Abstract
Background: Homozygosity for the variant 677T allele in the methylenetetrahydrofolate reductase (MTHFR) gene increases the requirement for folate and may alter the metabolic use of choline. The choline adequate intake is 550 mg/d for men, although the metabolic consequences of consuming extra choline are unclear.
Objective: Deuterium-labeled choline (d9-choline) as tracer was used to determine the differential effects of the MTHFR C677T genotype and the effect of various choline intakes on the isotopic enrichment of choline derivatives in folate-compromised men.
Design: Mexican American men with the MTHFR 677CC or 677TT genotype consumed a diet providing 300 mg choline/d plus supplemental choline chloride for total choline intakes of 550 (n = 11; 4 with 677CC and 7 with 677TT) or 1100 (n = 12; 4 with 677CC and 8 with 677TT) mg/d for 12 wk. During the last 3 wk, 15% of the total choline intake was provided as d9-choline.
Results: Low but measurable enrichments of the choline metabolites were achieved, including that of d3-phosphatidylcholine (d3-PtdCho)—a metabolite produced in the de novo pathway via choline-derived methyl groups. Men with the MTHFR 677TT genotype had a higher urinary enrichment ratio of betaine to choline (P = 0.041), a higher urinary enrichment of sarcosine (P = 0.041), and a greater plasma enrichment ratio of d9-betaine to d9-PtdCho with the 1100 mg choline/d intake (P = 0.033).
Conclusion: These data show for the first time in humans that choline itself is a source of methyl groups for de novo PtdCho biosynthesis and indicate that the MTHFR 677TT genotype favors the use of choline as a methyl donor.
INTRODUCTION
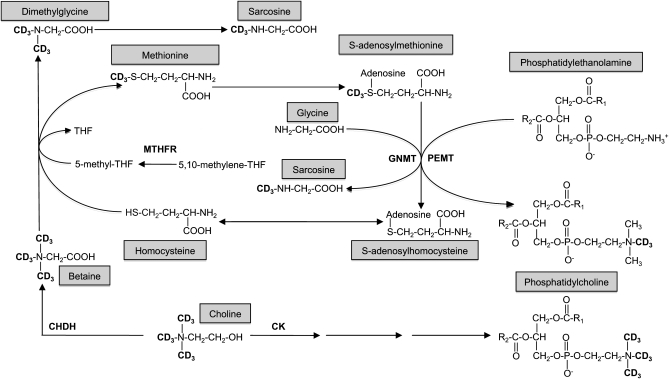
Choline is an essential micronutrient that serves as the precursor molecule for several important compounds, including the phospholipids phosphatidylcholine (PtdCho) and sphingomyelin, the neurotransmitter acetylcholine, and the methyl donor betaine (1) (Figure 1). PtdCho and sphingomyelin are abundant in cellular membranes and have structural and signaling functions; PtdCho is also the major phospholipid in lipoproteins, with influences on lipid metabolism and transport (2). Most PtdCho (≈70%) is synthesized by the cytidine diphosphate (CDP)-choline pathway in which choline itself serves as the substrate; the remainder is synthesized de novo via phosphatidylethanolamine N-methyltransferase (PEMT), an S-adenosylmethionine (SAM)-dependent enzyme that sequentially methylates phosphatidylethanolamine to PtdCho (3). The methyl groups associated with SAM are originally derived from folate, choline (betaine), and/or methionine (4). In addition to PtdCho biosynthesis, these one-carbon units may be used for the biosynthesis of several metabolites, hormones, and neurotransmitters and for the methylation of DNA with subsequent effects on gene expression and genome stability (5). Severe choline deficiency can cause metabolic disturbances such as fatty liver (6, 7), muscle damage (8), and aberrant gene expression as a result of alterations in DNA methylation patterns (9, 10). Associations between low dietary choline intake and increased risk of inflammation (11), birth defects (12), and breast cancer (13) have also been reported.
FIGURE 1.
Metabolic fate of the orally consumed deuterium-labeled choline. The d9-choline tracer contained deuterium-labeled methyl groups facilitating the examination of the metabolic fate of choline-derived methyl groups in addition to the intact molecule. THF, tetrahydrofolate; MTHFR, 5,10-methylenetetrahydrofolate reductase; PEMT, phosphatidylethanolamine N-methyltransferase; GNMT, glycine N-methyltransferase; CK, choline kinase; CHDH, choline dehydrogenase.
Based primarily on the amount of choline needed to prevent liver dysfunction, choline adequate intakes (AIs) of 425 and 550 mg/d for women and men, respectively, were established in 1998 (14). The metabolic requirement for choline is likely higher in individuals with compromised folate status because betaine (oxidized from choline) shares the homocysteine remethylation step in one-carbon metabolism with 5-methyltetrahydrofolate (5-methylTHF) (14). 5-MethylTHF is derived from 5,10-methyleneTHF in a reaction catalyzed by 5,10-methyleneTHF reductase (MTHFR). The availability of 5-methylTHF, the main folate coenzyme in circulation, is modified by a common single nucleotide polymorphism, 677C→T, in the MTHFR gene (15).
