Abstract
Background
The in vivo recovery of recombinant factor IX (rFIX) is reported to be lower than that of plasma-derived products, with potential clinical implications for dosing. In clinical practice, a conversion (augmentation) factor is suggested to calculate the necessary doses of rFIX. The aim of this study was to assess the range of values for the conversion factor in usual clinical practice in Italy.
Materials and methods
The study was questionnaire-based and proposed to all Italian Haemophilia centres treating patients with haemophilia B. Age, weight, dosage used in the last effective infusion, treatment regimen (prophylaxis versus on-demand), human immunodeficiency virus (HIV) and hepatitis C virus (HCV) status, and years of previous therapy with rFIX were recorded for patients with severe haemophilia B treated with rFIX. Mean, standard deviation, median and range were calculated for demographic and treatment data for the overall population and for subgroups. The conversion factor for the theoretical dosage of 40 IU/Kg was calculated.
Results
Among 207 patients with severe haemophilia B being followed in 24 centres, 138 (66.7%) were being treated with rFIX. The sample of 207 patients represents 83.1% of the population of Italian patients with severe haemophilia B. The age range of the studied patients was 0–72 years (mean, 24 years) and the weight range was 3–108 kg (mean, 60 kg). Nineteen patients (14.4%) were positive for HIV and 51 (42.9%) were positive for HCV. The mean dosage of rFIX was 44 IU/Kg, with no significant difference between those receiving the product as prophylaxis or on-demand. A reduction in dosage was observed with increasing age (0.23 IU/kg/year). The mean value for the conversion factor was 1.10 ± 0.36 (median 1.00, range 0.51–2.08), when estimated for the whole population. No effect of HIV and HCV status was found on the dose prescribed. No evident correlation was found with the underlying genetic mutation.
Discussion
We found that dosing of rFIX in clinical practice is very close to that of plasma-derived FIX concentrates. As a consequence, dosing in the non-surgical setting should be started using the same criteria as those for plasma-derived FIX and treatment effectiveness verified on a clinical basis rather than relying on in vivo recovery assessments.
Keywords: factor IX, haemophilia B, recombinant FIX, recovery
Introduction
Haemophilia B is an X-linked bleeding disorder caused by factor IX (FIX) deficiency. Its incidence is approximately 1 in 25,000 males1,2. The pathology is distinguished into three levels of severity, based on the levels of activity of the circulating FIX: severe (FIX:C below 1%), moderate (FIX:C from 1 to below 5%) and mild haemophilia (FIX:C from 5 to the lower end of normal range). In the calendar year 2007, the Italian National Database included 638 cases of haemophilia B (about 17% of all haemophiliacs), 249 (39%) of whom had severe disease, 153 (24%) a moderate form, and 236 (37%) mild disease3.
The treatment of bleeding episodes in patients with haemophilia B is based on replacement of the deficient factor. A large range of plasma-derived FIX concentrates is now available. High-purity concentrates have a lower thrombogenic risk4, and all products now undergo viral attenuation methods during manufacture, which leads to greater safety in terms of viral transmission5. These plasma-derived products are not, however, completely free of the risk of transmission of non-enveloped viruses, such as hepatitis A virus and parvovirus, or blood-borne prions associated with spongiform encephalopathy6,7. The cloning of the human F9 gene in 19828,9 enabled the manufacture of recombinant (r) FIX10–12: the first product became commercially available in the late 1990s, following clinical studies that had proven its safety and efficacy13–14.
Based on pharmacokinetic parameters, the comparison between plasma-derived and rFIX demonstrated similar half-lives and mean residence times, but showed a difference in in vivo recovery (post-infusional levels of FIX related to the administered dose), resulting in the need for higher doses of rFIX in order to obtain an equal concentration of circulating plasma factor15–17. Furthermore, a wide inter-individual variability emerged from pharmacokinetic studies, with in vivo recovery being lower in patients under 15 years old14–16.
