik D. The antihaemorrhagic vitamin of the chick. Biochem J. 1935;29:1273–85. doi: 10.1042/bj0291273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shearer MJ. Vitamin K in parenteral nutrition. Gastroenterology. 2009;137 (5 Suppl):S105–18. doi: 10.1053/j.gastro.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 3.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–47. [PubMed] [Google Scholar]
- 4.Otten JJ, Hellwig JP, Meyers LD, editors. US Government Food and Nutrition Information. The Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. National Academies Press; Washington, DC: 2008. [Google Scholar]
- 5.Scientific Committee for Food. Nutrient and energy intakes for the European Community Reports of the Scientific Committee for Food, Thirty First Series. European Commission; Luxembourg: 1993. [Last accessed: 05 February 2010]. http://ec.europa.eu/food/fs/sc/scf/out89.pdf. [Google Scholar]
- 6.Food and Nutrition Board. Institute of Medicine: Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 7.Furie B, Bouchard BA, Furie BC. Vitamin K-dependent biosynthesis of gamma-carboxyglutamic acid. Blood. 1999;93:1798–808. [PubMed] [Google Scholar]
- 8.Brenner B, Kuperman AA, Watzka M, Oldenburg J. Vitamin K-dependent coagulation factors deficiency. Semin Thromb Hemost. 2009;35:439–46. doi: 10.1055/s-0029-1225766. [DOI] [PubMed] [Google Scholar]
- 9.Cranenburg EC, Schurgers LJ, Vermeer C. Vitamin K: the coagulation vitamin that became omnipotent. Thromb Haemost. 2007;98:120–5. [PubMed] [Google Scholar]
- 10.Lippi G, Franchini M, Favaloro EJ. Pharmacogenetics of vitamin K antagonists: useful or hype? Clin Chem Lab Med. 2009;47:503–15. doi: 10.1515/CCLM.2009.140. [DOI] [PubMed] [Google Scholar]
- 11.Lippi G, Salvagno GL, Rugolotto S, et al. Routine coagulation tests in newborn and young infants. J Thromb Thrombolysis. 2007;24:153–5. doi: 10.1007/s11239-007-0046-4. [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, Franchini M, Montagnana M, Guidi GC. Coagulation testing in pediatric patients: the young are not just miniature adults. Semin Thromb Hemost. 2007;33:816–20. doi: 10.1055/s-2007-1000373. [DOI] [PubMed] [Google Scholar]
- 13.Van Winckel M, De Bruyne R, Van de Velde S, Van Biervliet S. Vitamin K, an update for the paediatrician. Eur J Pediatr. 2009;168:127–34. doi: 10.1007/s00431-008-0856-1. [DOI] [PubMed] [Google Scholar]
- 14.Sutor AH, von Kries R, Cornelissen EAM, et al. ISTH Pediatric/Perinatal Subcommittee International Society on Thrombosis and Haemostasis. Vitamin K deficiency bleeding (VKDB) in infancy. Thromb Haemost. 1999;81:456–61. [PubMed] [Google Scholar]
- 15.Shearer MJ. Vitamin K deficiency bleeding (VKDB) Blood Rev. 2009;23:49–59. doi: 10.1016/j.blre.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Pichler E, Pichler L. The neonatal coagulation system and the vitamin K deficiency bleeding – a mini review. Wien Med Wochenschr. 2008;158:385–95. doi: 10.1007/s10354-008-0538-7. [DOI] [PubMed] [Google Scholar]
- 17.Deblay MF, Vert P, Andre M, Marchal F. Transplacental vitamin K prevents haemorrhagic disease of infant of epileptic mother. Lancet. 1982;I:1242. doi: 10.1016/s0140-6736(82)92371-6. [DOI] [PubMed] [Google Scholar]
- 18.Mountain KR, Hirsh J, Gallus AS. Neonatal coagulation defect due to anticonvulsant drug treatment in pregnancy. Lancet. 1970;I:265–8. doi: 10.1016/s0140-6736(70)90636-7. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Pediatrics, Committee on Nutrition. Vitamin K compounds and their water soluble analogues: use in therapy and prophylaxis in pediatrics. Pediatrics. 1961;28:501–7. [Google Scholar]
- 20.von Kries R. Vitamin K prophylaxis – a useful public health measure? Paediatr Perinat Epidemiol. 1992;6:7–13. doi: 10.1111/j.1365-3016.1992.tb00736.x. [DOI] [PubMed] [Google Scholar]