We previously reported diminished serum folate and elevated plasma homocysteine concentrations in men with the MTHFR 677TT genotype, relative to those with the 677CC genotype, after 12 wk of consuming the Recommended Dietary Allowance (RDA) for folate (16). The diminished 5-methylTHF in men with the MTHFR 677TT genotype may result in a higher reliance on betaine as the methyl group donor for one carbon metabolism and therefore alter choline partitioning/metabolism. Our finding of lower plasma PtdCho concentrations in this group of men with the MTHFR 677TT genotype (17) is consistent with this working hypothesis.
The objective of this study was to determine the effects of the MTHFR C677T genotype and/or varied choline intakes on the metabolic use of orally consumed choline. To accomplish this aim, deuterium-labeled choline (d9-choline) was consumed orally by men with the MTHFR 677CC or TT genotype during the last 3 wk of a 12-wk controlled feeding study. Isotopic enrichment [enrichment = labeled metabolite/(labeled + unlabeled metabolite)] and the enrichment ratio (enrichment ratio = enrichment of product/enrichment of precursor) of one-carbon metabolites derived from the orally consumed labeled choline were evaluated.
SUBJECTS AND METHODS
Subjects and study design
The men in this study (n = 23; 8–42 y of age) represent a subsample of healthy Mexican American men (n = 60; 18–55 y of age) preselected for the MTHFR 677CC or TT genotype and were recruited between June 2005 and September 2006. Additional inclusion criteria were described elsewhere (16). The study was approved by the Institutional Review Board for Human Study Participants at Cal Poly Pomona University, and written informed consent was given by each participant. Approval to use de-identified samples for the measurements made in this study was granted by the Cornell Institutional Review Board for Human Subjects.
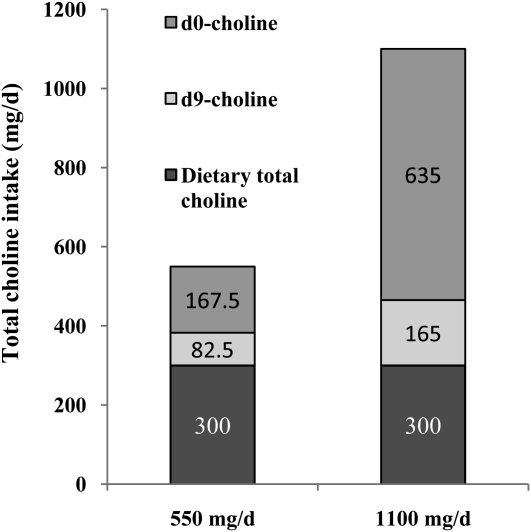
This was a 12-wk choline intervention study in which study participants (n = 60) with the MTHFR 677CC (n = 31) or 677TT (n = 29) genotype were randomly assigned at baseline to receive 300, 550, 1100, or 2200 mg choline/d. The diet, consumed by all study participants throughout the study, provided 300 mg total choline/d, 173 mg betaine/d, and 319 μg natural food folate/d (16, 17). The study participants also consumed 70 μg supplemental folic acid/d for total folate intakes of 438 μg dietary folate equivalents/d (16). To achieve the target choline intakes of 550, 1100, or 2200 mg/d, unlabeled commercially available choline chloride (BCP Ingredients Inc, Verona, MO) was administered for the first 9 wk as previously detailed (16). During the last 3 wk of the study (weeks 10–12), a subsample of the men in the 550 mg/d (n = 11; 4 with 677CC, 7 with 677TT) and 1100 mg/d (n = 12; 4 with 677CC, 8 with 677TT) choline intake groups received 15% of the target dose as d9-choline prepared from commercially available [trimethyl-d9]choline chloride (Cambridge Isotope Laboratories Inc, Andover, MA; Figure 2). In the 550 mg/d choline intake group, choline was derived from the diet (300 mg/d), unlabeled supplemental choline chloride (167.5 mg/d), and d9-labeled supplemental choline chloride (82.5 mg/d). The supplemental choline chloride (d0+d9) was consumed daily at the breakfast meal. In the 1100 mg/d choline intake group, choline was derived from the diet (300 mg/d), unlabeled supplemental choline chloride (635 mg/d), and d9-labeled supplemental choline chloride (165 mg/d). The supplemental choline chloride (d0+d9) was divided into 2 equal portions (317.5 mg d0-choline plus 82.5 mg d9-choline) and consumed at breakfast and dinner.
FIGURE 2.
Mean intake of dietary total choline and choline chloride either as unlabeled (d0) or labeled (d9) choline.
The isotopic enrichment of choline and its metabolites was assessed at the end of the study (week 12) in plasma and urine. Adherence to the study protocol was previously established by measurements of serum folate (a sensitive marker of dietary intake) and plasma free choline (16, 17).
Sample collection and MTHFR C677T genotyping
Baseline and weekly fasting (10 h) venous blood samples were collected into serum separator gel and clot-activated tubes (Vacutainer; Becton Dickinson, Rutherford, NJ) and EDTA-coated tubes (Vacutainer), processed, and stored at −80°C as previously described (18). Twenty-four–hour urine samples were collected at 0, 6, and 12 wk; processed; and stored at −20°C as described previously (18). Determination of the MTHFR C677T gene involved polymerase chain reaction, digestion with Hinf1, and electrophoretic separation on an agarose gel (19).