The nationwide-adopted guidelines of the Italian Association of Haemophilia Centres18 make the following suggestions about FIX treatment of bleeding episodes in patients with haemophilia B: mild or moderate haemarthrosis or haematoma should be treated using 20 to 40 IU/kg of body weight; severe haemarthrosis or haematoma, external bleeding resulting in acute anaemia, or trauma of moderate severity should be treated using 40 to 60 IU/kg; head trauma, brain haemorrhage, or pre-surgical haemostasis requires a dose of 50 to 100 IU/kg. These recommendations are issued assuming that each unit per kilogram of body weight of infused FIX will raise the plasma levels by approximately 1%. Given that in vivo recovery of rFIX is approximately 0.8 in adults and 0.7 in children under 15 years old, the World Federation of Hemophilia guidelines recommend using one of the following formulae to dose rFIX19:
- dose to be administered (IU/kg) = expected plasma level (IU/mL)/0.8 or expected plasma level (IU/mL) x 1.25 (adults);
- dose to be administered (IU/kg) = expected plasma level (IU/mL)/0.7 or expected plasma level (IU/mL) x 1.43 (children).
The only available population-wide assessment of the extent of adoption of this recommendation is the Canadian study by Poon et al.16, which assessed dosing of rFIX when most of the Canadian patients were switched from plasma-derived FIX to rFIX. The authors assessed in vivo plasma recovery of FIX, and calculated its mean population value and difference from the recovery of plasma-derived FIX.
The aim of our study was to assess the range of values for the conversion factor in usual clinical practice in Italy.
Materials and methods
The study was questionnaire-based and proposed to all Italian Haemophilia Centres reporting records about patients with haemophilia B to the Italian Database of Haemophilia. A presentation letter was prepared, explaining the aim of the study and the unresolved issues about the choice of dosage for treating patients with severe haemophilia B with rFIX. Participation in the study was voluntary and no funds were available to cover any related expense.
Data collection
A mock table for data collection was prepared. The information requested was: the name of the centre, the total number of patients with severe haemophilia B treated with rFIX and a progressive number given to each patient by the centre, the main data (age or date of birth, body weight in kg, last effective dose administered in total IU) and additional optional data (treatment regimen - prophylaxis versus on demand, years of previous therapy with rFIX, positivity or negativity for HIV and HCV and genetic characterisation - mutated exon, specific mutation). As an alternative, we asked for consent to access and use the data (HIV, HCV, genetics) in the National Database, through the patients' identification code. The above information was requested for all patients with severe haemophilia B treated with rFIX.
The presentation letter and the table for data collection were e-mailed to the Heads of each Haemophilia Centre. The filled in records received from the centres were pooled in a single database.
Data analysis
Descriptive analysis
Birth dates were converted into ages and doses administered were calculated as IU/kg of body weight; age at the beginning of treatment was calculated from years of previous therapy. The following data were also described: number of patients for whom years of previous treatment with rFIX were known; number of patients who started being treated with rFIX before 6 years of age.
The distribution of dose administered (as IU/kg) was preliminarily investigated by box-plot analysis to search for outliers.
Mean, standard deviation, median and range of age, body weight, dose administered (total IU and IU/kg) and years of previous treatment were calculated. Demographic and treatment data were calculated for the overall population and for the following subgroups: population ≤15 years old, population >15 years old, patients treated on demand, patients on prophylaxis, HIV-positive and -negative patients, HCV-positive and -negative patients, and co-infected patients.
Statistical analysis
The conversion factor was calculated referring to the theoretical dosage of 40 IU/kg, which is the dose suggested by guidelines for the prophylaxis regimen and is the mean dosage indicated for the treatment of mild to severe bleeding episodes18.
The formula used to calculate the conversion factor is the following: 1 + [(administered dose – theoretical dose)/theoretical dose].
All doses were expressed as IU/kg.
Mean, standard deviation, median and range of the conversion factor for the dosage of 40 IU/kg were calculated for the overall population and for the following subgroups: population ≤15 years old, population >15 years old, patients treated on demand, patients on prophylaxis, HIV-positive and -negative patients, HCV-positive and -negative patients, and co-infected patients.
