Abstract
Background: Cross-sectional data indicate that central adiposity is associated with cardiovascular disease risk, independent of total adiposity. The use of longitudinal data to investigate the relation between changes in fat distribution and the emergence of risk factors is limited.
Objective: We tested the hypothesis that age-related change in waist circumference (to reflect central adiposity) during adolescence is a significant predictor of longitudinal change in cardiovascular disease risk, after adjustment for change in body mass index (BMI) z score (to reflect total adiposity) in a cohort of postmenarcheal adolescent females. We also tested whether race modified this relation.
Design: We analyzed publicly available data from the National Heart, Lung, and Blood Institute Growth and Health Study. Longitudinal regression models were fitted to investigate the independent effects of changes in waist circumference on cardiovascular disease risk factors.
Results: Steeper age-related increases in waist circumference over time were associated with a greater increase in LDL-cholesterol concentrations, systolic blood pressure, diastolic blood pressure, and homeostasis model assessment of insulin resistance, after adjustment for BMI z score, in white but not in black females. Change in waist circumference was not a statistically significant predictor of age-related changes in HDL-cholesterol, triglyceride, insulin, and glucose concentrations, after adjustment for changes in BMI z score, in either white or black females.
Conclusions: Our research suggests that monitoring waist circumference in addition to BMI z score has the potential to identify adolescents at risk of the emergence of cardiovascular disease risk factors, at least in white females. The data also suggest that race may modify the relation between fat distribution pattern and cardiovascular disease risk factors.
INTRODUCTION
Obesity, a leading contributor to cardiovascular disease morbidity and mortality in the United States, places a heavy burden on society (1). Obesity's effect on cardiovascular disease risk often emerges in childhood and adolescence; those with excess adiposity during these critical periods have an increased risk of later cardiovascular disease and obesity (2–5). Because these risk factors track from childhood into adulthood, it is important to study them early, even if frank heart disease rarely presents before adulthood (6–15).
In adults, the intraindividual distribution of fat has long been recognized as important, with central adiposity conferring greater disease risk, perhaps because of the visceral fat depot (16–24). Although visceral fat stores in children are small before puberty, they increase and show considerable variability after puberty (25–29). The importance of fat distribution is evident in children and adolescents, with visceral fat (measured by magnetic resonance imaging) positively related to blood pressure and total cholesterol (TC), LDL-cholesterol, triglyceride, and basal insulin concentrations and inversely associated with HDL-cholesterol concentrations and insulin sensitivity (30–38). Not all studies, however, have yielded significant results (39).
Significant relations with cardiovascular disease risk factors are also seen when central adiposity is measured indirectly with an anthropometric measure such as waist circumference (WC). Such measures are commonly used in epidemiologic studies that investigate the role of central adiposity in disease risk of adults and have been used as a proxy for visceral fat in children (40). Cross-sectionally, in simple regression models, WC is a significant predictor of blood pressure and TC, LDL-cholesterol, HDL-cholesterol, and triglyceride concentrations (41–45). Because WC is correlated with total body fat, both variables must be included in regression models to investigate their independent effects (40). Some evidence indicates that cross-sectional relations between WC (or associated ratios such as waist-to-hip ratio or waist-to-height ratio) and cardiovascular disease risk (blood pressure and TC, LDL-cholesterol, HDL-cholesterol, and triglyceride concentrations) are independent of total adiposity, as measured by body mass index (BMI) (46–54). Other research, however, has suggested that BMI and WC do not have strong independent effects on risk factors (55). WC and related ratios predict cardiovascular disease risk, motivating the publication of percentiles from many pediatric populations (56–64). Racial differences in patterns of fat distribution emerge as early as age 7–10 y, and some, but not all, studies (35, 65) have found that race modifies the effect of central adiposity on cardiovascular disease risk (52, 66). We identified racial differences in the deposition of central adiposity during adolescence in previous analyses of this cohort (67).
The use of longitudinal data to investigate the relation between central adiposity and cardiovascular disease risk has been limited. We aimed to determine whether changes in central adiposity during adolescence, independent of changes in total adiposity, are significant predictors of cardiovascular disease risk in a large, biracial cohort. We tested the hypothesis that age-related change in WC (reflecting central adiposity) during adolescence was a significant predictor of longitudinal change in cardiovascular disease risk factors after adjusting for change in BMI z score (reflecting total adiposity). We also tested whether race modified this relation.
SUBJECTS AND METHODS
Data
We analyzed publicly available data from the subjects in the National Heart, Lung, and Blood Institute Growth and Health Study (NGHS)—a prospective cohort that was established to investigate how dietary patterns, physical activity levels, and psychosocial factors are related to the development of obesity in girls. This study was described previously in detail (68). Briefly, this multicenter, annual, longitudinal study consisted of 2379 girls enrolled in 1987–1988 at the age of 9–10 y in racially concordant households (race self-declared as black or white; Hispanic children were excluded) from public and parochial schools (recruited from the Richmond School district near Berkeley, CA, and from Cincinnati, OH) and a large Health Maintenance Organization in the Washington, DC area. Of the eligible girls, 78% were enrolled. At each study center, the respective Institutional Review Board approved the NGHS protocol, and all participants and their parents gave informed consent. Our investigation was approved by the Institutional Review Board of Tufts Medical Center.
Measurements
All NGHS measurements were made according to study protocol by certified, trained staff who were annually retrained and were monitored for consistency (68). At each annual visit, height and weight were measured in duplicate. Height was measured to the nearest 0.1 cm in subjects wearing socks, using custom-made stadiometers, and weight was measured by using Health-O-Meter electronic scales, to the nearest 0.1 kg, with subjects in gowns. The minimum above-waist circumference (the smallest circumference of the torso, at the “natural waist,” against the skin) was measured annually, in duplicate, beginning with NGHS visit 2. BMI was calculated as weight (in kg) divided by the square of height (in m). Study participants were asked annually whether they had started menstruation; the data set included self-reported age at which menstruation started, measured in years to one decimal place.
Fasting TC, HDL-cholesterol, and triglyceride concentrations were measured in the morning of annual visits 3, 5, 7, and 10. TC, HDL-cholesterol, and triglyceride concentrations were analyzed enzymatically with a commercially available method, and LDL cholesterol was calculated by using a modified Friedewald formula. Fasting insulin and glucose concentrations were measured at NGHS visits 7 and 10. Blood pressure was measured in triplicate at each annual visit with a standard mercury sphygmomanometer while subjects were seated with feet resting flat and the right arm resting at heart level. Cuff sizes appropriate to upper arm circumferences were used.