Measurements of plasma and urinary choline metabolites
Plasma and urinary concentrations of free choline [unlabeled (d0) and d9], betaine (d0 and d9), and dimethylglycine (d0 and d6) were measured by liquid chromatography tandem mass spectrometry according to the method of Holm et al (20). The system included an LCQ Advantage Mass Spectrometry system (Thermo Finnigan, San Jose, CA) with an electrospray ionization source operated in positive ion mode, a Surveyor HPLC system (Thermo Finnigan), and a refrigerated Surveyor autosampler (Thermo Finnigan). Urine or plasma (50 μL) was transferred to a 1.5-mL Eppendorf tube, and 100 μL of acetonitrile containing 0.1% (vol:vol) formic acid was added to precipitate proteins; d13-choline, d3-betaine, and d3-dimethylglycine (0.2 nmol/L of each) were used as internal standards. The mixture was mixed and centrifuged at 4°C. An aliquot of supernatant fluid (120 μL) was transferred to vials containing 120 μL 0.1% (vol:vol) formic acid in acetonitrile. The separation of choline, betaine, and dimethylglycine was achieved by using an Alltech Prevail Silica analytic column (2.1 × 150 mm, 5 μm) and an Alltech Prevail Silica guard column (2.1 × 7.5 mm, 5 μm) with a mobile phase of 81% filtered acetonitrile and 19% of 0.1% formic acid (vol:vol) in 15 mmol ammonium formate/L. The flow rate was 500 μL/min, the injection volume was 10 μL, and the column temperature was maintained at 25°C. The external standard curve for choline (d0 and d9), betaine (d0 and d9), and dimethylglycine (d0 and d6) was constructed in 25:75 (vol:vol) 15 mmol ammonium formate/L and acetonitrile solution.
Plasma PtdCho (d0, d3, d6, and d9) was measured by liquid chromatography mass spectrometry as described by Koc et al (21) with modifications based on our instrumentation (22) with d4-PtdCho as an internal standard. The calculation of enrichment percentage (d3-Ptdcho/total Ptdcho; d9-Ptdcho/total Ptdcho) was based on the peak area under the curve.
Plasma methionine (d0, d3) was measured by gas chromatography–mass spectrometry (GC-MS) in negative ion electron capture mode as the n-propyl ester N-heptafluorobutyryl derivative with chemical ionization (23) as previously described (24).
A panel of urinary one-carbon metabolites (ie, sarcosine, homocysteine, cystathionine, cysteine, methionine, aminobutyric acid, glycine, and serine) was quantified by GC-MS following the established protocol (25, 26). The enrichments of urinary dimethylglycine (DMG) and sarcosine were also determined by GC-MS in a separate run in which internal standard was not added.
Statistical analysis
To test for differences in the dependent variables of interest (eg, plasma d9-choline) between the MTHFR C677T genotypes and between the choline intake groups, a 2-factor ANOVA (choline intake and MTHFR C677T genotype) was performed on each dependent variable. Dependent variables whose residuals were not normally distributed were log or square root transformed to fit the assumption of the analysis of variance model. Effects were considered to be significant at P < 0.05, whereas a P < 0.10 was indicative of trends. Data are presented as means ± SEMs for all dependent variables and were analyzed by SPSS software (version 15; SPSS Inc, Chicago, IL).
RESULTS
Characteristics of the study population
Indicators of folate and choline status and concentrations of urinary metabolites related to one-carbon metabolism at the end of the 12-wk study are shown in Table 1. The ages and body mass indexes of the study population are also provided. Compared with men with the MTHFR 677CC genotype, those with the 677TT genotype had lower serum folate (P = 0.003) and higher plasma homocysteine (P = 0.001) concentrations. The mean age was lower in men with the MTHFR 677TT genotype (P = 0.038) than in men with the 677CC genotype. Compared with men consuming 550 mg choline/d, the 1100 mg/d intake group had higher plasma betaine concentrations (P = 0.014) and tended to have higher plasma free choline concentrations (P = 0.052) and greater urinary cystathionine excretion (P = 0.086). The MTHFR C677T genotype tended to interact with choline intake (P = 0.057) to affect urinary homocysteine, with the highest concentration detected in men with the MTHFR 677TT genotype in the 1100 mg/d choline intake group. No other differences were detected.
TABLE 1.