Linear regression analysis was used to test the effect of age and weight on dose administered (IU/kg). Univariable and multivariable analysis of variance was used to asses the effect on dose administered (IU/kg) of treatment regimen (prophylaxis/on demand), HIV or HCV status. A sensitivity analysis was performed, dividing the overall population in two groups: patients who had received a dose ≥40 IU/kg and patients who had received a dose <40 IU/kg. Mean, standard deviation, median and range of conversion factor were calculated for the first group related to a dosage of 40 IU/kg and for the second group related to a dosage of 20 IU/kg.
The role of the underlying genetic mutations was investigated by searching for clusters of specific mutations in the lowest and the highest dose tiers of the population of patients.
All the calculations were performed with Stata version 9.1 (Statacorp, College Station TX, USA).
Results
Database composition and coverage
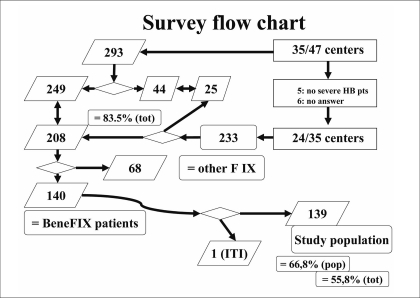
The National Haemophilia Database contains data on 293 patients affected by severe haemophilia B. Forty-four of these patients are registered in more than one centre, which makes a total population of 249 univocal patients. The patients are distributed in 35 out of 47 Italian Haemophilia Centres.
We received filled in questionnaires from 29 of the 35 centres.
Five centres were not treating patients with severe haemophilia B with rFIX. The other 24 centres sent information about 144 patients, 139 of whom univocal. One of these patients was treated with a protocol of immune-tolerance induction and was not included in the total count. The data included in the study was, therefore, derived from 138 patients.
As assessed by the National Database3, the total population with severe haemophilia B treated in the 29 centres that adhered to the study comprises 233 patients, 207 of whom are univocal cases.
The database of the study, therefore, contained data on 83.1% of the patients affected by severe haemophilia B in Italy (207/249) and the patients treated with rFIX account for 66.7% of all patients with severe haemophilia B treated in these centres (138/207) and 55.4% of the whole population of patients with severe haemophilia B in Italy (138/249) (Figure 1).
Figure 1.
Survey flow chart.
Data analysis
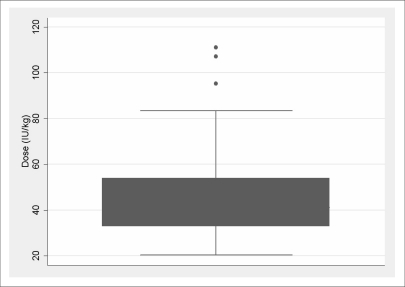
As a first step, the box-plot for administered dose (IU/kg) in the overall population (Figure 2) showed three outliers (95.24 IU/kg, 107.14 IU/kg, 111.11 IU/kg): these three patients were excluded from the subsequent analysis, so that the analysed population consisted of 135 patients.
Figure 2.
Box-plot of dose (IU/kg).
Demographic and treatment data are shown in Tables I and II. Years of previous treatment were known for 82 patients (60.7%): 25 of these (18.5% of the overall study population) started being treated with rFIX before the age of 6 years old. Furthermore, four other patients (without specified years of treatment) were less than 6 years old at study enrolment, so that the whole population treated before 6 years of age amounted to 29 patients (21.5 % of the overall population) treated for 103 patient-years (4±2 each one).
Table I.
Demographic data.
