Abstract
Background: Dietary flavonoids have beneficial effects on blood pressure in intervention settings, but there is limited information on habitual intake and risk of hypertension in population-based studies.
Objective: We examined the association between habitual flavonoid intake and incident hypertension in a prospective study in men and women.
Design: A total of 87,242 women from the Nurses' Health Study (NHS) II, 46,672 women from the NHS I, and 23,043 men from the Health Professionals Follow-Up Study (HPFS) participated in the study. Total flavonoid and subclass intakes were calculated from semiquantitative food-frequency questionnaires collected every 4 y by using an updated and extended US Department of Agriculture database.
Results: During 14 y of follow-up, 29,018 cases of hypertension in women and 5629 cases of hypertension in men were reported. In pooled multivariate-adjusted analyses, participants in the highest quintile of anthocyanin intake (predominantly from blueberries and strawberries) had an 8% reduction in risk of hypertension [relative risk (RR): 0.92; 95% CI: 0.86, 0.98; P < 0.03] compared with that for participants in the lowest quintile of anthocyanin intake; the risk reduction was 12% (RR: 0.88; 95% CI: 0.84, 0.93; P < 0.001) in participants ≤60 y of age and 0.96 (0.91, 1.02) in participants >60 y of age (P for age interaction = 0.02). Although intakes of other subclasses were not associated with hypertension, pooled analyses for individual compounds suggested a 5% (95% CI: 0.91, 0.99; P = 0.005) reduction in risk for the highest compared with the lowest quintiles of intake of the flavone apigenin. In participants ≤60 y of age, a 6% (95% CI: 0.88, 0.97; P = 0.002) reduction in risk was observed for the flavan-3-ol catechin when the highest and the lowest quintiles were compared.
Conclusions: Anthocyanins and some flavone and flavan-3-ol compounds may contribute to the prevention of hypertension. These vasodilatory properties may result from specific structural similarities (including the B-ring hydroxylation and methyoxylation pattern).
INTRODUCTION
Current guidelines for the prevention and treatment of hypertension focus on the additional benefit of lifestyle modifications to drug therapy (1) with increasing focus on the public health importance of Dietary Approaches to Stop Hypertension (DASH) diet, which emphasizes the importance of an increased consumption of fruit, vegetables, and low-fat dairy products (2–4). Further research on the components of plant foods that may play a role in reducing hypertension would potentially result in more targeted public health recommendations to allow consumers to make more informed choices.
Dietary flavonoids are a diverse range of bioactive polyphenolic compounds that naturally occur in plant-based foods and are present in significant amounts in many commonly consumed fruit, vegetables, grains, herbs, and drinks (including tea, wine, and juices) (5). Their structural complexity has led to their subclassification as flavonols, flavones, flavanones, flavan-3-ols and their oligomeric and polymeric forms (ie, procyanidins), isoflavones, and anthocyanins and other polymeric flavonoids. The differences in the chemical structures of these subclasses alter both their biological efficacy and bioavailability (6, 7). Experimental evidence suggests that some subclasses exert beneficial effects on blood pressure by increasing endothelial-derived nitric oxide (NO) via the modulation of endothelial nitric oxide synthase (eNOS) activity and expression, changes in eNOS substrate availability, or the prevention of radical-induced NO conversion caused by enzymes such as NADPH oxidase (8–11).
Results from a recent meta-analysis of randomized controlled trials on flavonoids and flavonoid-rich foods provided evidence that some subclasses of flavonoids are associated with a significant reduction in blood pressure (12). For example, short-term interventions (ie, a duration of 1–18 wk) with cocoa flavan-3-ols significantly reduced systolic blood pressure (SBP) by a mean of 5.9 mm Hg and diastolic blood pressure by a mean of 3.3 mm Hg (12). However, for a number of flavonoid subclasses, including anthocyanins, there are too few published studies to systematically examine their potential effects on blood pressure, whereas for other flavonoid subclasses, the amounts of flavonoids administered in the interventions were beyond the range typically consumed in the diet (12). To our knowledge, there have been no population-based studies that examined the relative effect of a habitual or usual intake of different subclasses of flavonoids on incident hypertension.
Therefore, we examined the association between the habitual intakes of each of the flavonoid subclasses and risk of incident hypertension in 3 prospective studies in middle-aged and older US women and men.
SUBJECTS AND METHODS
Study populations
In 1976 the Nurses' Health Study (NHS I) enrolled 121,700 female nurses aged 30–55 y who returned a mailed questionnaire regarding lifestyle and medical histories (13). In 1986 the Health Professionals Follow-Up Study (HPFS) enrolled 51,529 men aged 40–75 y, and in 1991, a younger cohort of 116,430 women aged 25–42 y were enrolled in the NHS II by using similar questionnaires (14, 15). Participants of these 3 cohorts received follow-up questionnaires biennially to record newly diagnosed illnesses and to update lifestyle factors, and every 4 y, participants received semiquantitative food-frequency questionnaires (FFQs) (16, 17). Participants who self-reported hypertension, cancer, stroke, or ischemic heart disease before baseline (returns of the 1990 questionnaire for the NHS I and HPFS and the 1991 questionnaire for the NHS II), used antihypertensive medication, were missing dietary data at baseline, or had implausible values for total caloric intake (<500 or >3500 cal for women and <800 or >4200 cal for men) were excluded. Given the prevalence of hypertension in middle age, we also conducted analyses in participants stratified by age (≤60 and >60 y). The institutional review board at Brigham and Women's Hospital reviewed and approved this study, and participants provided implied consent by returning their questionnaires.
Outcome assessment
At baseline and at each biennial questionnaire, participants reported if they had been professionally diagnosed with high blood pressure in the previous 2 y. The self-reported year of diagnosis was used to estimate an approximate date of diagnosis. Self-reported hypertension was shown to be highly reliable in all 3 cohorts (18–20). During 14 y of follow-up, 5636 men and 29,018 women reported incident hypertension.
Assessment of flavonoid intakes
Dietary intake data were collected from participants in 1990 in the NHS I and HPFS and in 1991 in the NHS II and every 4 y thereafter. FFQs administered before 1990 contained fewer questions on specific flavonoid-rich fruit and vegetables (for example, onions were absent from questionnaires before 1990). A database for the assessment of intakes of different flavonoid subclasses was constructed. We used the updated and expanded US Department of Agriculture (USDA) flavonoid content of foods and the proanthocyanidin databases (21, 22) as our primary sources. For foods in the FFQ where there were no values available in the USDA database, we searched a European database (EuroFIR eBASIS; http://www.eurofir.org) and other sources to ensure all available high-quality data on flavonoid values could be included in the database. However, although these other sources served as a validation to the USDA database, the addition of the EuroFIR data and the published literature did not contribute to >5–10% of the overall data used in the calculation of intakes for this analysis. Values for the individual flavonoid compounds were assigned to each of the foods listed in the FFQ, and if values for specific foods were not available, we imputed from similar foods if appropriate. For recipes, we assigned values for the specific flavonoids for each ingredient in the mixed dishes (please contact the authors for further information on amounts of flavonoids in individual foods or composite foods).