Statistical analyses
Subjects who had data from ≥2 valid postmenarcheal visits were included in this analysis. We calculated age-specific BMI z score, using the Centers for Disease Control and Prevention growth reference, to serve as a measure of total adiposity (69, 70). The homeostasis model assessment (HOMA) was calculated as an index of insulin resistance, and used to investigate the longitudinal change thereof (71, 72). Blood pressure z scores were calculated as recommended by the National High Blood Pressure Education Program (73).
For each subject, individual change trajectories for each outcome were constructed. We examined each trajectory for outliers and investigated whether a linear model was appropriate for age-related changes in risk. Separately for each risk factor, linear longitudinal growth was modeled in SAS by using PROC MIXED, with the risk factor level as the continuous time-varying outcome variable. Age (in mo) relative to menarche was the continuous “time” variable, which was calculated by subtracting the subject's age at menarche from their age at the study visit. WC and BMI z score were modeled as time-varying covariates. Data were stratified a priori on race, given the racial differences in age-related growth in central adiposity in this cohort; formal statistical tests of the interaction by race are still provided (67). All analyses were conducted in SAS (version 9.1; SAS Institute, Cary, NC).
We generated hypothetical trajectories for the risk factors of interest to represent the experiences of prototypical “average” NGHS subjects. First, the average BMI z score was calculated, by race and age (in y). Then, the WC values representing the 25th, 50th, and 75th percentiles were extracted. Using these values and the parameter estimates from the regression models, we created theoretical risk factor trajectories for girls with an average BMI z score, who differed only with respect to WC.
RESULTS
Subjects who had data from ≥2 valid postmenarcheal visits were included in this analysis. For analyses on HDL-cholesterol, LDL-cholesterol, and triglyceride concentrations, the sample size was 678 white females and 797 black females, with data from 1753 and 2200 subject visits, respectively, from visit 3, 5, 7, and 10 (Table 1). For analyses on longitudinal changes in blood pressure, the sample size was 1071 white females and 1153 black females, with 6070 and 7372 subject visits, respectively. For analyses on insulin, glucose, and HOMA, the sample size was 424 white females and 439 black females, with data from visits 7 and 10, respectively. Biologically implausible values for 14 measurements were excluded from the analyses.
TABLE 1.
Subject characteristics by visit: postmenarcheal data only1
| Visit |
|||||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| White | |||||||||
| Age (y) | 11.39 ± 0.52 | 12.29 ± 0.55 | 13.10 ± 0.54 | 14.02 ± 0.56 | 15.00 ± 0.56 | 15.99 ± 0.57 | 17.01 ± 0.59 | 17.94 ± 0.58 | 19.03 ± 0.64 |
| WC (cm) | 68.98 ± 7.72 (93) | 69.10 ± 8.47 (305) | 70.05 ± 8.64 (587) | 70.14 ± 8.97 (794) | 70.73 ± 8.99 (783) | 71.27 ± 9.52 (854) | 72.41 ± 9.88 (900) | 73.35 ± 10.23 (926) | 74.71 ± 11.19 (977) |
| BMI z score | 0.93 ± 0.77 (94) | 0.69 ± 0.85 (304) | 0.66 ± 0.84 (590) | 0.51 ± 0.88 (802) | 0.46 ± 0.87 (791) | 0.37 ± 0.92 (871) | 0.32 ± 0.95 (904) | 0.31 ± 1.00 (944) | 0.29 ± 1.01 (920) |
| LDL-C (mg/dL) | — | 90.51 ± 23.95 (221) | — | 91.51 ± 24.28 (581) | — | 93.17 ± 26.43 (645) | — | — | 98.62 ± 30.58 (625) |
| HDL-C (mg/dL) | — | 50.96 ± 10.35 (222) | — | 53.86 ± 10.50 (582) | — | 52.09 ± 10.10 (646) | — | — | 52.44 ± 11.27 (627) |
| TG (mg/dL) | — | 83.18 ± 40.85 (221) | — | 84.71 ± 43.95 (580) | — | 85.11 ± 41.80 (644) | — | — | 92.85 ± 43.54 (627) |
| SBP z score | −0.20 ± 0.80 (93) | −0.03 ± 0.81 (305) | −0.10 ± 0.79 (589) | −0.17 ± 0.82 (802) | −0.41 ± 0.79 (793) | −0.32 ± 0.86 (876) | −0.38 ± 0.88 (917) | −0.33 ± 0.86 (951) | −0.36 ± 0.85 (940) |
| DBP z score | −0.33 ± 1.09 (91) | −0.10 ± 0.83 (301) | −0.26 ± 0.79 (583) | −0.09 ± 0.81 (800) | −0.29 ± 0.89 (792) | −0.01 ± 0.88 (875) | −0.20 ± 0.85 (917) | −0.20 ± 0.84 (951) | −0.02 ± 0.84 (940) |
| log Insulin (μU/mL) | — | — | — | — | — | 2.33 ± 0.51 (624) | — | — | 1.94 ± 0.75 (706) |
| Glucose (mg/dL) | — | — | — | — | — | 75.22 ± 13.21 (609) | — | — | 91.99 ± 69.21 (735) |