Clinical and biochemical variables at the end of the controlled feeding study (week 12) in Mexican American men with the MTHFR 677CC or 677TT genotype who had choline intakes of 550 or 1100 mg/d1
| Choline intake |
P values |
|||||
| Variable | 550 mg/d | 1100 mg/d | All | Genotype | Choline | Interaction |
| Age (y) | 0.038 | 0.927 | 0.604 | |||
| 677CC | 28 (22–42)2 | 26 (19–35) | 27 (19–42)3 | |||
| 677TT | 21 (18–27) | 22 (20–28) | 22 (18–28) | |||
| Total | 24 (18–42) | 24 (19–35) | — | |||
| BMI (kg/m2) | 0.652 | 0.317 | 0.179 | |||
| 677CC | 24.5 (21–27) | 28.8 (20–36) | 26.6 (20–36) | |||
| 677TT | 26.1 (22–30) | 25.5 (21–30) | 25.8 (21–30) | |||
| Total | 25.5 (21–30) | 26.6 (20–36) | — | |||
| Serum folate (nmol/L) | 0.003 | 0.676 | 0.915 | |||
| 677CC | 12.3 ± 1.74 | 11.6 ± 2.7 | 11.9 ± 1.53 | |||
| 677TT | 7.3 ± 0.9 | 6.9 ± 0.8 | 7.1 ± 0.6 | |||
| Total | 9.1 ± 1.1 | 8.5 ± 1.2 | — | |||
| Plasma tHcy (μmol/L) | 0.001 | 0.328 | 0.336 | |||
| 677CC | 11.7 ± 0.7 | 11.8 ± 0.7 | 11.7 ± 0.53 | |||
| 677TT | 28.5 ± 6.7 | 40.7 ± 5.7 | 35 ± 4.5 | |||
| Total | 22.4 ± 4.9 | 31.1 ± 5.6 | — | |||
| Plasma choline (μmol/L) | 0.655 | 0.052 | 0.841 | |||
| 677CC | 7.0 ± 0.8 | 9.4 ± 2.2 | 8.2 ± 1.2 | |||
| 677TT | 7.4 ± 0.7 | 10.2 ± 1.1 | 8.9 ± 0.8 | |||
| Total | 7.2 ± 0.5 | 10.0 ± 1.05 | — | |||
| Plasma betaine (μmol/L) | 0.418 | 0.014 | 0.755 | |||
| 677CC | 46.0 ± 5.2 | 66.0 ± 14.0 | 56 ± 7.9 | |||
| 677TT | 42.6 ± 3.1 | 58.3 ± 4.7 | 51 ± 3.5 | |||
| Total | 43.8 ± 2.6 | 60.9 ± 5.45 | — | |||
| Plasma total PtdCho (d0+d3+d9) (μmol/L) | 0.498 | 0.733 | 0.079 | |||
| 677CC | 1784 ± 166 | 1532 ± 93 | 1658 ± 100 | |||
| 677TT | 1492 ± 84 | 1665 ± 101 | 1585 ± 68 | |||
| Total | 1599 ± 88 | 1621 ± 74 | — | |||
| Urinary choline (μmol/g Cr) | 0.135 | 0.085 | 0.249 | |||
| 677CC | 16.9 ± 6.3 | 22.7 ± 5.5 | 19.4 ± 4.1 | |||
| 677TT | 20.3 ± 5.5 | 48.2 ± 10 | 35.3 ± 7.1 | |||
| Total | 19.0 ± 4.0 | 40.6 ± 8.2 | — | |||
| Urinary betaine (μmol/g Cr) | 0.290 | 0.853 | 0.925 | |||
| 677CC | 80.2 ± 29 | 67.6 ± 18 | 74.8 ± 17 | |||
| 677TT | 124.6 ± 55 | 120.5 ± 31 | 122.4 ± 29 | |||
| Total | 106.9 ± 34 | 104.7 ± 23 | — | |||
| Urinary DMG (μmol/g Cr) | 0.159 | 0.957 | 0.088 | |||
| 677CC | 86.6 ± 23 | 35.7 ± 2 | 69.6 ± 18 | |||
| 677TT | 76.7 ± 20 | 130.8 ± 26 | 105.8 ± 18 | |||
| Total | 80.7 ± 10 | 109.7 ± 9 | — | |||
| Urinary sarcosine (μmol/g Cr) | 0.909 | 0.123 | 0.627 | |||
| 677CC | 12.1 ± 1.2 | 17.7 ± 5.3 | 14.5 ± 2.4 | |||
| 677TT | 13.1 ± 2.5 | 16.1 ± 1.7 | 14.7 ± 1.5 | |||
| Total | 12.7 ± 1.6 | 16.5 ± 1.7 | — | |||
| Urinary tHcy (μmol/g Cr) | 0.012 | 0.041 | 0.057 | |||
| 677CC | 4.5 ± 0.9 | 5.2 ± 0.4 | 4.8 ± 0.53 | |||
| 677TT | 7.7 ± 3.4 | 25.8 ± 9.1 | 18.9 ± 5.2 | |||
| Total | 6.3 ± 2.1 | 18.9 ± 5.55 | — | |||
| Urinary cystathionine (μmol/g Cr) | 0.787 | 0.086 | 0.859 | |||
| 677CC | 4.9 ± 1.2 | 7.2 ± 1.2 | 6.0 ± 0.9 | |||
| 677TT | 5.4 ± 1.0 | 7.3 ± 1.1 | 6.4 ± 0.8 | |||
| Total | 5.2 ± 0.7 | 7.3 ± 0.85 | — | |||
| Urinary cysteine (mmol/g Cr) | 0.731 | 0.163 | 0.833 | |||
| 677CC | 0.2 ± 0.03 | 0.3 ± 0.02 | 0.2 ± 0.02 | |||
| 677TT | 0.2 ± 0.02 | 0.2 ± 0.02 | 0.2 ± 0.01 | |||
| Total | 0.2 ± 0.01 | 0.2 ± 0.01 | — | |||
| Urinary methionine (μmol/g Cr) | 0.356 | 0.135 | 0.673 | |||
| 677CC | 6.3 ± 1.7 | 8.6 ± 1.9 | 7.4 ± 1.2 | |||
| 677TT | 7.3 ± 2.3 | 11.4 ± 1.7 | 9.5 ± 1.5 | |||
| Total | 6.9 ± 1.5 | 10.4 ± 1.3 | — | |||
| Urinary aminobutyric acid (μmol/g Cr) | 0.944 | 0.517 | 0.713 | |||
| 677CC | 5.7 ± 1.1 | 5.9 ± 1.4 | 5.8 ± 0.8 | |||
| 677TT | 5.4 ± 0.6 | 6.4 ± 0.7 | 5.9 ± 0.5 | |||
| Total | 5.9 ± 0.5 | 6.2 ± 0.6 | — | |||
| Urinary glycine (mmol/g Cr) | 0.417 | 0.197 | 0.506 | |||
| 677CC | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.8 ± 0.1 | |||
| 677TT | 1.1 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.1 | |||
| Total | 1.0 ± 0.1 | 0.8 ± 0.1 | — | |||
| Urinary serine (mmol/g Cr) | 0.408 | 0.543 | 0.929 | |||
| 677CC | 0.2 ± 0.04 | 0.2 ± 0.03 | 0.2 ± 0.02 | |||
| 677TT | 0.3 ± 0.04 | 0.2 ± 0.04 | 0.2 ± 0.03 | |||
| Total | 0.2 ± 0.03 | 0.2 ± 0.03 | — | |||
n = 3–8 per group. Cr, creatinine; DMG, dimethylglycine; PtdCho, phosphatidylcholine; tHcy, total homocysteine.