Intakes of individual compounds were calculated as the sum of the consumption frequency of each food multiplied by the content of the specific flavonoid for the specified portion size. We derived intakes of the 6 main subclasses commonly consumed in the US diet, specifically flavanones (eriodictyol, hesperetin, and naringenin), anthocyanins (cyanidin, delphinidin, malvidin, pelargonidin, petunidin, and peonidin), flavan-3-ols (catechins and epicatachins), flavonoid polymers (including proanthocyanidins, theaflavins, and thearubigins), flavonols (quercetin, kaempferol, myricetin, and isohamnetin), and flavones (luteolin and apigenin). Total flavonoid intakes were derived by the addition of component subclasses (flavanones, anthocyanins, flavan-3-ols, polymers, flavonols, and flavones).
Cumulative intakes (energy adjusted) were calculated for a given questionnaire cycle by averaging the intakes for the FFQs from 1990 and categorized into cohort-specific quintiles for analyses. Because of the wide variability in flavonoid intakes, cumulative intakes were chosen to more accurately represent usual intakes. The relative effect of the major food sources of flavonoids was also examined. The validity and reproducibility of the FFQs were previously reported; eg, correlations between several major dietary sources of flavonoids including apples, tea, and wine measured by diet-records and FFQs were 0.70, 0.77, and 0.83, respectively (23, 24).
Statistical methods
Participants contributed the person time of follow-up from the date of return of the baseline questionnaire to the earliest of the date of hypertension diagnosis, death, or end of follow-up (June 2004 for the NHS I, June 2005 for the NHS II, and January 2004 for the HPFS). In age-stratified analyses, participants were censored at the earliest of the date of diagnosis, death, end of follow-up, or upon reaching age 61 y. Cox proportional hazards analyses were used to assess the multivate relative risk (RR)adjusted for other risk factors for hypertension. Potential confounders included body mass index [BMI (in kg/m2) <23, 23 to <25, 25 to <30, or ≥30], physical activity (metabolic equivalents/wk in quintiles), alcohol consumption (0, 0.1–4.9, 5–14.9, 15–29.9, or >30 g alcohol/d), energy intake (kcal/d in quintiles), use of multivitamin supplements (yes or no), use of aspirin (yes or no), postmenopausal hormone use (never, past, or current), parental history of hypertension (yes or no), parental history of myocardial infarction before 60 y of age (yes or no), and intakes of sodium, magnesium, potassium, fiber, whole grains, folate, and caffeine (quintiles). Wald's test was used to determine whether there was heterogeneity across studies for each RR, and pooled RRs were calculated by weighting the study-specific log RR by the inverse of its variance by using a random-effects model (25). All analyses were conducted with SAS software (version 9; SAS Institute Inc, Cary, NC). All P values were 2 sided.
RESULTS
Baseline characteristics of each cohort overall and at extreme quintiles of total flavonoid intake are presented in Table 1. In the NHS II, the mean age of participants was 36 y (range: 26–42 y), and the mean BMI of participants was 24.3. During 822,839 person-years of follow-up in NHS II, 11,402 participants reported incident hypertension (13.9 cases/1000 person-years). With increasing intake of flavonoids, we observed a higher physical activity, lower intakes of sodium, and higher intakes of magnesium, potassium, fiber, and folate.
TABLE 1.
Age-adjusted baseline characteristics of total flavonoid intakes (means and interquartile ranges) in women and men in the Nurses' Health Study (NHS) I, the NHS II, and the Health Professionals Follow-Up Study (HPFS)1
| NHS II (1991) |
NHS I (1990) |
HPFS (1990) |
|||||||
| Total | Q1 | Q5 | Total | Q1 | Q5 | Total | Q1 | Q5 | |
| All participants | |||||||||
| n | 87,242 | 17,408 | 17,299 | 46,672 | 9578 | 9278 | 23,043 | 4690 | 4448 |
| No. of cases | 11,402 | — | — | 17,616 | 5629 | — | — | ||
| Incidence rate (per 100,000 person-years) | 13.9 | — | — | 34.5 | 26.5 | — | — | ||
| Age ≤60 y | |||||||||
| n | 87,242 | — | — | 33,041 | — | — | 15,434 | — | — |
| No. of cases | 11,402 | — | — | 6423 | — | — | 2289 | — | — |
| Incidence rate (per 100,000 person-years) | 13.9 | — | — | 27.5 | — | — | 20.8 | — | — |
| Age (y)2 | 36 (25–42) | 36 | 36 | 55 (30–55) | 55 | 55 | 56 (40–75) | 55 | 56 |
| Smoking (%) | |||||||||
| Past | 22.1 | 22.0 | 20.6 | 38.1 | 35.5 | 36.5 | 39.8 | 41.3 | 39.9 |
| Current | 12.1 | 17.8 | 11.6 | 17.3 | 28.3 | 14.3 | 7.6 | 13.9 | 6.0 |
| Family history of hypertension (%) | 51.8 | 51.7 | 52.4 | 45.3 | 44.1 | 45.9 | 28.6 | 27.1 | 20.9 |
| Postmenopausal hormone use (%) | |||||||||
| Never | — | — | — | 17.7 | 19.6 | 17.9 | — | — | — |
| Past | — | — | — | 16.8 | 15.4 | 16.8 | — | — | — |
| Current | — | — | — | 10.6 | 10.8 | 10.8 | — | — | — |
| BMI (kg/m2) | 24.3 ± 4.33 | 24.7 | 24.4 | 24.8 ± 5.0 | 25.1 | 24.7 | 25.2 ± 3.0 | 25.5 | 25.2 |
| Physical activity (METs/wk) | 21.0 ± 22.3 | 17.1 | 21.0 | 16.2 ± 27.3 | 13.6 | 15.7 | 39.2 ± 42.2 | 34.6 | 39.0 |
| Caffeine intake (mg/d)4 | 237 (61–370) | 245 | 268 | 279 (82–391) | 314 | 280 | 227 (44–318) | 269 | 234 |
| Total flavonoids (mg/d)4 | 413 (160–460) | 103 | 1122 | 358 (145–409) | 93 | 944 | 376 (176–429) | 115 | 933 |
| Flavonols (mg/d)4 | 18.4 (9.9–23) | 9.0 | 36.2 | 17.9 (9.7–22.4) | 9.4 | 34.1 | 18.9 (11.2–23.0) | 10.7 | 33.6 |
| Flavones (mg/d)4 | 1.5 (1.2–2.0) | 0.9 | 1.7 | 1.7 (0.9–2.3) | 1.0 | 1.9 | 2.2 (1.1–2.9) | 1.2 | 2.6 |
| Flavonones (mg/d)4 | 33.4 (11.0–44.7) | 16.1 | 38.7 | 37.3 (12.1–53.0) | 18.7 | 43.3 | 52.2 (18.9–72.1) | 24.5 | 65.0 |
| Flavan-3-ols (mg/d)4 | 61.7 (13.7–72.0) | 9.5 | 196 | 55.0 (12.0–64.3) | 8.6 | 175.9 | 50.1 (15.0–56.6) | 11.8 | 150 |
| Anthocyanins (mg/d)4 | 14.0 (5.4–17.8) | 6.7 | 18.0 | 12.5 (4.6–15.9) | 5.7 | 16.2 | 15.2 (5.8–19.3) | 6.8 | 21.9 |
| Polymers (mg/d)4 | 283 (92–310) | 60 | 831 | 233 (66–147) | 49 | 673 | 238 (99–292) | 60 | 660 |
| Sodium (mg/d)4 | 2153 (1921–2373) | 2211 | 2159 | 1840 (1627–2028) | 1895 | 1838 | 2092 (1838–2316) | 2180 | 2052 |
| Magnesium (mg/d)4 | 316 (266–354) | 292 | 323 | 302 (252–338) | 281 | 307 | 376 (318–420) | 350 | 389 |
| Potassium (mg/d)4 | 2934 (2578–3262) | 2667 | 3087 | 2853 (2515–3159) | 2610 | 2966 | 3350 (2939–3710) | 3026 | 3558 |
| Fiber (g/d)4 | 18.1 (12.8–22.0) | 16.0 | 17.1 | 19.3 (13.9–23.2) | 16.7 | 18.6 | 21.4 (15.2–25.8) | 17.9 | 21.4 |
| Whole grains (g/d)4 | 20.1 (8.6–27.4) | 18.0 | 17.8 | 20.8 (7.9–29.4) | 17.8 | 19.4 | 26.2 (11.2–36.4) | 22.5 | 25.0 |
| Folate (μg/d)4 | 480 (279–612) | 414 | 502 | 426 (267–555) | 366 | 457 | 500 (316–637) | 426 | 541 |
Q, quintile; METs, metabolic equivalent tasks.