| HOMA | — | — | — | — | — | 2.20 ± 1.50 (606) | — | — | 2.07 ± 3.64 (706) |
| Black | |||||||||
| Age (y) | 11.37 ± 0.52 | 12.28 ± 0.54 | 13.13 ± 0.56 | 14.09 ± 0.59 | 15.07 ± 0.58 | 16.09 ± 0.58 | 17.07 ± 0.58 | 18.04 ± 0.58 | 19.16 ± 0.65 |
| WC (cm) | 69.87 ± 9.47 (271) | 71.31 ± 10.21 (602) | 71.69 ± 10.43 (877) | 72.93 ± 11.20 (1004) | 73.49 ± 11.97 (937) | 75.10 ± 12.58 (952) | 76.17 ± 13.30 (971) | 77.50 ± 13.95 (969) | 79.19 ± 14.41 (1041) |
| BMI z score | 0.97 ± 0.90 (272) | 0.88 ± 0.96 (599) | 0.83 ± 0.94 (879) | 0.79 ± 0.98 (1006) | 0.75 ± 1.00 (957) | 0.75 ± 1.00 (962) | 0.73 ± 1.07 (957) | 0.71 ± 1.11 (969) | 0.70 ± 1.12 (932) |
| LDL-C (mg/dL) | — | 97.17 ± 24.38 (421) | — | 91.65 ± 25.17 (691) | — | 94.60 ± 29.31 (687) | — | — | 97.94 ± 28.55 (718) |
| HDL-C (mg/dL) | — | 54.41 ± 12.03 (422) | — | 57.38 ± 11.41 (691) | — | 55.66 ± 11.61 (687) | — | — | 54.68 ± 12.33 (718) |
| TG (mg/dL) | — | 70.65 ± 30.30 (423) | — | 66.73 ± 26.68 (691) | — | 68.85 ± 30.63 (689) | — | — | 70.49 ± 32.35 (718) |
| SBP z score | −0.17 ± 0.79 (272) | 0.05 ± 0.79 (602) | −0.054 ± 0.80 (878) | −0.08 ± 0.84 (1006) | −0.21 ± 0.91 (958) | −0.12 ± 0.83 (968) | −0.16 ± 0.86 (971) | −0.10 ± 0.86 (988) | −0.05 ± 0.92 (963) |
| DBP z score | −0.19 ± 0.88 (266) | −0.07 ± 0.86 (588) | −0.20 ± 0.85 (873) | −0.06 ± 0.83 (1005) | −0.09 ± 0.88 (953) | 0.08 ± 0.87 (966) | 0.01 ± 0.93 (969) | 0.00 ± 0.89 (984) | 0.18 ± 0.85 (962) |
| log Insulin (μU/mL) | — | — | — | — | — | 2.60 ± 0.61 (662) | — | — | 2.25 ± 0.81 (777) |
| Glucose (mg/dL) | — | — | — | — | — | 77.36 ± 21.23 (658) | — | — | 89.95 ± 39.09 (798) |
| HOMA | — | — | — | — | — | 3.55 ± 5.96 (647) | — | — | 2.98 ± 4.19 (767) |
All values are means ± SDs; n in parentheses. HOMA, homeostasis model assessment; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; TG, triglycerides; WC, waist circumference.
WC increased during adolescence, as expected, with steeper increases seen in black females. On average, BMI z scores decreased during adolescence, and the decrease was relatively larger in white females than in black females. Both LDL-cholesterol and triglyceride concentrations increased in white females, but not in black females. The HDL-cholesterol concentration did not change significantly in either group. Glucose concentrations increased in both groups. Insulin concentrations and HOMA decreased, which reflected the end of pubertal development and its associated insulin resistance.
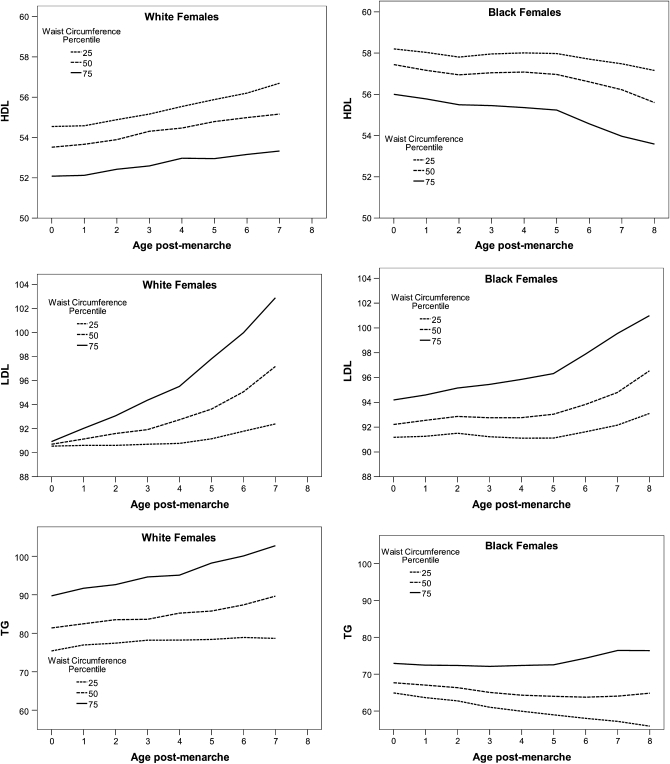
Multilevel regression models were fitted to test whether the change in central adiposity was a significant predictor of change in each risk factor, after adjustment for change in total adiposity. Steeper age-related increases in WC over time were associated with an increase in LDL-cholesterol concentrations, after adjustment for BMI z score, but only in white females (Table 2; P < 0.001). This significant relation with LDL-cholesterol concentration was not seen in black females (the formal test for the interaction by race was statistically significant, P = 0.008). Prototypical trajectories representing the characteristics of subjects with an average BMI z score at 3 contrasting levels of WC indicate steeper age-related increase in white females (Figure 1).
TABLE 2.
Race-specific longitudinal regression models predicting changes in lipid concentrations from age-related changes in waist circumference: postmenarcheal data only for visits 3, 5, 7, and 101
| LDL cholesterol (mg/dL) |
HDL cholesterol (mg/dL) |
Triglycerides (mg/dL) |
||||
| White females | Black females | White females | Black females | White females | Black females | |
| n | 678 (1753 obs) | 797 (2200 obs) | 679 (1758 obs) | 797 (2201 obs) | 678 (1753 obs) | 798 (2204 obs) |
| Intercept | 86.94** | 73.03** | 71.21** | 72.00** | −19.34 | 21.54 |
| Age (y postmenarche) | −7.05* | −1.55 | 0.34 | −0.06 | −3.56 | −4.67* |
| Waist (cm) | 0.04 | 0.26 | −0.26** | −0.19* | 1.51** | 0.69** |
| Age × waist | 0.11** | 0.02 | −4.9 × 10minus3 | −1.1 × 10minus3 | 0.05 | 0.05 |
| BMI z score | 2.65 | 2.25 | −0.90 | −2.37* | 0.25 | −0.47 |
| Age × BMI z score | −0.40 | 0.06 | 0.32* | 0.18 | −0.86 | −0.52 |
For SEs, see the supplemental tables under “Supplemental data” in the online issue. obs, observations. *P < 0.05, **P < 0.001.