| Age | Weight | Years of treatment | |||||
|---|---|---|---|---|---|---|---|
| Number of patients* | Mean (SD) | Median (range) | Mean (SD) | Median (range) | Mean (SD) | Median (range) | |
| Overall population | 135 [82] | 23.9 (15.2) | 22.0 (0–72) | 59.9 (22.3) | 65.0 (3.2–108) | 6.0 (2.6) | 7 (0–11) |
| Population =15 years | 47 [30] | 8.9 (4.4) | 9 (0–15) | 36.9 (18.1) | 39.0 (3.2–78) | 5.8 (3.1) | 6.5 (0–11) |
| Population >15 years | 88 [52] | 32 (12.6) | 29 (16–72) | 72.1 (12.6) | 71.5 (46–108) | 6.1 (12.9) | 7 (1–10) |
| Prophylaxis | 57 [39] | 19.1 (12.6) | 15.0 (3–56) | 54.8 (21.3) | 59.0 (15–92) | 5.8 (2.6) | 6 (1–11) |
| On-demand | 74 [43] | 27.9 (16.1) | 28.0 (0–72) | 64.6 (22.3) | 69.5 (3.2–108) | 6.1 (2.5) | 7 (0–10) |
| HIV+ | 19 [11] | 32.6 (13.0) | 33 (12–51) | 68.7 (15.5) | 72 (40–95) | 6.4 (1.9) | 7 (4–9) |
| HIV− | 110 [65] | 22.9 (15.5) | 21.0 (0–72) | 58.6 (23.0) | 62.0 (3–108) | 6.2 (2.6) | 7 (1–11) |
| HCV+ | 51 [28] | 35.2 (13.3) | 33 (9–71) | 72 (12.6) | 72 (40–104) | 6.1 (2.0) | 6.5 (1–10) |
| HCV− | 65 [35] | 14.6 (8.5) | 14.0 (0–37) | 51.0 (24.1) | 53.0 (3–108) | 5.9 (3.1) | 7 (1–11) |
| Co-infected | 17 [9] | 30.6 (12.2) | 33 (12–51) | 68.8 (16) | 72.0 (40–95) | 6.2 (2.0) | 7 (4–9) |
Numbers in square brackets indicate patients with years of treatment data available.
Table II.
Treatment data.
| Dose administered (IU) | Dose administered (IU/kg) | ||||
|---|---|---|---|---|---|
| Number of patients | Mean (SD) | Median (range) | Mean (SD) | Median (range) | |
| Overall population | 135 | 2,566.7 (1,272.3) | 2,000 (250–7,000) | 44.0 (14.6) | 40 (20.0–83.3) |
| Population =15 years | 47 | 1,702.1 (1,168.7) | 1,250 (250–6,000) | 47.6 (16.9) | 44.4 (20.4–83.3) |
| Population >15 years | 88 | 3,028.4 (1,073.1) | 3,000 (1,500–7,000) | 42.1 (12.9) | 40.0 (20.2–72.7) |
| Prophylaxis | 57 | 2,368.4 (1,216.6) | 2,000 (500–6,000) | 44.5 (14.9) | 40 (20.0–80.0) |
| On-demand | 74 | 2,729.7 (1,318.8) | 3,000 (250–7,000) | 43.3 (14.7) | 40 (20.3–83.3) |
| HIV+ | 19 | 2,737.8 (991.2) | 2,500 (1,000–5,000) | 39.9 (11.3) | 40 (20.4–63.6) |
| HIV− | 110 | 2,540.9 (1,330.9) | 2,000 (250–7,000) | 44.5 (15.1) | 40 (20.3–83.3) |
| HCV+ | 51 | 3,019.6 (932.5) | 3,000 (1,000–6,000) | 42.2 (11.9) | 40 (20.4–83.3) |
| HCV− | 65 | 2,407.7 (1,515.2) | 2,000 (250–7,000) | 47.8 (16.1) | 47.1 (20.3–80.0) |
| Co-infected | 17 | 2,823.5 (1,014.6) | 3,000 (1,000–5,000) | 41.1 (11.3) | 40 (20.4–63.6) |
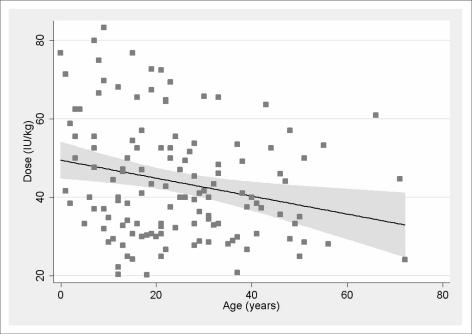
The linear regression with dose (IU/kg) as the dependent variable and patient's age as the independent variable is shown in Figure 3. The regression was statistically significant (p = 0.006, R2 = 0.057).
