Abstract
Background: Loss of muscle mass with aging is a major public health concern. Omega-3 (n–3) fatty acids stimulate protein anabolism in animals and might therefore be useful for the treatment of sarcopenia. However, the effect of omega-3 fatty acids on human protein metabolism is unknown.
Objective: The objective of this study was to evaluate the effect of omega-3 fatty acid supplementation on the rate of muscle protein synthesis in older adults.
Design: Sixteen healthy, older adults were randomly assigned to receive either omega-3 fatty acids or corn oil for 8 wk. The rate of muscle protein synthesis and the phosphorylation of key elements of the anabolic signaling pathway were evaluated before and after supplementation during basal, postabsorptive conditions and during a hyperaminoacidemic-hyperinsulinemic clamp.
Results: Corn oil supplementation had no effect on the muscle protein synthesis rate and the extent of anabolic signaling element phosphorylation in muscle. Omega-3 fatty acid supplementation had no effect on the basal rate of muscle protein synthesis (mean ± SEM: 0.051 ± 0.005%/h compared with 0.053 ± 0.008%/h before and after supplementation, respectively; P = 0.80) but augmented the hyperaminoacidemia-hyperinsulinemia–induced increase in the rate of muscle protein synthesis (from 0.009 ± 0.005%/h above basal values to 0.031 ± 0.003%/h above basal values; P < 0.01), which was accompanied by greater increases in muscle mTORSer2448 (P = 0.08) and p70s6kThr389 (P < 0.01) phosphorylation.
Conclusion: Omega-3 fatty acids stimulate muscle protein synthesis in older adults and may be useful for the prevention and treatment of sarcopenia. This trial was registered at clinical trials.gov as NCT00794079.
INTRODUCTION
Loss of muscle mass with aging is a major public health concern because it impairs physical function, which reduces quality of life and may lead to frailty and premature death (1–4). A major cause for the loss of muscle mass with advanced age is the inability of aging muscle to adequately increase the rate of muscle protein synthesis in response to nutritional stimuli (eg, amino acids and insulin) (5–7). The blunted anabolic response to nutritional stimuli is at least in part due to defects in the anabolic signaling cascade in muscle (ie, decreased activation of the mTOR-p70s6k signaling pathway) (5, 6), which may be mediated by increased inflammatory activity (8–10). Interventions that can overcome this anabolic resistance are therefore likely to prevent or slow the progression of muscle loss with aging.
Some evidence suggests that fish-oil–derived omega-3 fatty acids (n−3 polyunsaturated fatty acids) might be a potentially useful therapeutic agent for the treatment and prevention of sarcopenia. It has been shown (11) that providing feed enriched in menhaden oil to growing steers increases the activation (phosphorylation) of anabolic signaling proteins in muscle during administration of insulin and amino acids and increases the nonoxidative whole-body disposal of amino acids—an index of increased whole-body protein synthesis (the effect on muscle protein synthesis was not determined). Furthermore, omega-3 fatty acid supplementation has been shown to prevent loss of muscle mass in burned guinea pigs (12). In addition, omega-3 fatty acids have antiinflammatory properties (13), which may also help alleviate the muscle anabolic resistance in older adults. However, the effect of omega-3 fatty acid intake on human muscle protein metabolism and the intracellular signaling pathways that might regulate it are not known.
The purpose of the present study therefore was to determine the effect of dietary omega-3 fatty acid supplementation on the rate of muscle protein synthesis and the anabolic signaling cascade in older adults. We hypothesized that dietary omega-3 fatty acid supplementation increases the stimulatory effect of hyperaminoacidemia-hyperinsulinema on anabolic signaling and protein synthesis in muscle.
SUBJECTS AND METHODS
Subjects and preliminary testing
Sixteen older adults (10 men and 6 women ≥65 y of age) participated in this randomized controlled trial (ClinicalTrials.gov number NCT00794079). Subjects were considered eligible for the trial if they 1) were in good health (ie, no evidence of significant cardiovascular disease or organ dysfunction, including hypertension, dyslipidemia, and diabetes mellitus) after completing a comprehensive medical evaluation, which included a history and physical examination and standard blood tests; 2) were not obese [body mass index <30.0 (in kg/m2)]; 3) did not engage in regular physical activities (ie, they exercised ≤1 h/wk); 4) did not consume fish-oil supplements; 5) did not take medications that could affect muscle protein metabolism; 6) did not consume tobacco products; and 7) did not report excessive alcohol intake. Each subject's total body fat-free mass (FFM) and fat mass were measured by using dual-energy X-ray absorptiometry (DXA; Delphi-W densitometer; Hologic, Waltham, MA) as part of the screening evaluation to help us adjust the amino acid infusion to FFM during the muscle protein study. We decided a priori to not perform DXA analysis after treatment to measure changes in appendicular lean body mass in this study because we determined that it would require a much bigger sample size to reliably measure the changes that could be achieved during the 8-wk intervention period in our study, or, alternatively, require a much longer duration of the intervention. On completion of the screening procedure, subjects were randomly assigned to either the omega-3 fatty acid or corn oil (control) group. One individual randomly assigned to the control group withdrew from the study after completing the initial assessment. Therefore, data from only 7 subjects are presented in the control group.