FIGURE 1.
Prototypical LDL-cholesterol, HDL-cholesterol, and triglyceride (TG) (mg/dL) trajectories for girls with an average BMI z score and select waist circumferences.
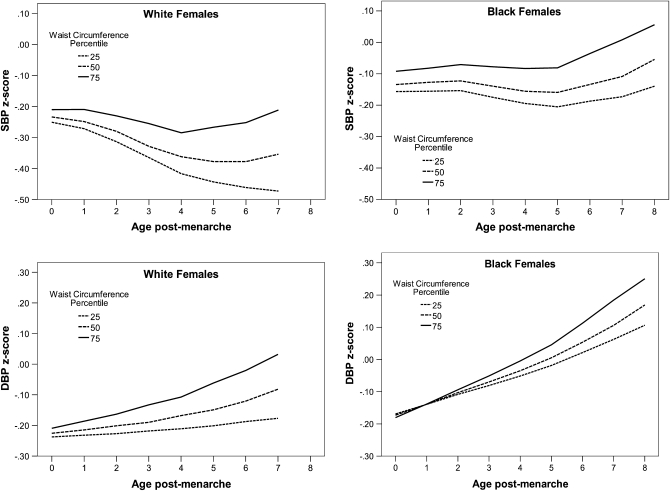
For age-related changes in HDL-cholesterol and triglyceride concentrations, change in WC was not a statistically significant predictor after adjustment for changes in BMI z score, in either white or black females (Table 2; HDL: P = 0.74 for white females and P = 0.90 for black females; triglyceride: P = 0.45 for white females and P = 0.06 for black females). The estimates for black and white females were similar in magnitude, and the formal test of the interaction was not statistically significant for HDL-cholesterol or triglyceride concentrations (P = 0.50 and 0.29, respectively;). Postmenarcheal age-related increases in blood pressure (both systolic blood pressure and diastolic blood pressure z score) were significantly predicted by increases in WC after adjustment for changes in BMI z score; however, again, only in white females (systolic blood pressure: P = 0.002 in white females and P = 0.21 in black females; diastolic blood pressure: P = 0.018 in white females and P = 0.07 in black females, Table 3, Figure 2).
TABLE 3.
Race-specific longitudinal regression models predicting changes in blood pressure (z scores) from age-related changes in waist circumference: postmenarcheal data only for visits 2–101
| Systolic blood pressure |
Diastolic blood pressure |
|||
| White females | Black females | White females | Black females | |
| n | 1071 (6086 obs) | 1153 (7412 obs) | 1071 (6070 obs) | 1153 (7372 obs) |
| Intercept | −0.64* | −0.71** | −0.43 | −0.16 |
| Age (y postmenarche) | −0.18** | −0.04 | −0.12* | −0.04 |
| Waist (cm) | 4.3 × 10minus3 | 5.6 × 10minus3 | 3.0 × 10minus3 | −9.9 × 10minus4 |
| Age × waist | 2.3 × 10minus3* | 6.0 × 10minus4 | 1.9 × 10minus3* | 1.1 × 10minus3 |
| BMI z score | 0.28** | 0.26** | 0.02 | 0.07 |
| Age × BMI z score | −0.02* | −0.01* | −0.01 | −0.01 |
For SEs, see the supplemental tables under “Supplemental data” in the online issue. obs, observations. *P < 0.05, **P < 0.001.
FIGURE 2.
Prototypical systolic blood pressure (SBP) and diastolic blood pressure (DBP) z score trajectories for girls with an average BMI z score and select waist circumferences.
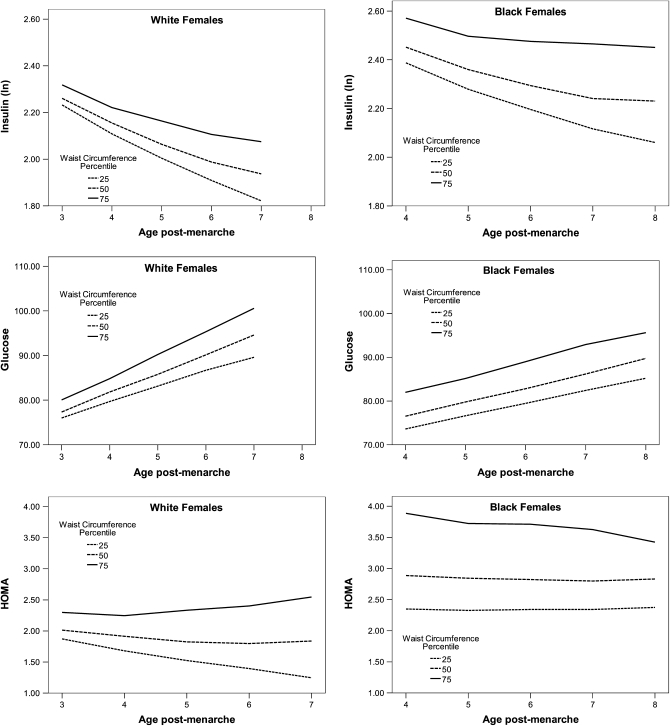
For postmenarcheal changes in insulin and glucose concentrations and in HOMA, models were fitted to data from NGHS visits 7 and 10. Changes in WC did not predict changes in insulin or glucose concentrations, after adjustment for changes in BMI z score (insulin: P = 0.14 for white females and 0.23 for black females; glucose: P = 0.22 for white females and 0.81 for black females; Table 4, ; formal tests for the interaction by race were not statistically significant. Steeper increases in WC predicted significantly higher HOMA after adjustment for BMI z score, but only in white females (P = 0.003; Table 4, Figure 3; the test for the interaction by race was significant, P = 0.014). The same relations remained statistically significant after a Bonferonni correction for the parameter estimates of interest.
TABLE 4.