Values are means; ranges in parentheses.
Mean ± SD (all such values).
Values are means; interquartile ranges in parentheses.
During 511,082 person-years of follow-up in the NHS I, 17,616 participants reported incident hypertension (34.5 cases/1000 person-years). At baseline, the mean age of participants was 55 y (range: 43–71 y), and the mean BMI of participants was 24.8. During 212,763 y of follow-up in the HPFS, 5629 men reported incident hypertension (26.5 cases/1000 person-years). At baseline, the mean age of participants was 56 y (range: 36–80 y), and the meant BMI of participants was 25.2. The distribution of covariates across quintiles of total flavonoids in the NHS I and HPFS were similar to those observed in the NHS II (Table 1).
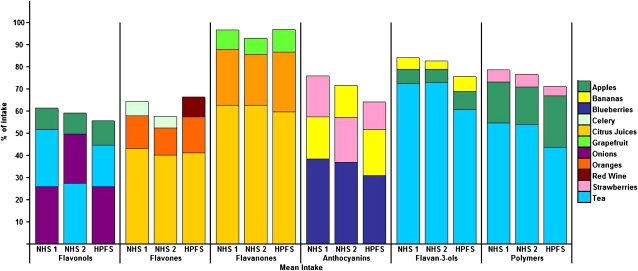
The variability in total flavonoid intakes within and across the 3 cohorts ranged from a mean of 358–413 mg flavonoids/d (interquartile range (IQR):145–429 mg flavonoids/d) (Table 1). Quantitatively, polymers contributed the highest intake in each cohort, whereas intakes from flavones were negligible. Mean amounts of flavan-3-ols ranged from 50.1 to 61.7 mg flavan-3-ols/d (IQR: 12.0–72.0 mg flavan-3-ols/d) across cohorts, whereas mean anthocyanin intakes ranged from 12.5 to 15.2 mg anthocyanin/d (IQR: 4.6–19.3 mg anthocyanin/d). Tea was the main contributor to the total flavonoid intake, with apples, orange juice, and strawberries as other significant contributors. Flavan-3-ols were predominantly consumed from tea, whereas blueberries and strawberries were the main sources of anthocyanins, and citrus fruit was the main contributor to flavanone and flavone intakes (Figure 1). We focused on the top 3 contributors to each subclass because all other foods contributed <4% of intakes.
FIGURE 1.
Major contributors to dietary intake by subclass of flavonoid in the Nurses' Health Study (NHS 1), the Nurses' Health Study 2 (NHS 2), and the Health Professionals Follow-Up Study (HPFS).
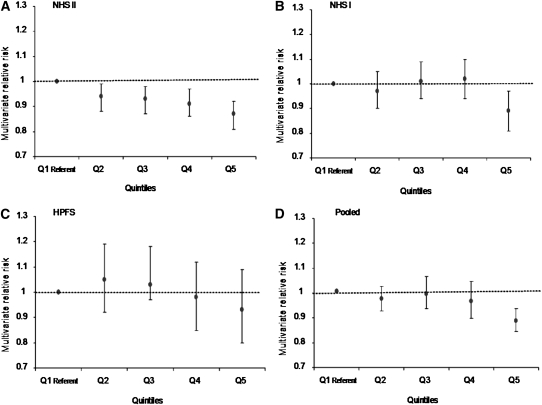
In pooled analyses, a high anthocyanin intake was associated with an 8% decreased risk of hypertension (quintile 5 compared with quintile1, RR: 0.92; 95% CI: 0.86, 0.98; P for trend < 0.03) (Table 2). The magnitude of the association was greater in participants ≤60 y of age (quintile 5 compared with quintile, RR: 0.88; 95% CI: 0.84, 0.93; P for trend < 0.001; P for age interaction = 0.02) (Table 3, Figure 2). The inverse association was observed in the NHS I and NHS II but not in the HPFS, and the linear inverse association was only observed in the younger women (NHS II; Figure 2). The point estimates for the dose response were modestly different across cohorts, but the 95% CIs for NHS I and NHS II were compatible with similar dose-response inverse associations.
TABLE 2.