Figure 3.
Effect of patients' age.
The estimated slope coefficient was −0.23 (95% C.I. −0.39 ~ −0.07), indicating a reduction of 1 IU/kg every 4.3 years of age.
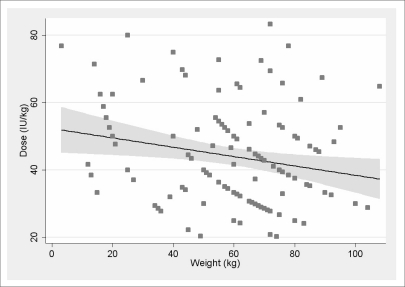
The linear regression with dose/kg as the dependent variable and the patient's weight as the independent variable is shown in Figure 4. The regression was statistically significant (p = 0.013, R2 = 0.045).
Figure 4.
Effect of patients' weight.
The estimated slope coefficient was −0.14 (95% C.I. −0.25 ~ −0.03), indicating a reduction of 1 IU/kg every 7 kg of body weight.
At univariable ANOVA analysis only HCV resulted significantly associated with difference in administered dose (IU/kg). When adjusting for age and weight at multivariable ANOVA, HCV lost its significance.
Conversion factor
The mean conversion factor for the theoretical dosage of 40 IU/kg was 1.10 (standard deviation 0.36, median 1, range 0.51–2.08) in the overall population. Table III shows the conversion factors for the population ≤15 years old, the population >15 years old, patients receiving on demand treatment, patients on prophylaxis, the HIV-positive and -negative populations, the HCV-positive and -negative populations, and those patients who were co-infected with HIV and HCV.
Table III.
Conversion factor for a theoretical dosage of 40 IU/kg.
| Conversion factor for a theoretical dosage of 40 IU/kg | |||
|---|---|---|---|
| Number of patients | Mean (SD) | Median (range) | |
| Overall population | 135 | 1.10 (0.36) | 1.00 (0.51–2.08) |
| Population =15 years | 47 | 1.19 (0.42) | 1.11 (0.51–2.08) |
| Population >15 years | 88 | 1.05 (0.32) | 1.00 (0.51–1.82) |
| Prophylaxis | 57 | 1.11 (0.37) | 1.00 (0.51–2.00) |
| On-demand | 74 | 1.08 (0.37) | 1.00 (0.51–2.08) |
| HIV+ | 19 | 1.00 (0.28) | 1.00 (0.51–1.59) |
| HIV− | 110 | 1.11 (0.37) | 1.00 (0.51–2.08) |
| HCV+ | 51 | 1.05 (0.29) | 1.00 (0.51–2.08) |
| HCV− | 65 | 1.19 (0.40) | 1.18 (0.51–2.00) |
| Co-infected | 17 | 1.02 (0.28) | 1.00 (0.51–1.59) |
Sensitivity analysis
Seventy-six (56.3%) patients had received a dose ≥40 IU/kg, whereas 59 (43.7%) patients had received a dose <40 IU/kg. Table IV shows the conversion factor for both groups and details about calculations. The mean conversion factor for the dosage of 40 IU/kg for the former group was 1.35 ± 0.29 (range, 1.00–2.08; 95% CI, 0.78–1.92); the mean conversion factor for the dosage of 20 IU/kg for the latter group was 1.56 ± 0.24 (range, 1.01–1.97; 95% CI, 1.08–2.03). Consideration of the genetic mutations responsible for the FIX deficiency did not provide meaningful information for this study: no correlation was found between the entity of the dose (IU/kg) administered to treat the bleeding episode and the patient's genetic mutation.
Table IV.