Mean; range in parentheses (all such values).
Significantly different from the corresponding MTHFR 677TT genotype, P < 0.05 (2-factor ANOVA).
Mean ± SEM (all such values).
Significantly different from the corresponding 550 mg/d choline intake group, P < 0.05 (2-factor ANOVA).
Effects of choline intake and MTHFR C677T genotype on the isotopic enrichment and enrichment ratios of choline metabolites
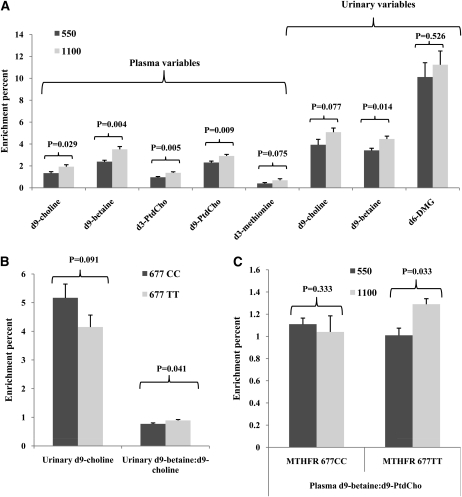
Label was detected at acceptable signal-to-noise ratios in most choline metabolites (Figure 3). Greater enrichments of plasma choline (P = 0.029), plasma betaine (P = 0.004), plasma d3-PtdCho (P = 0.005), plasma d9-PtdCho (P = 0.009), and urinary betaine (P = 0.014) were observed in the 1100 mg/d than in the 550 mg/d choline intake group (Figure 3A). In addition, enrichments of plasma methionine (P = 0.075) and urinary choline (P = 0.077) tended to be greater in the higher choline intake group (Figure 3A). However, choline intake did not affect the enrichment ratios of product:precursor metabolites (ie, betaine:choline, methionine:betaine, methionine:choline, d3-PtdCho:choline,and d3-PtdCho:betaine), which indicated that the enrichments of precursors and products were equivalently affected.
FIGURE 3.
Mean (±SEM) isotopic enrichment of plasma and urinary choline metabolites [labeled metabolite/(labeled + unlabeled metabolite)] grouped by choline intake (A; n = 10–12 per choline intake group) or 5,10-methylenetetrahydrofolate reductase (MTHFR) C677T genotype (B; n = 7 with 677CC; n = 13 with 677TT) or both (C; n = 4–8 per group) at week 12 in folate-compromised Mexican American men who consumed 15% of their total choline intake as d9-choline from weeks 10 to 12. Data were analyzed by 2-factor ANOVA; P < 0.05 was considered significant. For the enrichment ratio of d9-betaine to d9-phosphatidylcholine (d9-PtdCho), an interaction between MTHFR C677T and choline intake was detected (P = 0.03; C). Thus, the data are presented after stratification by MTHFR C677T genotype. DMG, dimethylglycine.
The MTHFR 677TT genotype yielded a lower enrichment of urinary choline (P = 0.091) and a greater urinary betaine to choline enrichment ratio (P = 0.041), which suggests that flux through choline dehydrogenase may be greater in men with the 677TT than with the 677CC genotype (Figure 3B). In addition, the MTHFR C677T genotype and choline intake interacted to affect the plasma enrichment ratio of betaine to PtdCho derived from the CDP-choline pathway (Figure 3C). In men with the MTHFR 677TT genotype, the higher choline intake yielded a greater betaine to PtdCho enrichment ratio, which suggests that betaine oxidation was favored over the use of choline by the CDP-choline pathway; no effect of choline intake on this ratio was observed in the group with the 677CC genotype. No other significant effects of either choline intake, MTHFR C677T genotype, or the interaction term were detected on the measured variables.