Incident hypertension and cumulative average intakes of flavonoids (total and subclasses in quintiles) in participants in the Nurses' Health Study (NHS) I, the NHS II, and the Health Professionals Follow-Up Study (HPFS)1
| Flavonoid | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend |
| Flavonols | ||||||
| NHS II | ||||||
| No. of cases | 2282 | 2214 | 2253 | 2313 | 2340 | — |
| Person-years | 160,275 | 167,691 | 168,830 | 166,985 | 159,058 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.97 (0.91, 1.03) | 0.97 (0.92, 1.04) | 0.98 (0.92, 1.05) | 1.00 (0.94, 1.07) | 0.58 |
| NHS I | ||||||
| No. of cases | 3560 | 3714 | 3623 | 3514 | 3205 | — |
| Person-years | 103,645 | 105,671 | 103,580 | 101,502 | 96,684 | — |
| Relative risk (95% CI) | 1.0 (ref) | 1.02 (0.97, 1.07) | 1.01 (0.96, 1.06) | 1.00 (0.95, 1.05) | 0.96 (0.91, 1.01) | 0.02 |
| HPFS | ||||||
| No. of cases | 1087 | 1170 | 1134 | 1141 | 1104 | — |
| Person-years | 42,553 | 44,709 | 43,682 | 42,792 | 39,026 | — |
| Relative risk (95% CI) | 1.0 (ref) | 1.02 (0.94, 1.12) | 1.01 (0.93, 1.11) | 1.01 (0.92, 1.11) | 1.07 (0.97, 1.18) | 0.17 |
| Pooled relative risk (95% CI) | 1.0 (ref) | 1.00 (0.97, 1.04) | 1.00 (0.96, 1.03) | 1.00 (0.96, 1.03) | 1.00 (0.95, 1.01) | 0.99 |
| Flavones | ||||||
| NHS II | ||||||
| No. of cases | 2360 | 2396 | 2228 | 2301 | 2117 | — |
| Person-years | 155,081 | 165,536 | 166,431 | 169,171 | 166,619 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.98 (0.93, 1.04) | 0.93 (0.87, 0.98) | 0.97 (0.91, 1.04) | 0.95 (0.88, 1.01) | 0.17 |
| NHS I | ||||||
| No. of cases | 3625 | 3830 | 3606 | 3394 | 3161 | — |
| Person-years | 104,945 | 108,204 | 103,930 | 100,494 | 93,509 | — |
| Relative risk (95% CI) | 1.0 (ref) | 1.00 (0.96, 1.05) | 0.98 (0.93, 1.02) | 0.95 (0.90, 1.00) | 0.97 (0.92, 1.02) | 0.09 |
| HPFS | ||||||
| No. of cases | 1107 | 1222 | 1130 | 1085 | 1092 | — |
| Person-years | 42,932 | 43,795 | 43,113 | 42,509 | 40,414 | — |
| Relative risk (95% CI) | 1.0 (ref) | 1.12 (1.03, 1.22) | 1.05 (0.96, 1.15) | 1.05 (0.96, 1.15) | 1.09 (0.98, 1.20) | 0.44 |
| Pooled relative risk (95% CI) | 1.0 (ref) | 1.02 (0.96, 1.09) | 0.98 (0.92, 1.04) | 0.98 (0.93, 1.03) | 0.99 (0.93, 1.06) | 0.28 |
| Flavonones | ||||||
| NHS II | ||||||
| No. of cases | 2334 | 2347 | 2285 | 2267 | 2169 | — |
| Person-years | 156,829 | 166,072 | 166,546 | 167,819 | 165,573 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.97 (0.92, 1.03) | 0.96 (0.91, 1.02) | 0.98 (0.92, 1.04) | 0.98 (0.92, 1.05) | 0.92 |
| NHS I | ||||||
| No. of cases | 3497 | 3892 | 3679 | 3424 | 3124 | — |
| Person-years | 103,246 | 108,915 | 104,344 | 101,934 | 92,642 | — |
| Relative risk (95% CI) | 1.0 (ref) | 1.04 (0.99, 1.09) | 1.01 (0.97, 1.06) | 0.97 (0.92, 1.02) | 0.99 (0.94, 1.05) | 0.14 |
| HPFS | ||||||
| No. of cases | 1161 | 1215 | 1168 | 1084 | 1008 | — |
| Person-years | 43,591 | 45,209 | 43,482 | 41,617 | 38,864 | — |
| Relative risk (95% CI) | 1.0 (ref) | 1.05 (0.96, 1.14) | 1.04 (0.92, 1.13) | 1.05 (0.95, 1.15) | 1.04 (0.94, 1.14) | 0.63 |
| Pooled relative risk (95% CI) | 1.0 (ref) | 1.02 (0.97, 1.07) | 1.00 (0.96, 1.04) | 0.99 (0.95, 1.02) | 0.99 (0.96, 1.03) | 0.39 |
| Anthocyanins | ||||||
| NHS II | ||||||
| No. of cases | 2379 | 2344 | 2301 | 2276 | 2102 | — |
| Person-years | 150,085 | 163,823 | 168,435 | 170,786 | 169,711 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.94 (0.88, 0.99) | 0.93 (0.87, 0.98) | 0.91 (0.86, 0.97) | 0.87 (0.81, 0.92)* | <0.0001 |
| NHS I | ||||||
| No. of cases | 3531 | 3569 | 3640 | 3616 | 3260 | — |
| Person-years | 100,201 | 102,378 | 103,767 | 104,063 | 100,673 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.96 (0.91, 1.00) | 0.97 (0.92, 1.02) | 0.98 (0.93, 1.03) | 0.93 (0.88, 0.98)* | 0.02 |
| HPFS | ||||||
| No. of cases | 1137 | 1168 | 1140 | 1112 | 1079 | — |
| Person-years | 41,162 | 42,906 | 43,290 | 43,756 | 41,648 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.99 (0.91, 1.08) | 0.97 (0.89, 1.06) | 0.98 (0.89, 1.07) | 0.99 (0.90, 1.09) | 0.82 |
| Pooled relative risk (95% CI) | 1.0 (ref) | 0.96 (0.93, 0.99) | 0.96 (0.92, 0.99) | 0.95 (0.91, 1.01) | 0.92 (0.86, 0.98)* | 0.03 |
| Flavan-3-ols | ||||||
| NHS II | ||||||
| No. of cases | 2332 | 2134 | 2170 | 2311 | 2455 | — |
| Person-years | 159,269 | 166,255 | 170,197 | 165,959 | 161,159 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.97 (0.91, 1.03) | 0.96 (0.90, 1.02) | 1.00 (0.94, 1.06) | 1.03 (0.97, 1.09) | 0.08 |
| NHS I | ||||||
| No. of cases | 3406 | 3533 | 3562 | 3597 | 3518 | — |
| Person-years | 98,181 | 103,210 | 103,130 | 102,945 | 103,615 | — |
| Relative risk (95% CI) | 1.0 (ref) | 1.00 (0.95, 1.05) | 1.01 (0.96, 1.06) | 1.01 (0.96, 1.06) | 0.98 (0.93, 1.03) | 0.31 |
| HPFS | ||||||
| No. of cases | 1031 | 1104 | 1220 | 1141 | 1140 | — |
| Person-years | 40,501 | 43,027 | 44,372 | 43,659 | 41,204 | — |
| Relative risk (95% CI) | 1.0 (ref) | 1.06 (0.97, 1.16) | 1.14 (1.04, 1.24) | 1.07 (0.98, 1.18) | 1.12 (1.02, 1.22) | 0.10 |
| Pooled relative risk (95% CI) | 1.0 (ref) | 1.00 (0.96, 1.04) | 1.03 (0.95, 1.12) | 1.01 (0.98, 1.05) | 1.03 (0.97, 1.11) | 0.40 |
| Polymers | ||||||
| NHS II | ||||||
| No. of cases | 2331 | 2155 | 2234 | 2253 | 2429 | — |
| Person-years | 159,191 | 166,861 | 168,412 | 166,666 | 161,709 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.93 (0.88, 0.99) | 0.96 (0.91, 1.02) | 0.96 (0.91, 1.02) | 1.00 (0.94, 1.06) | 0.34 |
| NHS I | ||||||
| No. of cases | 3456 | 3576 | 3551 | 3469 | 3564 | — |
| Person-years | 97,859 | 103,301 | 103,129 | 102,945 | 103,848 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.98 (0.94, 1.03) | 0.98 (0.93, 1.03) | 0.96 (0.91, 1.01) | 0.97 (0.93, 1.02) | 0.56 |
| HPFS | ||||||
| No. of cases | 1125 | 1127 | 1153 | 1091 | 1140 | — |
| Person-years | 40,257 | 44,229 | 44,312 | 43,379 | 40,586 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.98 (0.90,1.07) | 1.04 (0.95,1.13) | 0.99 (0.90,1.08) | 1.08 (0.99,1.18) | 0.04 |
| Pooled relative risk (95% CI) | 1.0 (ref) | 0.96 (0.93, 1.00) | 0.98 (0.95, 1.02) | 0.96 (0.93, 1.00) | 1.01 (0.95, 1.06) | 0.36 |
| Total flavonoids | ||||||
| NHS II | ||||||
| No. of cases | 2330 | 2192 | 2253 | 2216 | 2411 | — |
| Person-years | 159,191 | 166,861 | 168,412 | 166,666 | 161,709 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.95 (0.90, 1.01) | 0.99 (0.93, 1.05) | 0.96 (0.90, 1.02) | 1.01 (0.95, 1.07) | 0.41 |
| NHS I | ||||||
| No. of cases | 3615 | 3574 | 3468 | 3433 | 3526 | — |
| Person-years | 102,510 | 103,094 | 101,273 | 101,300 | 102,905 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.96 (0.91, 1.00) | 0.94 (0.89, 0.98) | 0.92 (0.88, 0.97) | 0.94 (0.90, 0.99)* | 0.40 |
| HPFS | ||||||
| No. of cases | 1166 | 1124 | 1124 | 1094 | 1128 | — |
| Person-years | 41,618 | 44,231 | 44,329 | 42,267 | 40,318 | — |
| Relative risk (95% CI) | 1.0 (ref) | 0.96 (0.88, 1.05) | 0.99 (0.91, 1.08) | 0.99 (0.91, 1.09) | 1.06 (0.97, 1.16) | 0.04 |
| Pooled relative risk (95% CI) | 1.0 (ref) | 0.96 (0.93, 0.99) | 0.97 (0.93, 1.00) | 0.95 (0.91, 0.98) | 1.00 (0.93, 1.07) | 0.46 |
ref, reference. Multivariate model included age and smoking, BMI, physical activity, alcohol consumption, family history of hypertension, aspirin use, multivitamin use, and intakes of sodium, magnesium, potassium, fiber, whole grain, folate, and caffeine. *Quintile 5 compared with quintile 1, P < 0.001.