Sensitivity analysis.
| Dose IU/Kg | ≥40 (Conversion factor for a theoretical dosage of 40 IU/kg) | <40 (Conversion factor for a theoretical dosage of 20 IU/kg) |
|---|---|---|
| Patients (%) | 76 (56.3%) | 59 (43.7%) |
| Mean dose±SD | 53.9±11.6 | 31.2±4.9 |
| Mean conversion factor (95% CI) | 1.35 (0.78–1.92) | 1.56 (1.08–2.03) |
| Median of conversion factor (range) | 1.31 (1.00–2.08) | 1.54 (1.01–1.97) |
Discussion and conclusions
We analysed data from 135 patients with severe haemophilia B treated with rFIX, 57 of whom were on prophylaxis. The mean administered dose was 44 ± 15 IU/kg (median 40 IU/kg; range, 20–83 IU/kg). The mean conversion factor used in clinical practice to dose rFIX was 1.10 ± 0.36.
The strength of our results is that they are based on a representative sample of the Italian population of patients with haemophilia B and on clinical data collected trying to minimise bias; as a trade-off, its major limit lies in the uncertainty deriving from the lack of some additional information about the severity of bleeding and the targeted FIX plasma levels. In fact, in our survey, we declared that we were interested in knowing the last effective administered dose, meaning a dose that had healed the patient's symptoms. We did not ask about the severity of the treated bleeding episode, as this information would have been difficult to standardise, nor did we ask about the target plasma level of FIX that it was intended to reach. In fact, this was a retrospective survey based on data recorded on a clinical practice base. We did not expect that data about severity of bleeding episodes and targeted FIX plasma level had been routinely recorded on standard clinical records in a homogeneous way for the entire population. We, therefore, assumed that asking the participant physicians to detail the target level they had wanted to achieve would have lead them to post-hoc recalculation aiming to stick to the commonly agreed conversion factor and guidelines. Furthermore, we assumed that the choice of the dosage to be administered is more commonly based on a comprehensive evaluation of a patient's treatment history than on a formal calculation, and that asking for the theoretical sought effect would have biased the survey. We considered that getting the information needed to stratify the prescribed dosages directly from the participating physicians would have produced an advantage inferior to the risk of any bias introduced.
On the other hand, it was not plausible to consider that all patients who were prescribed doses lower than 40 IU/kg had been treated targeting the post-infusion FIX as 40 IU/mL. The empirical way we chose to get a better approximation of the real clinical use of a conversion factor for prescribing rFIX was by assuming that a dosage of 20 IU/kg would have been prescribed to every patient who actually received a dose <40 IU/kg. We then recalculated the mean conversion factors for the two resulting populations (patients who were administered a dosage ≥40 IU/kg). The results showed a mean dose of 54 IU/kg for patients who had been administered ≥40 IU/kg (76 patients, 56.3%) and 31 IU/kg for patients who had been administered <40 IU/kg (59 patients, 43.7%). The conversion factors were 1.35±0.29 and 1.56±0.24, respectively. On the other hand, it should be noted that this was a very conservative choice; in fact, it is unlikely that 43.7% of the bleeding episodes were so mild to require the prescription of a dose as low as 20 IU/kg of FIX. More likely the careful observation of the clinical and therapeutic history of some patients made their physicians safely prescribe a low, but still effective, dose when treating moderately severe bleeding events.
We can safely conclude that the mean values of conversion factor used in clinical practice are distributed between 1.10 and 1.56 and that the mean dosage prescribed to the whole population is approximately 44 IU/kg, which is very close to the 40 IU/kg suggested by national and international guidelines for FIX replacement therapy, both for prophylaxis and on demand treatment of bleeding episodes of moderate severity.
Two secondary results of our study deserve to be mentioned: first, the subgroup analysis did not show a correlation between HIV status, HCV status or genetic mutations and the dose needed to treat the bleeding episodes effectively; second, we registered the treatment of 29 patients under 6 years old, which confirms the wide extent of the off-label use of rFIX in small children. In fact, the use of rFIX is not licensed for children under 6 years of age, even if, in a prospective clinical study, Monahan et al.20 described the safety and efficacy of rFIX for prophylaxis in patients in this age range.
Our data are at variance with others emphasising the lower recovery obtained with rFIX as compared with plasma-derived FIX14–18. These data are usually cited to anticipate an increase in FIX usage when the recombinant factor is preferred. The results we found in the Italian population do not support this concept, since the increase is not above 10%. This fits very well with our estimated mean for the conversion factor (1.1). Our clinical results are in agreement with a recovery evaluation by Martorell et al.21 in which the mean recovery value was 0.98±0.19 IU/dL per IU/kg (32 determinations from 23 patients with haemophilia B).