Effects of choline intake and MTHFR C677T genotype on the proportion of PtdCho enrichment derived from the PEMT and CDP-choline pathways
Under the conditions of this study, only d3-PtdCho and d9-PtdCho (but not d6-PtdCho) were detected at quantifiable levels; therefore, it can be assumed that all of the d9-PtdCho was derived from the CDP-choline pathway. Notably, neither choline intake nor MTHFR C677T genotype affected the plasma enrichment ratio of d3-PtdCho to d9-PtdCho (data not shown). This observation suggests that the proportion of PtdCho derived from each of these pathways was unaffected by these variables.
Effects of choline intake and MTHFR genotype on urinary sarcosine enrichment
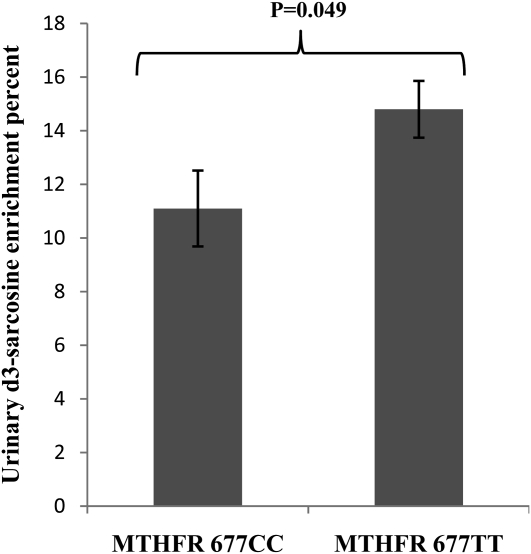
Urinary sarcosine enrichment was higher (P = 0.049) in men with the MTHFR 677TT genotype than in men with the 677CC genotype (Figure 4). Choline intake did not affect urinary sarcosine enrichment, and the interaction term for MTHFR C677T genotype and choline intake was not significant.
FIGURE 4.
Mean (±SEM) urinary d3-sarcosine enrichment at week 12 according to MTHFR C677T genotype (n = 7 with 677CC, n = 13 with 677TT) in folate-compromised Mexican American men who consumed 15% of their total choline intake (550 or 1100 mg/d) as d9-choline from weeks 10 to 12. Data were analyzed by a 2-factor ANOVA; P < 0.05 was considered significant. Choline intake did not affect urinary sarcosine enrichment, and the interaction term for MTHFR C677T genotype and choline intake was not significant.
DISCUSSION
To the best of our knowledge, this is the first human study to use stable-isotope methods to investigate the effects of choline intake and the MTHFR C677T genotype (677CC or 677TT) on the metabolic use of exogenous choline. The d9-choline tracer contained deuterium-labeled methyl groups and facilitated our examination of the metabolic fate of choline-derived methyl groups in addition to the intact molecule. In turn, distinctions could be made between PtdCho derived from the sequential methylation of phosphatidylethanolamine via PEMT (which produces d3-PtdCho, d6-PtdCho, and less likely d9-PtdCho) and PtdCho derived from intact choline by the CDP-choline pathway, which yields only d9-PtdCho (Figure 1). Because d6-PtdCho was below the detection limits in the present study, it was assumed that all of the d9-PtdCho was derived from the CDP-choline pathway.
The d3 labeling of PtdCho indicates for the first time in humans that choline itself is a source of methyl groups for the de novo biosynthesis of the choline moiety through the PEMT pathway—a finding consistent with a cell culture model using rat primary hepatocytes (27). The observed d3 labeling of plasma methionine suggests that cellular pools of SAM were similarly labeled and, thus, served as methyl donors in d3-PtdCho synthesis by PEMT. Although the PEMT pathway is traditionally considered to be most important when dietary choline intake is low (28), d3 labeling of the PtdCho pool occurred under conditions of high choline intake (ie, 1100 mg/d or 2 times the AI). Notably, the PtdCho molecules derived from the PEMT and CDP-choline pathways are molecularly distinct (29), with PEMT-derived PtdCho containing more polyunsaturated fatty acids, including docosahexaenoic acid (DHA). Hence, a possible caveat of increasing dietary intake of choline may be reducing the activity of the PEMT pathway, which, in mice, results in decreased concentrations of circulating DHA (30). However, on the basis of the enrichment ratio of d3-PtdCho to d9-PtdCho, the relative proportions of PtdCho derived from each pathway were not altered by the choline intakes used in this study.
In the current study, we hypothesized that varied choline intakes would affect the partitioning of choline such that a higher intake would favor its oxidation. However, the enrichment of products and precursors (ie, d3-PtdCho/betaine, betaine/choline, and DMG/choline) were affected to a similar extent, which suggests that a doubling of choline intake did not alter the metabolic use of orally consumed choline (ie, proportion of choline entering the oxidative pathway or the extent of its catabolism).
Simple one-pool constant infusion kinetics require that enrichments decrease as the label moves from pool to pool. Our observed increase in isotope enrichment from precursor d9-choline to several products in blood and urine indicates that pools are in flux and that more than one precursor pool exists for derivation of subsequent products. The high enrichment of betaine, relative to that of choline, may also reflect an increased release of more enriched products as a result of gut-level oxidation of choline to methylamines.