TABLE 3.
Incident hypertension for total flavonoid intakes and flavonoid subclasses (quintiles of cumulative intakes: quintile 5 compared with quintile 1) in the Nurses' Health Study (NHS) I, the NHS II, and the Health Professionals Follow-Up Study (HPFS) restricted to participants ≤60 y of age1
| NHS I | NHS II | HPFS | Pooled | P for age interaction | |
| Flavonols | |||||
| Age and smoking adjusted | 0.88 (0.81, 0.95) | 1.01 (0.95, 1.07) | 0.94 (0.83, 1.08) | 0.96 (0.90, 1.02) | 0.26 |
| Multivariate adjusted | 0.91 (0.84, 0.99) | 1.00 (0.94, 1.07) | 0.96 (0.82, 1.11) | — | — |
| Flavones | |||||
| Age and smoking adjusted | 0.94 (0.87, 1.02) | 0.81 (0.76, 0.86) | 0.87 (0.76, 0.99) | — | — |
| Multivariate adjusted | 1.06 (0.97, 1.16) | 0.95 (0.88, 1.01) | 1.03 (0.88, 1.21) | 1.00 (0.93, 1.09) | 0.97 |
| Flavonones | |||||
| Age and smoking adjusted | 0.93 (0.86, 1.01) | 0.86 (0.81, 0.92) | 0.84 (0.73, 0.94) | 1.00 (0.95, 1.05) | 0.56 |
| Multivariate adjusted | 1.04 (0.95, 1.14) | 0.98 (0.92, 1.05) | 1.02 (0.87, 1.19) | — | — |
| Flavan-3-ols | |||||
| Age and smoking adjusted | 0.92 (0.86, 1.00) | 1.03 (0.97, 1.09) | 1.00 (0.88, 1.14) | — | — |
| Multivariate adjusted | 0.94 (0.81, 1.02) | 1.03 (0.97, 1.09) | 1.05 (0.92, 1.21) | 1.00 (0.94, 1.07) | 0.44 |
| Anthocyanins | |||||
| Age and smoking adjusted | 0.79 (0.73, 0.85) | 0.76 (0.72, 0.81) | 0.79 (0.69, 0.90) | — | — |
| Multivariate adjusted | 0.89 (0.81, 0.97)*** | 0.87 (0.81, 0.92)*** | 0.93 (0.80, 1.09) | 0.88 (0.84, 0.93)*** | 0.02* |
| Polymers | |||||
| Age and smoking adjusted | 0.92 (0.85, 0.99) | 1.02 (0.96, 1.08) | 0.96 (0.84, 1.09) | — | — |
| Multivariate adjusted | 0.95 (0.88, 1.03) | 1.00 (0.94, 1.06) | 1.05 (0.91, 1.20) | 0.99 (0.95, 1.03) | 0.48 |
| Total flavonoids | |||||
| Age and smoking adjusted | 0.90 (0.83, 0.97) | 1.00 (0.96, 1.08) | 0.94 (0.83, 1.07) | — | — |
| Multivariate adjusted | 0.95 (0.88, 1.03) | 1.01 (0.95, 1.07) | 1.06 (0.92, 1.22) | 1.00 (0.95, 1.05) | — |
All values are relative risks; 95% CIs in parentheses. ***P < 0.001, *P < 0.05.
FIGURE 2.
A–D: Incident hypertension by quintiles (Q) of anthocyanin intake (stratified by age <60 y). NHS II, Nurses' Health Study II; NHS I, Nurses' Health Study; HPFS, Health Professionals Follow-Up Study. P for trend < 0.001.
Although there was no evidence that other subclasses were significantly associated with a reduction in incident hypertension, some individual flavonoid compounds from subclasses with specific structural characteristics (such as a B-ring hydroxylation and methyoxylation pattern) (Table 4) were associated with lower rates of hypertension. In the flavone subclass, there was a 5% reduction in rates of hypertension in participants in the highest compared with lowest quintiles of apigenin intake (95% CI: 1%, 9%; P = 0.005). In the flavan-3-ol subclass, in analyses restricted to participants ≤60 y of age, lower rates of hypertension were observed in participants in the highest quintiles of catechin (7%; 95% CI: 3%, 12%; P = 0.002) and epicatechin (5%; 95% CI: 0%, 9%; P = 0.05) intakes than in participants in the lowest quintiles of catechin and epicatechin intakes (Table 4). In all participants, there was evidence of an effect modification of the epicatechin and hypertension association (P for sex interaction = 0.03); in women, the RR was 0.95 (95% CI: 0.92, 0.99; P = 0.015). No other effect modification by sex was observed for other subclasses (data not shown). Among individual anthocyanins, cyanidin, malvidin, and pelargonidin were associated with reduced rates of hypertension, and results were generally stronger in participants ≤60 y of age (P for age interaction < 0.05 for cyanidin, <0.01 for malvidin, and <0.001 for peonidin and petunidin) (Table 4).
TABLE 4.