As a consequence, the role of the laboratory can be reassessed: optimising the dosage of rFIX to achieve the same recovery as that after the use of plasma-derived FIX may not be necessary in order to obtain an effective clinical result; on the contrary, considering the wide variability observed in clinical practice, evaluation of the single patient's pharmacokinetic parameters could be useful for optimising the regimen and the interval of administrations for patients with incomplete or insufficient responses.
In conclusion, in a large and representative population of treated patients with haemophilia B, we found that dosing of rFIX in clinical practice is very close to that of plasma-derived FIX concentrates. As a consequence, our advice is to start dosing in the non-surgical setting using the same criteria as for plasma-derived FIX and to verify treatment effectiveness on a clinical basis rather than relying on in vivo recovery assessments. As far as regards the long-term effectiveness of replacement therapy in preventing joint arthropathy in patients with haemophilia B, long-term follow-up data are needed to define both clinically and pharmacokinetically driven protocols. In the meanwhile, we showed that when clinical efficacy is considered, rather than laboratory data, rFIX behaves very similarly to plasma-derived FIX.
Appendix 1: Full list of authors
Laura Contino (Alessandria), Arianna Accorsi (Arezzo), Cosimo Ettorre (Bari), Francesco Antonio Scaraggi (Bari), Giuseppina Rodorigo, Lelia Valdrè (Bologna), Roberto Targhetta (Cagliari), Giuseppe Tagariello, Paolo Radossi (Castelfranco Veneto), Gaetano Muleo (Catanzaro), Chiara Biasioli (Cesena), Massimo Morfini (Firenze), Angelo Claudio Molinari (Genova), Guglielmo Mariani (L'Aquila), Maria Teresa Carloni, Isabella Cantori (Macerata), Pier Mannuccio Mannucci (Milano), Giovanni Di Minno, Antonio Coppola (Napoli), Michele Schiavulli (Napoli), Ezio Zanon (Padova), Annarita Tagliaferri, Franca Rivolta (Parma), Emanuela Marchesini, Maura Marcucci (Perugia), Alfredo Dragani (Pescara), Marco D'Incà (Reggio Emilia), Matteo Luciani (Roma, Ospedale Bambino Gesù), Maria Gabriella Mazzucconi, Cristina Santoro (Roma, Policlinico Umberto I), Piercarla Schinco (Torino), Gina Rossetti (Trento), Giovanni Barillari (Udine), Giorgio Gandini (Verona), Giancarlo Castaman (Vicenza).
References
- 1.Soucie JM, Jackson D, Evatt B. Occurrence of hemophilia in the United States: the Hemophilia Surveillance System Project Invest. Am J Hematol. 1998;59:288–94. doi: 10.1002/(sici)1096-8652(199812)59:4<288::aid-ajh4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 2.Iorio A, Oliovecchio E, Morfini M, et al. on behalf the Association of Italian Haemophilia Centres Directors. Report from the Italian National Haemophilia Database. Objectives, Methodology and Data Analysis as at December 2006. Haemophilia. 2008;14:444–53. doi: 10.1111/j.1365-2516.2008.01679.x. [DOI] [PubMed] [Google Scholar]
- 3.National haemophilia registry data summary. 2007. [Accessed on line on 15/01/2010]. Available at: http://www.aiceonline.it/emocard/2007/Hemophilia%20B.pdf.