The observed greater enrichment of urinary metabolites than its plasma counterpart was likely due to the timing differences between the urine and blood collections. Similar to the kinetic curve in plasma, urinary metabolite enrichment is probably highest within several hours after dosing and declines thereafter, reaching its lowest value the next day before dosing. Therefore, the 24-h urine and fasting blood collections probably captured different phases of the kinetic curves with plasma enrichment reflecting the lowest point but urinary enrichment reflecting an average status.
MTHFR produces 5-methylTHF which, like betaine, can be used to remethylate homocysteine to methionine. The MTHFR 677TT genotype tends to yield diminished tissue concentrations of 5-methylTHF, particularly under conditions of folate inadequacy. Notably, in the current study, men with the MTHFR 677TT genotype had markedly diminished concentrations of serum folate (consisting mostly of 5-methylTHF; Table 1) such that 47% (7 of 15 participants) had serum folate concentrations in the deficient range (ie, ≤6.8 nmol/L; data not shown). Although, serum folate was also diminished in men with the MTHFR 677CC genotype (Table 1), only one participant had serum folate in the deficient range. As a result, the demand for betaine as a source of labile methyl groups for homocysteine remethylation may be higher in persons with the MTHFR 677TT genotype. The higher urinary enrichment ratio of betaine to choline in men with the 677TT genotype is consistent with this working hypothesis, because it implies enhanced flux through choline dehydrogenase—the enzyme responsible for oxidizing choline to betaine. The higher plasma enrichment ratio of betaine to CDP-choline derived PtdCho in men with the MTHFR 677TT (relative to the 677CC genotype) with the higher choline intake is also consistent with an increased demand for betaine among persons with the MTHFR 677TT genotype, because it suggests that more of the free choline was converted to betaine as opposed to entering the CDP-choline pathway. Increased demand for betaine during MTHFR deficiency has been reported in rodent studies, as evidenced by lower concentrations of hepatic phosphocholine and betaine concentrations in MTHFR+/− mice than in +/+ mice (31).
The higher urinary enrichment of d3-sarcosine in men with the 677TT genotype was likely due to the faster oxidation of exogenous choline (choline→betaine→dimethylglycine→sarcosine) in these men and was consistent with their increased reliance on choline as a source of methyl groups. Sarcosine is also produced from glycine in a cytosolic reaction catalyzed by glycine N-methyltransferase (GNMT)—an enzyme subject to reciprocal regulation by SAM (positive cooperativity) and 5-methylTHF (allosteric inhibition) (32, 33). Given the lower serum folate in this group of men (Table 1), it is possible that a less inhibited GNMT led to greater production of sarcosine (and homocysteine) in those with the 677TT genotype.
Finally, these data show that daily oral administration of a choline tracer over a 3-wk period leads to measurable enrichments in choline and its derivatives in humans. As such, these data can provide guidance for future studies designed to quantitatively assess the effect of varied choline intakes or genetic variation on choline metabolism. The multiple methyl labeling strategy that we used also suggests that inputs from SAM for PEMT-derived PtdCho can be estimated by conventional mass isotopomer distribution analysis (34), although somewhat higher labeling would be required to enable measurements of the d6 isotopomer, which was below the detection limit in the present study. In principle, the mass spectra yield relative abundances of d0, d3, d6, and d9 choline isotopomers. The d3 and d6 concentrations represent the minimum of 2 isotopomers required to calculate enrichment of the precursor methyl pool. Analysis of this labeling would enable an estimate of the dilution and net flux of dietary choline–derived methyl groups as they pass through the PEMT pathway.
Acknowledgments
The authors’ responsibilities were as follows—MAC and JFG: designed the research; WW, JY, OM, SPS, and RHA: conducted the research; JY: analyzed the data; MAC and JY: wrote the paper with significant input from JFG and JTB; and MAC: had primary responsibility for the final content. All authors read and approved the final manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Zeisel SH, Blusztajn J. Choline and human nutrition. Annu Rev Nutr 1994;14:269–96 [DOI] [PubMed] [Google Scholar]
- 2.Cui Z, Houweling M. Phosphatidylcholine and cell death. Biochim Biophys Acta 2002;1585:87–96 [DOI] [PubMed] [Google Scholar]
- 3.Reo NV, Adinehzadeh M, Foy B. Kinetic analyses of liver phosphatidylcholine and phosphatidylethanolamine biosynthesis using 13C NMR spectroscopy. Biochim Biophys Acta 2002;1580:171–88 [DOI] [PubMed] [Google Scholar]
- 4.Niculescu MD, Zeisel S. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr 2002;132:2333S–5S [DOI] [PubMed] [Google Scholar]
- 5.Roje S. S-Adenosyl-L-methionine: beyond the universal methyl group donor. Phytochemistry 2006;67:1686–98 [DOI] [PubMed] [Google Scholar]
- 6.Yao ZM, Vance DE. The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J Biol Chem 1988;263:2998–3004 [PubMed] [Google Scholar]