Race-specific longitudinal regression models predicting change in insulin (ln), glucose, and homeostasis model assessment (HOMA) from age-related change in waist circumference: postmenarcheal data only for visits 7 and 101
| Insulin (μU/mL) |
Glucose (mg/dL) |
HOMA |
||||
| White females | Black females | White females | Black females | White females | Black females | |
| n | 428 | 453 | 436 | 453 | 424 | 439 |
| Intercept | 2.4742** | 2.42** | 61.14* | 24.82 | 2.27 | −7.97 |
| Age (y postmenarche) | −0.2917* | −0.25* | −3.55 | 2.97 | −1.10* | 0.75 |
| Waist (cm) | 4.9 × 10minus4 | 5.5 × 10minus3 | 8.1 × 10minus2 | 0.65* | 2.1 × 10minus4 | 0.16* |
| Age × waist | 2.7 × 10minus3 | 1.9 × 10minus3 | 0.11 | −1.1 × 10minus2 | 0.01* | −0.01 |
| BMI z score | 0.1865* | 0.09 | −0.56 | −5.76 | 0.46* | −0.32 |
| Age × BMI z score | −0.01 | 0.01 | −1.02 | 0.43 | −0.09 | 0.09 |
For SEs, see the supplemental tables under “Supplemental data” in the online issue. *P < 0.05, **P < 0.001.
FIGURE 3.
Prototypical glucose (mg/dL), insulin (μU/mL), and homeostasis model assessment (HOMA) trajectories for girls with an average BMI z score and select waist circumferences.
DISCUSSION
We tested the hypothesis that change in the WC of females during adolescence, as a measure of central adiposity, was a significant predictor of longitudinal change in cardiovascular disease risk, after adjusting for change in BMI z score (a proxy for total adiposity). We found that increases in WC significantly predicted increases in LDL-cholesterol concentrations, systolic and diastolic blood pressure, and HOMA, independent of changes in total adiposity, but only in white females. HDL-cholesterol, triglyceride, glucose, and insulin concentrations were not related to changes in WC after adjustment for BMI z score in either white or black females.
In cross-sectional data on children and adolescents, LDL-cholesterol concentrations, blood pressure, and insulin resistance have been linked to central adiposity, independent of total adiposity For example, children aged 5–17 y in the Bogalusa Study who had high WC had significantly higher LDL-cholesterol concentrations than did those of comparable height and weight with a low WC (74). Results similar to ours have also been seen for blood pressure; in prepubertal Italian children, WC was associated with higher blood pressure readings after adjustment for BMI (46). Other evidence also supports this BMI-independent link between WC and blood pressure (52). Finally, insulin resistance, which we approximated on the basis of HOMA, has been shown to be related to WC, after adjustment for BMI z score (47).
We found no evidence that changes in WC were predictive of changes in HDL-cholesterol, triglyceride, insulin, or glucose concentrations. Some cross-sectional studies have yielded results similar to ours. In Spanish females aged 13–18 y, WC was not related to HDL-cholesterol concentration after control for BMI (48). Other researchers, however, have found that central adiposity is a significant predictor of these risk factors, such as the aforementioned study on prepubertal Italian children (46). In Portuguese children of ≈13 y of age, high central adiposity (measured by skinfold thickness) was a significant predictor of low HDL-cholesterol concentration, after control for total body fat as measured by dual-energy X-ray absorptiometry (49). In prepubertal children in the nationally representative National Health and Nutrition Examination Survey (NHANES), central adiposity (based on waist-to-hip ratio) was associated with increased triglyceride concentrations, after adjustment for BMI (50). In addition, in the aforementioned Bogalusa Study, WC predicted insulin after adjustment for height and weight (74).
In some cross-sectional studies, race modified the effect of WC on cardiovascular disease risk, after adjustment for BMI. In girls aged 7–10 y, increased subcutaneous abdominal adipose tissue (measured by magnetic resonance imaging; n = 40) was significantly related to insulin concentrations in black, but not in white, females (66). This racial difference, however, was not evident with respect to the subjects’ lipid profiles. Similarly, in prepubertal girls who had visceral fat measured by computed tomography (n = 61), no evidence that the relation between central adiposity and cardiovascular disease risk (insulin sensitivity, triglycerides, and HDL cholesterol) differed by race was observed, after adjustment for total fat (35). In adults, central adiposity confers greater disease risk in white woman than in black women, independent of total adiposity (75, 76), which suggests that differences arise during adolescence.
One explanation for the racial differences in our analysis is that central adiposity comprises both visceral and subcutaneous fat depots. In adults, black women have less visceral fat than whites after total adiposity is accounted for (77, 78). Similarly, white children have more total and intraabdominal fat than do black children of similar BMI (26, 29, 79, 80). If the visceral adipose depot is important in the relation between fat distribution and cardiovascular disease risk factors, differential deposition of fat by black and white female adolescents could explain our findings.
A major strength of our analysis was the collection of longitudinal data during adolescence to investigate changes in cardiovascular disease risk over this critical period. This permits investigations about changes in body composition and their effects on risk factors—hypotheses not testable with cross-sectional data. The NGHS provides data for the longitudinal analysis of a large sample of girls with an exceptionally high follow-up rate (89% at the final visit), which allows statistical control for variables of interest.
We elected to use commonly used clinical measures: WC as a proxy for central adiposity and BMI z score to reflect total adiposity. A limitation of our analyses was the absence of dual-energy X-ray absorptiometry and computed tomography data—direct measures of visceral fat. If these tools were used, some common critiques of BMI would be avoided, such as the inability of BMI to distinguish between differences in body composition (eg, higher bone density of black females relative to whites) (81). These measures, however, are not feasible in a large epidemiologic study such as the NGHS (82). The use of BMI and WC allows for large sample sizes and provides ample statistical power to detect significant differences. Although BMI and WC could contribute to measurement error, it is likely to be random and thus does not introduce bias to the observed relations. Also, although the NGHS measurement protocol for WC does not accord to current recommendations by the National Institutes of Health or the World Health Organization, the use of a consistent protocol over the course of the cohort likely outweighs the small differences that arise from different measurement protocols (83, 84). Of note, visceral fat stores, which are small during adolescence, might not be the only factor linking central adiposity and cardiovascular disease risk (27). Thus, it is possible that WC is more appropriate in this situation, given that the measure also encompasses subcutaneous fat, which has been suggested to have a role in insulin resistance for children with small amounts of visceral fat (85).