Individual dietary flavonoids from the flavonol, flavone, flavan-3-ol, and anthocyanin subclasses (with specific structural similarities) and risk of incident hypertension in the Nurses' Health Study (NHS) I, the NHS II, and the Health Professionals Follow-Up Study (HPFS)1
| Cumulative intakes: Q5 vs Q1 |
All participants pooled | Participants ≤60 y of age pooled | P for age interaction | |||
| NHS I | NHS II | HPFS | ||||
| Flavonols | ||||||
| Quercetin | 0.93 (0.89, 0.99) | 0.96 (0.90,1.01) | 1.07 (0.97,1.18) | 0.97 (0.91,1.04) | 0.95 (0.91,1.00) | 0.48 |
| Kaempferol | 0.99 (0.94, 1.04) | 1.09 (1.03,1.16) | 1.03 (0.94,1.13) | 1.04 (0.97,1.10) | 1.00 (0.88,1.13) | 0.45 |
| Myricetin | 0.99 (0.94, 1.05) | 1.05 (0.99,1.12) | 1.12 (1.02,1.24) | 1.04 (0.98,1.11) | 1.00 (0.91,1.11) | 0.27 |
| Isorhamnetin | 0.96 (0.92, 1.01) | 1.01 (0.95,1.07) | 1.06 (1.00,1.13) | 1.01 (0.95,1.07) | 0.98 (0.91,1.05) | 0.25 |
| Flavones | ||||||
| Apigenin | 0.94 (0.90, 1.00) | 0.96 (0.89,1.02) | 0.95 (0.86,1.04) | 0.95 (0.91,0.99)*** | 0.94 (0.87,1.01) | 0.67 |
| Luteolin | 0.99 (0.93, 1.04) | 0.94 (0.88,1.00) | 1.05 (0.95,1.16) | 0.98 (0.93,1.04) | 0.99 (0.91,1.06) | 0.74 |
| Flavan-3-ols | ||||||
| Catechin | 0.94 (0.90, 0.99) | 0.95 (0.89,1.01) | 1.03 (0.93,1.12) | 0.96 (0.92,1.01) | 0.93 (0.88,0.97)** | 0.08 |
| Gallocatechins | 0.98 (0.93, 1.03) | 1.09 (1.02,1.15) | 1.05 (0.95,1.15) | 1.04 (0.96,1.12) | 1.00 (0.89,1.13) | 0.58 |
| Epicatechin | 0.96 (0.91, 1.01) | 0.95 (0.89,1.01) | 1.06 (0.97,1.16) | 0.98 (0.93,1.04) | 0.95 (0.91,1.00)* | 0.27 |
| Eigallocatechin | 0.99 (0.94, 1.04) | 1.06 (1.00,1.13) | 1.05 (0.96,1.15) | 1.03 (0.98,1.08) | 1.01 (0.93,1.10) | 0.56 |
| Epicatechin 3 gallate | 0.98 (0.93, 1.03) | 1.07 (1.01,1.14) | 1.09 (0.99,1.19) | 1.04 (0.97,1.04) | 1.01 (0.90,1.13) | 0.65 |
| Epigallocatechin 3 gallate | 0.99 (0.94, 1.04) | 1.08 (1.01, 1.14) | 1.04 (0.95,1.14) | 1.03 (0.98,1.04) | 1.01 (0.90,1.13) | 0.86 |
| Anthocyanins | ||||||
| Cyanidin | 0.93 (0.88, 0.98) | 0.84 (0.79, 0.90) | 0.96 (0.87,1.06) | 0.91 (0.84,0.98)* | 0.88 (0.82,0.94)*** | 0.04* |
| Delphinidin | 0.98 (0.93, 1.04) | 0.86 (0.81, 0.92) | 1.04 (0.95,1.15) | 0.95 (0.86,1.06) | 0.94 (0.84,1.05) | 0.33 |
| Malvidin | 0.97 (0.92, 0.98) | 0.88 (0.82, 0.93) | 0.95 (0.87,1.05) | 0.95 (0.90,1.00)* | 0.89 (0.85, 0.94)*** | 0.004** |
| Pelargonidin | 0.92 (0.88, 0.97) | 0.96 (0.90, 1.02) | 1.00 (0.92,1.10) | 0.95 (0.91,0.99)* | 0.95 (0.91,1.00)* | 0.93 |
| Peonidin | 0.97 (0.92, 1.02) | 0.88 (0.83, 0.93) | 0.97 (0.89,1.07) | 0.94 (0.87,1.00) | 0.89 (0.85,0.93)*** | 0.001*** |
| Petunidin | 0.98 (0.93, 1.03) | 0.87 (0.81, 0.92) | 0.96 (0.87,1.05) | 0.94 (0.86,1.01) | 0.89 (0.85,0.93)*** | 0.001*** |
All values are relative risks; 95% CIs in parentheses. Q, quintile. The multivariate model included age and smoking, BMI, physical activity, alcohol consumption, family history of hypertension, aspirin use, multivitamin use, and intakes of sodium, magnesium, potassium, fiber, whole grain, folate, and caffeine. ***P < 0.001, **P < 0.01, *P < 0.05.
The 2 main sources of anthocyanins across cohorts were blueberries and strawberries. In pooled analyses of the ≤60-y-old group, the consumption of more than one serving of blueberries per week compared with no blueberry intake was associated with a 10% reduction in hypertension (RR: 0.90; 95% CI: 0.81, 0.98; P = 0.02). The consumption of more than one serving of strawberries per week was not significantly associated with a reduction in hypertension (RR: 0.97; 95% CI: 0.94, 1.00; P = 0.34). The results were not materially different when we restricted analyses to nonsmokers or nondrinkers (data not shown).
DISCUSSION
In this large prospective study, we observed that a higher total anthocyanin intake was significantly associated with a reduced risk of incident hypertension even after controlling for a large number of covariates including family history, physical activity, BMI, and dietary factors previously associated with blood pressure; the magnitude of the reduction was greatest in the ≤60-y-old participants. Although the total intake of other subclasses was not associated with a significant reduction in hypertension, higher intakes of individual compounds were suggestive of a benefit in the ≤60-y-old participants. Interestingly all of these flavonoids (ie, the anthocyanins, flavan-3-ols, and flavones) have implicated vascular mechanisms of action on the basis of their structural characteristics (8, 11).
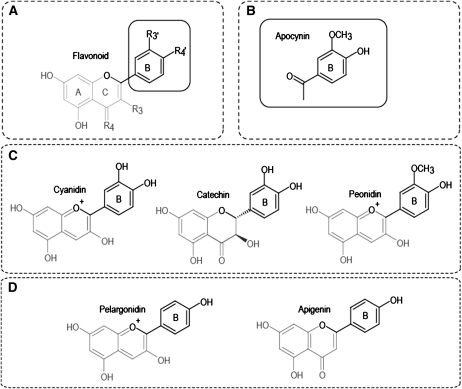
From a structural perspective, catechol flavonoids (such as cyanidin and catechin and epicatechin) may be more bioactive than other forms because they can become methylated via the action of catechol-O-methyl transferase through the course of metabolism (or naturally as in the case of the anthocyanin peonidin), which potentially enhances their bioactivity (12). This chemical change results in molecular forms that are structurally analogous to apocynin, which is an established vasoactive drug (Figure 3) (26). Structural activity relation studies conducted on numerous flavonoid species (by using cultured human vascular endothelial cells) have indicated that mono-O-methylated flavonoids and those possessing only one 4′ hydroxy (such as apigenin and the anthocyanin pelargonidin) can act as inhibitors of endothelial NADPH oxidase (8, 11), which is an enzyme that decreases the bioactivity of NO in the vascular endothelium and, thus, limits vasodilatory processes associated with blood-flow regulation (27).
FIGURE 3.