- 7.Buchman A, Ament M, Sohel M, et al. Choline deficiency causes reversible hepatic abnormalities in patients receiving parenteral nutrition: proof of a human choline requirement: a placebo-controlled trial. J Parenter Enteral Nutr 2001;25:260–8 [DOI] [PubMed] [Google Scholar]
- 8.da Costa K, Badea M, Fischer L, Zeisel S. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr 2004;80:163–70 [DOI] [PubMed] [Google Scholar]
- 9.da Costa KA, Niculescu M, Craciunescu C, Fischer L, Zeisel S. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am J Clin Nutr 2006;84:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niculescu MD, da Costa K, Fischer L, Zeisel S. Lymphocyte gene expression in subjects fed a low-choline diet differs between those who develop organ dysfunction and those who do not. Am J Clin Nutr 2007;86:230–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Detopoulou P, Panagiotakos D, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr 2008;87:424–30 [DOI] [PubMed] [Google Scholar]
- 12.Shaw GM, Carmichael S, Yang W, Selvin S, Schaffer D. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol 2004;160:102–9 [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Gammon M, Zeisel S, et al. Choline metabolism and risk of breast cancer in a population-based study. FASEB J 2008;22:2045–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine, National Academy of Sciences Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin and choline. Washington, DC: National Academy Press, 1998 [PubMed] [Google Scholar]
- 15.Christensen KE, Rozen R. Genetic variation: effect on folate metabolism and health. Bailey LB, Folate in health and disease. Boca Raton, FL: CRC Press, 2009;75–110 [Google Scholar]
- 16.Solis C, Veenema K, Ivanov AA, et al. Folate intake at RDA levels is inadequate for Mexican American men with the methylenetetrahydrofolate reductase 677TT genotype. J Nutr 2008;138:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veenema K, Solis C, Li R, et al. Adequate Intake levels of choline are sufficient for preventing elevations in serum markers of liver dysfunction in Mexican American men but are not optimal for minimizing plasma total homocysteine increases after a methionine load. Am J Clin Nutr 2008;88:685–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guinotte CL, Burns M, Axume J, et al. Methylenetetrahydrofolate reductase 677C-> T variant modulates folate status response to controlled folate intakes in young women. J Nutr 2003;133:1272–80 [DOI] [PubMed] [Google Scholar]
- 19.Frosst P, Blom H, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995;10:111–3 [DOI] [PubMed] [Google Scholar]
- 20.Holm PI, Ueland P, Kvalheim G, Lien E. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clin Chem 2003;49:286–94 [DOI] [PubMed] [Google Scholar]
- 21.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem 2002;74:4734–40 [DOI] [PubMed] [Google Scholar]
- 22.Abratte CM, Wang W, Li R, Moriarty DJ, Caudill MA. Folate intake and the MTHFR C677T genotype influence choline status in young Mexican American women. J Nutr Biochem 2008;19:158–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichtenstein AH, Cohn JS, Hachey DL, Millar JS, Ordovas JM, Schaefer EJ. Comparison of deuterated leucine, valine and lysine in the measurement of human apolipoprotein A-I and B-100 kinetics. J Lipid Res 1990;31:1693–701 [PubMed] [Google Scholar]
- 24.Davis SR, Stacpoole PW, Williamson J, et al. Tracer-derived total and folate- dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab 2004;286:E272–9 (Published erratum appears in Am J Physiol Endocrinol Metab 2004;286:E674) [DOI] [PubMed] [Google Scholar]
- 25.Stabler SP, Lindenbaum J, Savage DG, Allen RH. Elevation of serum cystathionine in patients with cobalamin and folate deficiency. Blood 1993;81:3404–13 [PubMed] [Google Scholar]
- 26.Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism 1993;42:1448–60 [DOI] [PubMed] [Google Scholar]
- 27.DeLong CJ, Hicks AM, Cui Z. Disruption of choline methyl group donation for phosphatidylethanolamine methylation in hepatocarcinoma cells. J Biol Chem 2002;277:17217–25 [DOI] [PubMed] [Google Scholar]
- 28.Cui Z, Vance DE. Expression of phosphatidylethanolamine N-methyltransferase-2 is markedly enhanced in long term choline-deficient rats. J Biol Chem 1996;271:2839–43 [DOI] [PubMed] [Google Scholar]
- 29.DeLong CJ, Shen Y-J, Thomas MJ, Cui Z. Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem 1999;274:29683–8 [DOI] [PubMed] [Google Scholar]
- 30.Watkins SM, Zhu X, Zeisel SH. Phosphatidylethanolamine N-methyltransferase activity and dietary choline regulate liver-plasma lipid flux and essential fatty acid metabolism in mice. J Nutr 2003;133:3386–91 [DOI] [PubMed] [Google Scholar]
- 31.Schwahn BC, Chen Z, Laryear MD, et al. Homocysteine-betaine interactions in a murine model of 5,10-methylenetetrahydrofolate reductase deficiency. FASEB J 2003;17:512–4 [DOI] [PubMed] [Google Scholar]
- 32.Wagner C, Briggs W, Cook R. Inhibition of glycine N-methyltransferase activity by folate derivatives: implications for regulation of methyl group metabolism. Biochem Biophys Res Commun 1985;127:746–52 [DOI] [PubMed] [Google Scholar]
- 33.Yeo EJ, Briggs WT, Wagner C. Inhibition of glycine N-methyltransferase by 5-methyltetrahydrofolate pentaglutamate. J Biol Chem 1999;274:37559–64 [DOI] [PubMed] [Google Scholar]
- 34.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic and experimental considerations. Am J Physiol 1999;276:E1146–70 [DOI] [PubMed] [Google Scholar]