Another potential limitation of our analysis was the generalizability of the NGHS data. This study was not designed to be representative of any specific population; thus, the descriptive statistics for this large cohort of black and white females—enrolled over 20 y ago from 3 parts of the country—do not necessarily reflect the contemporary US population, in which the prevalence of obesity is high. The NGHS sample did, however, have a baseline prevalence of obesity comparable with the contemporaneous NHANES III and a final-year prevalence similar to NHANES 1999–2000 (86, 87). For example, in NHANES 1999–2000, the prevalence of overweight (>85th percentile BMI z score) at age 12–19 y was 25.4% in white females and 45.4% in black females; in our NGHS analysis, the respective values were 22.9% in white females and 43.7% in black females. Also, the prevalences of obesity in NHANES 1999–2000 and the final year of NGHS were similar: 12.4% of white females and 26.6% of black females in NHANES compared with 10.9% of white females and 24.7% of black females in the NGHS. Furthermore, there is no reason to believe that our major finding—that changes in WC during adolescence can lead to changes in select risk factors—would not be replicated in other cohorts of postmenarcheal adolescent females.
In conclusion, we found that changes in central adiposity during adolescence were significant predictors of changes in LDL-cholesterol concentrations, blood pressure, and insulin resistance for white, but not black, females. These relations were independent of changes in total adiposity. This suggests that monitoring WC in addition to BMI z score has the potential to identify adolescents at risk of the emergence of cardiovascular disease risk factors, at least in white females. The data also suggest that race is a potential effect modifier in the relation between fat distribution patterns and cardiovascular disease risk—an observation that should be taken into consideration for future research.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—DJT: responsible for the study design, data analysis, and writing of the manuscript under the supervision of AM; GED: provided statistical consultation; and AHL and SRD: provided critical input and advice. All authors contributed manuscript revisions and approved the final version. None of the authors had any conflicts of interest to report.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics—2009 update. A report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation 2009;119:480–6 [DOI] [PubMed] [Google Scholar]
- 2.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents: a follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992;327:1350–5 [DOI] [PubMed] [Google Scholar]
- 3.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord 1999;23:S2–11 [DOI] [PubMed] [Google Scholar]
- 4.Daniels SR. Is there an epidemic of cardiovascular disease on the horizon? J Pediatr 1999;134:665–6 [DOI] [PubMed] [Google Scholar]
- 5.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–73 [DOI] [PubMed] [Google Scholar]
- 6.Kavey REW, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr 2003;142:368–72 [DOI] [PubMed] [Google Scholar]
- 7.Lauer RM, Connor WE, Leaverton PE. Coronary heart disease risk factors in school children: the Muscatine study. J Pediatr 1975;86:697–706 [DOI] [PubMed] [Google Scholar]
- 8.Laskarzewski P, Morrison JA, de Groot I. Lipid and lipoprotein tracking in 108 children over a four year period. Pediatrics 1979;64:584–91 [PubMed] [Google Scholar]
- 9.Freedman DS, Shear CL, Srinivasan SR. Tracking of serum lipids and lipoproteins in children over an 8-year period: the Bogalusa Heart Study. Prev Med 1985;14:203–16 [DOI] [PubMed] [Google Scholar]
- 10.Webber LS, Cresanta JL, Croft JB, Srinivasan SR, Berenson GS. Transitions of cardiovascular risk from adolescence to young adulthood—the Bogalusa Heart Study: II. Alterations in anthropomorphic blood pressure and serum lipoprotein variables. J Chronic Dis 1986;39:91–103 [DOI] [PubMed] [Google Scholar]
- 11.Mahoney LT, Lauer RM, Lee J, Clarke WR. Factors affecting tracking of coronary heart disease risk factors in children: the Muscatine study. Ann N Y Acad Sci 1991;623:120–32 [DOI] [PubMed] [Google Scholar]
- 12.Webber LS, Harsha DW, Phillips GT, Srinivasan SR, Simpson JW, Berenson GS. Cardiovascular risk factors in hispanic, white, and black children: the Brooks County and Bogalusa Heart Studies. Am J Epidemiol 1991;133:704–14 [DOI] [PubMed] [Google Scholar]
- 13.Berenson GS, Radhakrishnamurthy B, Bao W, Sriniwasan SR. Does adult-onset diabetes mellitus begin in childhood?: the Bogalusa Heart Study. Am J Med Sci 1995;310:S77–82 [DOI] [PubMed] [Google Scholar]
- 14.Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. N Engl J Med 1998;338:1650–6 [DOI] [PubMed] [Google Scholar]
- 15.Sivanandam S, Sinaiko AR, Jacobs DR, Jr, Steffen L, Moran A, Steinberger J. Relation of increase in adiposity to increase in left ventricular mass from childhood to young adulthood. Am J Cardiol 2006;98:411–5 [DOI] [PubMed] [Google Scholar]
- 16.Vague J. The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease. Am J Clin Nutr 1956;4:20–34 [DOI] [PubMed] [Google Scholar]
- 17.Bjorntorp P. The associations between obesity, adipose tissue distribution and disease. Acta Med Scand Suppl 1988;723:121–34 [DOI] [PubMed] [Google Scholar]
- 18.Despres JP, Moorjani S, Ferland M, et al. Adipose tissue distribution and plasma lipoprotein levels in obese womenL: importance of intra-abdominal fat. Arteriosclerosis 1989;9:203–10 [DOI] [PubMed] [Google Scholar]
- 19.Pouliot MC, Despres JP, Nadeau A, et al. Visceral obesity in men: associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes 1992;41:826–34 [DOI] [PubMed] [Google Scholar]
- 20.Kissebah AH, Vydelingum N, Murray R. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982;54:254–60 [DOI] [PubMed] [Google Scholar]