- 4.Shapiro AD, White GC, II, Kim HC, et al. Efficacy and safety of monoclonal antibody purified factor IX concentrate in haemophilia B patients undergoing surgical procedures. Haemophilia. 1997;3:247–53. doi: 10.1046/j.1365-2516.1997.00110.x. [DOI] [PubMed] [Google Scholar]
- 5.Seitz R, Dodt J. Virus safety of prothrombin complex concentrates and factor IX concentrates. Thromb Res. 1999;95 (4 Suppl 1):S19–23. doi: 10.1016/s0049-3848(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 6.Evatt BL, Farrugia A, Shapiro AD, Wilde JT. Haemophilia 2002: emerging risks of treatment. Haemophilia. 2002;8:221–9. doi: 10.1046/j.1365-2516.2002.00612.x. [DOI] [PubMed] [Google Scholar]
- 7.Hill FGH, Zaman A, Connor N, et al. Extent of exposure to and risk stratification of UK patients reated with plasma concentrates with contribution from a donor who developed vCJD. [abstract] J Thromb Haemost. (Suppl 2) 2009;7:OC-MO-033. [Google Scholar]
- 8.Kurachi k, Davie EW. Isolation and characterization of a cDNA coding for human factor IX. Proc Acad Sci USA. 1982;79:6461–4. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choo GH, Gould KG, Rees DJ, Brownlee GC. Molecular cloning of the gene for human anti-haemophilic factor IX. Nature. 1982;299:178–80. doi: 10.1038/299178a0. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman RJ, Wasley LC, Furie BC, et al. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986;261:9622–8. [PubMed] [Google Scholar]
- 11.Harrison S, Adamson S, Bonam D, et al. The manufacturing process for recombinant factor IX. Semin Hematol. 1998;35:4–10. [PubMed] [Google Scholar]
- 12.Bond M, Jankowski M, Patel H, et al. Biochemical characterization of recombinant factor IX. Semin Hematol. 1998;35:11–7. [PubMed] [Google Scholar]
- 13.White G, Shapiro A, Ragni M, et al. Clinical evaluation of recombinant factor IX. Semin Hematol. 1998;35 (2 Suppl 2):33–8. [PubMed] [Google Scholar]
- 14.Roth DA, Kessler CM, Pasi KJ, et al. Recombinant Factor IX Study Group. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood. 2001;98:3600–6. doi: 10.1182/blood.v98.13.3600. [DOI] [PubMed] [Google Scholar]
- 15.Bjorkman S, Shapiro AD, Berntorp E. Pharmacokinetics of recombinant factor IX in relation to age of the patient: implications for dosing in prophylaxis. Haemophilia. 2001;7:133–9. doi: 10.1046/j.1365-2516.2001.00465.x. [DOI] [PubMed] [Google Scholar]
- 16.Poon MC, Lillcrap D, Hensman C, et al. Recombinant factor IX recovery and inhibitor safety: a Canadian post-licensure surveillance study. Thromb Haemost. 2002;87:431–5. [PubMed] [Google Scholar]
- 17.Ewenstein BM, Joist JH, Shapiro AD, et al. for the Mononine Comparison Study Group. Pharmacokinetics analysis of plasma-derived and recombinant F IX concentrates in previously treated patients with moderate or severe hemophilia. Transfusion. 2002;42:190–7. doi: 10.1046/j.1537-2995.2002.00039.x. [DOI] [PubMed] [Google Scholar]
- 18.Linee guida per la terapia sostitutiva dell'emofilia e dei difetti ereditari della coagulazione edite a cura di E. Santagostino approvate dai Direttori dei Centri Emofilia Italiani. 2003. [Accessed on January 15th 2010]. Available at: http://www.aiceonline.it/documenti/LineeGuida/italia_Coagulopatie.pdf.
- 19.Guidelines for the Management of Hemophilia World Federation of Hemophilia. 2005. [Accessed on 15/01/2010]. Available at: http://www.wfh.org/2/docs/Publications/Diagnosis_and_Treatment/Guidelines_Mng_Hemophilia.pdf.
- 20.Monahan PE, Liesner R, Sullivan ST, et al. Safety and efficacy of investigator-prescribed BeneFIX® prophylaxis in children less than 6 years of age with severe haemophilia B. Haemophilia. 2010;16:460–8. doi: 10.1111/j.1365-2516.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 21.Martorell M, Altisent C, Parra R. Recovery of recombinant factor IX determined in clinical practice. Haemophilia. 2009;15:840–2. doi: 10.1111/j.1365-2516.2009.02000.x. [DOI] [PubMed] [Google Scholar]