Common flavonoid structures. Catechol flavonoids and their methyl- and 4′-hydroxy derivatives. A: Basic catechol flavonoid structure. B: Structure of apocynin, which is a vasoactive drug (similar in structure to methylated B-ring flavonoids). C: Catechol flavonoids in the diet (anthocyanin, cyanidin; flavan-3-ol, catechin) and a naturally occurring methyl derivative (anthocyanin, peonidin) in plants. D: 4′ Hydroxy flavonoids (anthocyanin, pelargonidin; flavone, apigenin).
The human diet may contain a significant diversity of flavonoids, and it is likely that the bioactivity relevant to endothelial function is limited to a few species or metabolic derivatives. Our prospective data provided evidence to suggest that after habitual intake, anthocyanins are most strongly inversely associated with hypertension, with suggestive evidence that specific compounds from the flavone and flavan-3-ol subclasses may also play a role in blood pressure reduction. Interestingly, the magnitude of the inverse association between total anthocyanin intake and hypertension was most pronounced in the ≤60-y-old participants. Similar observations have been made in these cohorts with other exposures, including folate and nonnarcotic analgesic use (28, 29). It is possible that the cumulative damage over many decades exceeds the capacity for flavonoids to beneficially affect endothelial function and blood pressure in older individuals. These data reinforce the importance of dietary intervention strategies for blood pressure reduction before middle age. Although an inverse association was observed in both the NHS I and NHS II, the effect was only linear in the younger women (NHS II; Figure 2), and no effect was observed in men who were, on average, older than the other cohorts.
Although plausible biological mechanisms and data from short-term interventions studies exist that would predict inverse associations between flavonoid intake and hypertension, to our knowledge, this is the first prospective study to report an association between the habitual intakes of different flavonoid subclasses and risk of incident hypertension. Two previous cohort studies have examined the effects of cocoa intake on blood pressure. In the Zutphen Elderly study of 470 men, cocoa intake was inversely related to blood pressure after multivariate adjustment, with a 3.8 mm Hg lower SBP in the highest tertile of cocoa intake, but no relation with tea intake was observed (30). In another study, cocoa intake was not related to new onset hypertension (31). Although we observed no relation between hypertension and the flavan-3-ol subclass overall, we did observe risk reductions in the ≤60-y-old participants for the main constituents of dark cocoa, catechin, and epicatechin (5% and 7% reduction, respectively) of a magnitude similar to that observed in a meta-analysis of randomized controlled trials on flavonoids (12). Significant reductions in blood pressure after interventions with cocoa flavan-3-ols were observed; interventions of 1–18 wk of duration resulted in a mean reduction in SBP of 5.9 mm Hg (95% CI: −9.55, −2.21 mm Hg; n = 5 studies) and in diastolic blood pressure of 3.3 mm Hg (95% CI: −5.77, −0.83 mm Hg; n = 4 studies) (12). Numerous intervention studies have shown that an increased intake of specific flavonoids improves endothelial function in vivo (8, 9, 11, 32–34) with a predominant focus on cocoa flavan-3-ols and soy isoflavones (12). For many of the other flavonoid subclasses, including anthocyanins, there was insufficient evidence to draw conclusions about efficacy.
According to experimental studies, the underlying biological mechanisms by which flavonoids regulate blood pressure include the effects of flavonoids on vascular blood flow, vascular reactivity, and glucose uptake. Growing mechanistic evidence suggests that endothelial NO regulation rather than a general antioxidant effect (ie, direct radical scavenging) is a major target for these compounds, and emerging data suggests that eNOS and NADPH oxidase activity are crucial sites of action for many flavonoids (9–11, 34). Data from experimental animal models and in vitro work support a blood pressure–lowering effect of a number of flavonoids; specifically, quercetin, delphinidin, cyanidin-3-glucoside, and epigallopcatechin-3-gallate enhanced NO output or bioactivity and improved endothelium-dependent vascular relaxation and lowered blood pressure in spontaneously hypertensive rats (35–43). These data are supported by human intervention studies. For example, the ingestion of pure epicatechin by healthy men augmented NO bioavailability and acutely reduced plasma concentrations of endothelin-1, which is a potent endothelium-derived vasoconstrictor (44). In another trial, the vascular effects observed after consumption of flavan-3-ol rich cocoa were linked to epicatechin intake (9). Other proposed mechanisms for the antihypertensive effects of flavan-3-ols include the ability to lower the activity of arginase-2, which is an enzyme that competes with NO synthase for l-arginine (33) and inhibitory activity on angiotension converting enzyme in vitro (45).
The diversity in structures of flavonoids contributes to differences in biological efficacy, and it is likely that the bioactivity relevant to endothelial function is limited to a few structurally similar compounds such as the catechol and 4′ hydroxy flavonoids as observed in our study. Our data suggested that several specific classes of flavonoids were associated with blood pressure reduction, specifically anthocyanins, which resulted in a 12% reduction in hypertension risk in multivariate analyses. These data are important because anthocyanins are present in commonly consumed fruit, such as blueberries, cranberries, and strawberries, which can be readily incorporated into the diet. An average portion of blueberries, blackcurrants, or blood orange juice can contain very high amounts of anthocyanins (>500 mg anthocyanins) (46, 47). Although the mean intakes of anthocyanins were somewhat low (12–15 mg anthocyanins/d) in the current study, considerable amounts were consumed by some individuals (≤1252 mg anthocyanins/d). We also believe that the amounts of anthocyanins reported in foods and food databases are still underestimations because of methodologic limitations in analysis methods such as a lack of characterization of anthocyanin intermediates or phenolic degradation products in processed foods, which may be potentially bioactive anthocyanin constituents (48, 49).
The limitations of the current study include that the blood pressures of participants were not directly measured, and the diagnosis of hypertension was self-reported; however, all participants were health professionals, and hypertension reporting by participants of these cohorts was highly sensitive (19). The specificity of hypertension reporting may have resulted in the misclassification of a few truly hypertensive individuals as control subjects, but such misclassification would tend to diminish the magnitude of the association. Therefore, our findings may be an underestimation of the true association. As with any observational study design, no causal associations could be made, and we could not exclude the possibility of residual confounding; however, we controlled for a range of dietary and lifestyle factors known to affect hypertension risk (including smoking, BMI, physical activity, alcohol consumption, family history, aspirin use, and a number of dietary variables). Furthermore, because we specifically observed an association with anthocyanins and not with all flavonoids, the possibility of residual confounding by a healthy lifestyle may have been reduced. Dietary flavonoid intakes were based on cumulative intakes and were calculated from a database developed by using the most recent USDA database (21), with significant additional input from other sources. These data sets allowed us to quantify flavonoid intakes more robustly than previous flavonoid-based analyses (5). However there is wide variability in the flavonoid content of foods depending on geographical origin, growing season, different cultivars, agricultural methods and processing, and a lack of biomarkers to integrate intakes with the extensive metabolism these compounds undergo in vivo.
In conclusion, these findings warrant further investigation, including intervention studies designed to test optimal doses of anthocyanin rich foods for the prevention of hypertension and to underpin guidelines for the prevention and treatment of hypertension. These data support the hypothesis that the antihypertensive bioactivity may be relevant to vasodilatory processes associated with specific flavonoid structural characteristics.
Acknowledgments
We are indebted to the participants of the NHS and NHS II and HPFS for their continued dedication and commitment.