- 21.Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E, Sjostrom L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. BMJ 1984;289:1257–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reis JP, Macera CA, Araneta MR, Lindsay SP, Marshall SJ, Wingard DL. Comparison of overall obesity and body fat distribution in predicting risk of mortality. Obesity (Silver Spring) 2009;17:1232–9 [DOI] [PubMed] [Google Scholar]
- 23.Janssen I. Influence of age on the relation between waist circumference and cardiometabolic risk markers. Nutr Metab Cardiovasc Dis 2009;19:163–9 [DOI] [PubMed] [Google Scholar]
- 24.Page JH, Rexrode KM, Hu F, Albert CM, Chae CU, Manson JE. Waist-height ratio as a predictor of coronary heart disease among women. Epidemiology 2009;20:361–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Ridder CM, Thijssen JH, Bruning PF, Van den Brande JL, Zonderland ML, Erich WB. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab 1992;75:442–6 [DOI] [PubMed] [Google Scholar]
- 26.Goran MI, Nagy TR, Treuth MS, et al. Visceral fat in white and African American prepubertal children. Am J Clin Nutr 1997;65:1703–8 [DOI] [PubMed] [Google Scholar]
- 27.Brambilla P, Manzoni P, Agostini G, et al. Persisting obesity starting before puberty is associated with stable intraabdominal fat during adolescence. Int J Obes Relat Metab Disord 1999;23:299–303 [DOI] [PubMed] [Google Scholar]
- 28.Fox KR, Peters DM, Sharpe P, Bell M. Assessment of abdominal fat development in young adolescents using magnetic resonance imaging. Int J Obes Relat Metab Disord 2000;24:1653–9 [DOI] [PubMed] [Google Scholar]
- 29.Huang TTK, Johnson MS, Figueroa-Colon R, Dwyer JH, Goran MI. Growth of visceral fat, subcutaneous abdominal fat, and total body fat in children. Obes Res 2001;9:283–9 [DOI] [PubMed] [Google Scholar]
- 30.Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation 1999;99:541–5 [DOI] [PubMed] [Google Scholar]
- 31.Brambilla P, Manzoni P, Sironi S, et al. Peripheral and abdominal adiposity in childhood obesity. Int J Obes Relat Metab Disord 1994;18:795–800 [PubMed] [Google Scholar]
- 32.Caprio S, Hyman LD, Limb C, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol 1995;269:E118–26 [DOI] [PubMed] [Google Scholar]
- 33.Caprio S, Hyman LD, McCarthy S, Lange R, Bronson M, Tamborlane WV. Fat distribution and cardiovascular risk factors in obese adolescent girls: importance of the intraabdominal fat depot. Am J Clin Nutr 1996;64:12–7 [DOI] [PubMed] [Google Scholar]
- 34.Owens S, Gutin B, Ferguson M, Allison J, Karp W, Le NA. Visceral adipose tissue and cardiovascular risk factors in obese children. J Pediatr 1998;133:41–5 [DOI] [PubMed] [Google Scholar]
- 35.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes 1999;48:1515–21 [DOI] [PubMed] [Google Scholar]
- 36.Owens S, Gutin B, Barbeau P, et al. Visceral adipose tissue and markers of the insulin resistance syndrome in obese black and white teenagers. Obes Res 2000;8:287–93 [DOI] [PubMed] [Google Scholar]
- 37.Gutin B, Johnson MH, Humphries MC, et al. Relationship of visceral adiposity to cardiovascular disease risk factors in black and white teens. Obesity (Silver Spring) 2007;15:1029–35 [DOI] [PubMed] [Google Scholar]
- 38.He Q, Zhang X, He S, et al. Higher insulin, triglycerides, and blood pressure with greater trunk fat in Tanner 1 Chinese. Obesity (Silver Spring) 2007;15:1004–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tershakovec AM, Kuppler KM, Zemel BS, et al. Body composition and metabolic factors in obese children and adolescents. Int J Obes 2003;27:19–24 [DOI] [PubMed] [Google Scholar]
- 40.Brambilla P, Bedogni G, Moreno LA, et al. Crossvalidation of anthropometry against magnetic resonance imaging for the assessment of visceral and subcutaneous adipose tissue in children. Int J Obes Relat Metab Disord 2006;30:23–30 [DOI] [PubMed] [Google Scholar]
- 41.Flodmark CE, Sveger T, Nilsson-Ehle P. Waist measurement correlates to a potentially atherogenic lipoprotein profile in obese 12-14-year-old children. Acta Paediatrica. Int J Paediatr 1994;83:941–5 [DOI] [PubMed] [Google Scholar]
- 42.Hara M, Saitou E, Iwata F, Okada T, Harada K. Waist-to-height ratio is the best predictor of cardiovascular disease risk factors in Japanese schoolchildren. J Atheroscler Thromb 2002;9:127–32 [DOI] [PubMed] [Google Scholar]
- 43.Savva SC, Tornaritis M, Savva ME, et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index. Int J Obes Relat Metab Disord 2000;24:1453–8 [DOI] [PubMed] [Google Scholar]
- 44.Chu NF, Rimm EB, Wang DJ, Liou HS, Shieh SM. Relationship between anthropometric variables and lipid levels among school children: the Taipei Children Heart Study. Int J Obes Relat Metab Disord 1998;22:66–72 [DOI] [PubMed] [Google Scholar]
- 45.Craig LCA, Love J, Ratcliffe B, McNeill G. Overweight and cardiovascular risk factors in 4-to 18-year-olds. Obes Facts 2008;1:237–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maffeis C, Pietrobelli A, Grezzani A, Provera S, Tato L. Waist circumference and cardiovascular risk factors in prepubertal children. Obes Res 2001;9:179–87 [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Bacha F, Gungor N, Arslanian SA. Waist circumference is an independent predictor of insulin resistance in black and white youths. J Pediatr 2006;148:188–94 [DOI] [PubMed] [Google Scholar]
- 48.Tresaco B, Moreno LA, Ruiz JR, et al. Truncal and abdominal fat as determinants of high triglycerides and low HDL-cholesterol in adolescents. Obesity (Silver Spring) 2009;17:1086–91 [DOI] [PubMed] [Google Scholar]
- 49.Teixeira PJ, Sardinha LB, Going SB, Lohman TG. Total and regional fat and serum cardiovascular disease risk factors in lean and obese children and adolescents. Obes Res 2001;9:432–42 [DOI] [PubMed] [Google Scholar]
- 50.Gillum RF. Indices of adipose tissue distribution, apolipoproteins B and AI, lipoprotein (a), and triglyceride concentration in children aged 4-11 years: the Third National Health and Nutrition Examination Survey. J Clin Epidemiol 2001;54:367–75 [DOI] [PubMed] [Google Scholar]