- 21.Autret-Leca E, Jonville-Béra AP. Vitamin K in neonates. Paediatr Drugs. 2001;3:1–8. doi: 10.2165/00128072-200103010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Hey E. Vitamin K – what, why, and when. Arch Child fetal Neonatal. 2003;88:F80–3. doi: 10.1136/fn.88.2.F80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golding J, Paterson M, Kinlen LJ. Factors associated with childhood cancer in a national cohort study. Br J Cancer. 1990;62:304–8. doi: 10.1038/bjc.1990.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golding J, Greenwood R, Birmingham K, Mott M. Childhood cancer, intramuscular vitamin K, and pethidine given during labour. BMJ. 1992;305:341–6. doi: 10.1136/bmj.305.6849.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klebanoff MA, Read JS, Mills JL, Shiono PH. The risk of childhood cancer after neonatal exposure to vitamin K. N Engl J Med. 1993;329:905–8. doi: 10.1056/NEJM199309233291301. [DOI] [PubMed] [Google Scholar]
- 26.Ekelund H, Finnstrom O, Gunnarskog J, Kallen B, Larsson Y. Administration of vitamin K to newborn infants and childhood cancer. BMJ. 1993;307:89–91. doi: 10.1136/bmj.307.6896.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roman E, Fear NT, Ansell P, et al. Vitamin K and childhood cancer: analysis of individual patient data from six case-control studies. Br J Cancer. 2002;86:63–9. doi: 10.1038/sj.bjc.6600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross JA, Davies SM. Vitamin K prophylaxis and childhood cancer. Med Pediatr Oncol. 2000;34:434–7. doi: 10.1002/(sici)1096-911x(200006)34:6<434::aid-mpo11>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.American Academy of Pediatrics Committee on Fetus and Newborn. Controversies concerning vitamin K and the newborn. Pediatrics. 2003;112:191–2. [PubMed] [Google Scholar]
- 30.American Academy of Pediatrics. Policy Statement-AAP publications retired and reaffirmed. Pediatrics. 2009;124:845. doi: 10.1542/peds.2009-1415. [DOI] [PubMed] [Google Scholar]
- 31.Routine administration of vitamin K to newborns. Joint position paper of the Canadian Paediatric Society and the Committee on Child and Adolescent Health of the College of Family Physicians of Canada. Can Fam Physician. 1998;44:1083–90. [PMC free article] [PubMed] [Google Scholar]
- 32.Department of Health. Vitamin K for newborn babies, PL/CMO(98)3. London: HMSO; 1998. [Google Scholar]
- 33.National Healthcare System. Administration of Vitamin K to Neonates Guidelines. 2008. [Last accessed: 5 February 2010]. Available at: http://www.dvh.nhs.uk/downloads/documents/NKCRKENUII_Vitamin_K_v2.pdf.
- 34.Profilassi con la vitamina K dell'emorragia da deficit di vitamina K. Acta Neonatol Pediatr; Consensus Conference; 2 Aprile 2004; Siena. 2004. pp. 375–7. [Google Scholar]
- 35.Kumar D, Greer FR, Super DM, Suttie JW, Moore JJ. Vitamin K status of premature infants: implications for current recommendations. Pediatrics. 2001;108:1117–2. doi: 10.1542/peds.108.5.1117. [DOI] [PubMed] [Google Scholar]
- 36.Costakos DT, Greer FR, Love LA, Dahlen LR, Sutte JW. Vitamin K prophylaxis for premature infants: 1 mg versus 0.5 mg. Am J Perinatol. 2003;20:485–90. doi: 10.1055/s-2003-45384. [DOI] [PubMed] [Google Scholar]
- 37.Puckett RM, Offringa M. Prophylactic vitamin K for vitamin K deficiency bleeding in neonates. Cochrane Database Syst Rev. 2000;4:CD002776. doi: 10.1002/14651858.CD002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Winckel M, De Bruyne R, Van de Velde S, Van Biervliet S. Vitamin K, an update for the paediatrician. Eur J Pediatr. 2009;168:127–34. doi: 10.1007/s00431-008-0856-1. [DOI] [PubMed] [Google Scholar]