The authors' responsibilities were as follows—AC, EJO, and EBR: conducted the statistical analyses, interpreted the data, and drafted the manuscript; AC, CK, LS, MF, and ERB: developed the flavonoid database; JPF, CK, and GC: provided critical review of the manuscript; and all authors: contributed to the manuscript and agreed on the final version of the manuscript. AC and CK have received funding from Unilever Research and GlaxoSmithKline to conduct trials and experimental (in vitro) studies on flavonoid-rich foods. GC has received funding from TAP Pharmaceuticals and Astellas Pharmaceuticals. EJO, LS, MF, JPF, and EB reported no conflicts of interest.
REFERENCES
- 1.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52 [DOI] [PubMed] [Google Scholar]
- 2.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997;336:1117–24 [DOI] [PubMed] [Google Scholar]
- 3.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308 [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001;344:3–10 [DOI] [PubMed] [Google Scholar]
- 5.Erdman JW, Jr, Balentine D, Arab L, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31-June 1, 2005, Washington, DC. J Nutr 2007;137:718S–37S [DOI] [PubMed] [Google Scholar]
- 6.Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr 2005;81:243S–55S [DOI] [PubMed] [Google Scholar]
- 7.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81:230S–42S [DOI] [PubMed] [Google Scholar]
- 8.Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys 2008;469:209–19 [DOI] [PubMed] [Google Scholar]
- 9.Schroeter H, Heiss C, Balzer J, et al. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 2006;103:1024–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 2003;290:1030–1 [DOI] [PubMed] [Google Scholar]
- 11.Steffen Y, Schewe T, Sies H. (−)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem Biophys Res Commun 2007;359:828–33 [DOI] [PubMed] [Google Scholar]
- 12.Hooper L, Kroon PA, Rimm EB, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2008;88:38–50 [DOI] [PubMed] [Google Scholar]
- 13.Colditz GA. The nurses' health study: a cohort of US women followed since 1976. J Am Med Womens Assoc 1995;50:40–4 [PubMed] [Google Scholar]
- 14.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9 [DOI] [PubMed] [Google Scholar]
- 15.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 1127–36 [DOI] [PubMed] [Google Scholar]
- 16.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62 [DOI] [PubMed] [Google Scholar]
- 17.Willett WC. Nutritional epidemiology. 2nd ed New York, NY: Oxford University Press, 1998 [Google Scholar]
- 18.Ascherio A, Rimm EB, Giovannucci EL, et al. A prospective study of nutritional factors and hypertension among US men. Circulation 1992;86:1475–84 [DOI] [PubMed] [Google Scholar]
- 19.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol 1986;123:894–900 [DOI] [PubMed] [Google Scholar]
- 20.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 2008;52:828–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.USDA Database for the Flavonoid Content of Selected Foods Release 2.1. Washington, DC: USDA, 2007 [Google Scholar]
- 22.USDA Database for the Proanthocyanidin Content of Selected Foods. Washington, DC: USDA, 2004 [Google Scholar]
- 23.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 24.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–67 [DOI] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88 [DOI] [PubMed] [Google Scholar]
- 26.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245–313 [DOI] [PubMed] [Google Scholar]
- 27.de Gasparo M. Angiotensin II and nitric oxide interaction. Heart Fail Rev 2002;7:347–58 [DOI] [PubMed] [Google Scholar]
- 28.Forman JP, Rimm EB, Stampfer MJ, Curhan GC. Folate intake and the risk of incident hypertension among US women. JAMA 2005;293:320–9 [DOI] [PubMed] [Google Scholar]
- 29.Forman JP, Stampfer MJ, Curhan GC. Non-narcotic analgesic dose and risk of incident hypertension in US women. Hypertension 2005;46:500–7 [DOI] [PubMed] [Google Scholar]
- 30.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med 2006;166:411–7 [DOI] [PubMed] [Google Scholar]
- 31.Alonso A, de la Fuente C, Beunza JJ, Sanchez-Villegas A, Martinez-Gonzalez MA. Chocolate consumption and incidence of hypertension. Hypertension 2005;46:e21–2; author reply e22 [DOI] [PubMed] [Google Scholar]
- 32.Heiss C, Kleinbongard P, Dejam A, et al. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol 2005;46:1276–83 [DOI] [PubMed] [Google Scholar]
- 33.Schnorr O, Brossette T, Momma TY, et al. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch Biochem Biophys 2008;476:211–5 [DOI] [PubMed] [Google Scholar]
- 34.Schewe T, Steffen Y, Sies H. How do dietary flavanols improve vascular function? A position paper. Arch Biochem Biophys 2008;476:102–6 [DOI] [PubMed] [Google Scholar]
- 35.Fitzpatrick DF, Hirschfield SL, Coffey RG. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am J Physiol 1993;265:H774–8 [DOI] [PubMed] [Google Scholar]
- 36.Andriambeloson E, Stoclet JC, Andriantsitohaina R. Mechanism of endothelial nitric oxide-dependent vasorelaxation induced by wine polyphenols in rat thoracic aorta. J Cardiovasc Pharmacol 1999;33:248–54 [DOI] [PubMed] [Google Scholar]
- 37.Duarte J, Perez-Palencia R, Vargas F, et al. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol 2001;133:117–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freedman JE, Parker C, 3rd, Li L, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 2001;103:2792–8 [DOI] [PubMed] [Google Scholar]
- 39.Leikert JF, Rathel TR, Wohlfart P, Cheynier V, Vollmar AM, Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation 2002;106:1614–7 [DOI] [PubMed] [Google Scholar]
- 40.Lorenz M, Wessler S, Follmann E, et al. A constituent of green tea, epigallocatechin-3-gallate, activates endothelial nitric oxide synthase by a phosphatidylinositol-3-OH-kinase-, cAMP-dependent protein kinase-, and Akt-dependent pathway and leads to endothelial-dependent vasorelaxation. J Biol Chem 2004;279:6190–5 [DOI] [PubMed] [Google Scholar]
- 41.Xu JW, Ikeda K, Yamori Y. Cyanidin-3-glucoside regulates phosphorylation of endothelial nitric oxide synthase. FEBS Lett 2004;574:176–80 [DOI] [PubMed] [Google Scholar]
- 42.Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension 2004;44:217–22 [DOI] [PubMed] [Google Scholar]
- 43.Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep 2009;61:67–75 [DOI] [PubMed] [Google Scholar]
- 44.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr 2008;88:1018–25 [DOI] [PubMed] [Google Scholar]
- 45.Actis-Goretta L, Ottaviani JI, Fraga CG. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem 2006;54:229–34 [DOI] [PubMed] [Google Scholar]
- 46.Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem 2006;54:4069–75 [DOI] [PubMed] [Google Scholar]
- 47.Clifford MN. Anthocyanins—nature, occurrence and dietary burden. J Sci Food Agric 2000;80:1063–72 [Google Scholar]
- 48.Sadilova E, Stintzing FC, Carle R. Thermal degradation of acylated and nonacylated anthocyanins. J Food Sci 2006;71:C504–12 [Google Scholar]
- 49.Kay CD, Kroon PA, Cassidy A. The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol Nutr Food Res 2009;53(suppl 1):S92–101 [DOI] [PubMed] [Google Scholar]