- 51.Gillum RF. Distribution of waist-to-hip ratio, other indices of body fat distribution and obesity and associations with HDL cholesterol in children and young adults aged 4-19 years: the Third National Health and Nutrition Examination Survey. Int J Obes Relat Metab Disord 1999;23:556–63 [DOI] [PubMed] [Google Scholar]
- 52.Lee S, Bacha F, Arslanian SA. Waist circumference, blood pressure, and lipid components of the metabolic syndrome. J Pediatr 2006;149:809–16 [DOI] [PubMed] [Google Scholar]
- 53.Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr 1999;69:308–17 [DOI] [PubMed] [Google Scholar]
- 54.Kahn HS, Imperatore G, Cheng YJ. A population-based comparison of BMI percentiles and waist-to-height ratio for identifying cardiovascular risk in youth. J Pediatr 2005;146:482–8 [DOI] [PubMed] [Google Scholar]
- 55.Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Combined influence of body mass index and waist circumference on coronary artery disease risk factors among children and adolescents. Pediatrics 2005;115:1623–30 [DOI] [PubMed] [Google Scholar]
- 56.Moreno LA, Fleta J, Mur L, Feja C. Sarría A, Bueno M. Indices of body fat distribution in Spanish children aged 4.0 to 14.9 years. J Pediatr Gastroenterol Nutr 1997;25:175–81 [DOI] [PubMed] [Google Scholar]
- 57.Zannolli R, Morgese G. Waist percentiles: a simple test for atherogenic disease? Acta Paediatrica. 1996;85:1368–9 [DOI] [PubMed] [Google Scholar]
- 58.McCarthy HD, Jarrett KV, Crawley HF. The development of waist circumference percentiles in British children aged 5.0-16.9 y. Eur J Clin Nutr 2001;55:902–7 [DOI] [PubMed] [Google Scholar]
- 59.Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 2004;145:439–44 [DOI] [PubMed] [Google Scholar]
- 60.Katzmarzyk PT. Waist circumference percentiles for Canadian youth 11-18y of age. Eur J Clin Nutr 2004;58:1011–5 [DOI] [PubMed] [Google Scholar]
- 61.Fredriks AM, Van Buuren S, Fekkes M, Verloove-Vanhorick SP, Wit JM. Are age references for waist circumference, hip circumference and waist-hip ratio in Dutch children useful in clinical practice? Eur J Pediatr 2005;164:216–22 [DOI] [PubMed] [Google Scholar]
- 62.Li C, Ford ES, Mokdad AH, Cook S. Recent trends in waist circumference and waist-height ratio among US children and adolescents. Pediatrics 2006;118:e1390–8 [DOI] [PubMed] [Google Scholar]
- 63.Senbanjo IO, Njokanma OF, Oshikoya KA. Waist circumference values of nigerian children and adolescents. Ann Nutr Metab 2009;54:145–50 [DOI] [PubMed] [Google Scholar]
- 64.Sung RYT, Yu CCW. Waist circumference and body mass index in Chinese children: cutoff values for predicting cardiovascular risk factors. Int J Obes (Lond) 2007;31:550–8 [DOI] [PubMed] [Google Scholar]
- 65.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab 2003;88:2534–40 [DOI] [PubMed] [Google Scholar]
- 66.Yanovski JA, Yanovski SZ, Filmer KM, et al. Differences in body composition of black and white girls. Am J Clin Nutr 1996;64:833–9 [DOI] [PubMed] [Google Scholar]
- 67.Tybor DJ, Lichtenstein AH, Dallal GE, Daniels S, Must A. Racial differences in central adiposity in a longitudinal cohort of black and white adolescent females. BMC Pediatr 2010;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morrison JA. Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. Am J Public Health 1992;82:1613–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention CDC growth charts: United States. Atlanta, GA: CDC, 2000 [Google Scholar]
- 70.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–92 [DOI] [PubMed] [Google Scholar]
- 71.Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 72.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–95 [DOI] [PubMed] [Google Scholar]
- 73.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–76 [PubMed] [Google Scholar]
- 74.Freedman DS, Dietz WH, Srinivasan SR, Berenson GS. The relation of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics 1999;103:1175–82 [DOI] [PubMed] [Google Scholar]
- 75.Stevens J, Keil JE, Rust PF, Tyroler HA, Davis CE, Gazes PC. Body mass index and body girths as predictors of mortality in black and white women. Arch Intern Med 1992;152:1257–62 [PubMed] [Google Scholar]
- 76.Dowling HJ, Pi-Sunyer FX. Race-dependent health risks of upper body obesity. Diabetes 1993;42:537–43 [PubMed] [Google Scholar]
- 77.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr 1995;61:765–71 [DOI] [PubMed] [Google Scholar]
- 78.Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African-Americans and whites. Obes Res 2005;13:66–74 [DOI] [PubMed] [Google Scholar]
- 79.Freedman DS, Kettel-Khan L, Srinivasan SR, Berenson GS. Black/white differences in relative weight and obesity among girls: the Bogalusa Heart Study. Prev Med 2000;30:234–43 [DOI] [PubMed] [Google Scholar]
- 80.Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 1997;99:804–7 [DOI] [PubMed] [Google Scholar]
- 81.Ortiz O, Russell M, Daley TL, et al. Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. Am J Clin Nutr 1992;55:8–13 [DOI] [PubMed] [Google Scholar]
- 82.Van Der Kooy K, Seidell JC. Techniques for the measurement of visceral fat: a practical guide. Int J Obes Relat Metab Disord 1993;17:187–96 [PubMed] [Google Scholar]
- 83.Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr 2003;77:379–84 [DOI] [PubMed] [Google Scholar]
- 84.Agarwal SK, Misra A, Aggarwal P, et al. Waist circumference measurement by site, posture, respiratory phase, and meal time: implications for methodology. Obesity (Silver Spring) 2009;17:1056–61 [DOI] [PubMed] [Google Scholar]
- 85.Gower BA, Nagy TR, Trowbridge CA, Dezenberg C, Goran MI. Fat distribution and insulin response in prepubertal African American and white children. Am J Clin Nutr 1998;67:821–7 [DOI] [PubMed] [Google Scholar]
- 86.Flegal KM, Ogden CL, Wei R, Kuczmarski RL, Johnson CL. Prevalence of overweight in US children: comparison of US growth charts from the Centers for Disease Control and Prevention with other reference values for body mass index. Am J Clin Nutr 2001;73:1086–93 [DOI] [PubMed] [Google Scholar]
- 87.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA 2002;288:1728–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.