Written informed consent was obtained from all subjects before their participation in the study, which was approved by the Human Research Protection Office and the Clinical Research Unit Advisory Committee at Washington University School of Medicine (St Louis, MO).
Experimental protocol
Each subject completed 2 stable-isotope-labeled tracer infusion studies to determine the effect of omega-3 fatty acid or corn oil (control group) supplementation on the rate of muscle protein synthesis and anabolic signaling in muscle during basal, postabsorptive conditions and combined amino acid and insulin infusions. The first study was performed within 1–3 wk of screening (before the intervention); the second study took place after 8 wk of dietary supplementation with omega-3 fatty acids [4 g Lovaza/d containing 1.86 g eicosapentaenoic acid (EPA, 20:5n−3) and 1.50 g docosahexaenoic acid (DHA, 22:6n−3)—both as ethyl esters—or an equal amount of corn oil]. We chose this dose because it is the dose approved by the Food and Drug Administration for lowering plasma triglyceride concentrations in hypertriglyceridemic subjects and has therefore previously been shown to be physiologically relevant in human subjects. Furthermore, similar doses (relative to body weight) increased whole-body protein synthesis (no muscle data were reported) in burned rats (14) and doubled the insulin-stimulated nonoxidative whole-body disposal of amino acids, a marker of increased whole-body protein synthesis (again, no measures of muscle protein synthesis were obtained), in growing steer (11), whereas much higher doses (and possibly even toxic amounts of fish oil) resulted in no effect on protein metabolism or even decreased muscle protein synthesis rates in rats (15–18). Both the omega-3 fatty acid and corn oil treatments were provided by Reliant Pharmaceuticals Inc, Liberty Corner, NJ (now GlaxoSmithKline, Research Triangle Park, NC) and packaged in identical-looking capsules, and subjects were blinded to the treatment. Compliance was evaluated by pill count. To minimize falsification of the pill count, subjects were given an unknown (to them) number of pills in excess of needs and were asked to return any remaining pills at the end of the study.
Before each muscle protein metabolism study, subjects were instructed to adhere to their usual diet and to refrain from vigorous physical activities for 3 d. On the evening before the study, subjects were admitted to the Clinical Research Unit at Washington University School of Medicine. At 2000, they consumed a standard meal providing 50.2 kJ/kg body wt (15% as protein, 55% as carbohydrates, and 30% as fat). Subjects then rested in bed and fasted (except for water) until completion of the study the next day. At ≈0600 h on the following morning, a cannula was inserted into an antecubital vein for the infusion of stable-isotope-labeled tracers (ie, a phenylalanine tracer to measure the rate of muscle protein synthesis and phenylalanine rate of appearance in the systemic circulation and a glucose tracer to measure glucose rate of appearance in the systemic circulation); another cannula was inserted into a vein of the contralateral hand, which was warmed to 55°C for blood sampling. At ≈0800 h, primed, constant infusions of [ring-2H5]phenylalanine (priming dose: 2.8 μmol/kg FFM, infusion rate: 0.08 μmol ⋅ kg FFM−1 ∙ min−1) and [6,6-2H2]glucose (priming dose: 18 μmol/kg body wt, infusion rate: 0.22 μmol ⋅ kg body wt−1 ⋅ min−1), both purchased from Cambridge Isotope Laboratories Inc (Andover, MA), were started and maintained for 7 h. Four hours after the start of the tracer infusions, a hyperaminoacidemic-hyperinsulinemic clamp was started and maintained for 3 h. Human insulin (Novolin R; Novo Nordisk, Princeton, NJ) was infused at a rate of 20 mU ⋅ m2 body surface area (BSA)−1 ⋅ min−1 (initiated with priming doses of 80 mU ⋅ m2 BSA−1 ⋅ min−1 for 5 min and then 40 mU ⋅ m2 BSA−1 ⋅ min−1 for an additional 5 min), and plasma amino acid availability was increased by providing an intravenous amino acid mixture (Travasol 10%; Baxter, Deerfield, IL) at a rate of 105 mg amino acids ⋅ kg FFM−1 ⋅ h−1 (priming dose: 35 mg amino acids/kg FFM) to raise plasma insulin and amino acid concentrations to within the range normally seen after meal consumption (19, 20). Euglycemia (blood glucose concentration of ≈5.5 mmol/L) was maintained during the clamp procedure by variable rate infusion of 20% dextrose (Baxter) enriched to 2.5% with [6,6-2H2]glucose. To minimize changes in plasma phenylalanine and glucose isotopic enrichment during the clamp due to the increased amino acid rate of appearance in plasma and reduced hepatic glucose production, the [ring-2H5]phenylalanine infusion rate was increased to 0.12 μmol ⋅ kg FFM−1 ⋅ min−1 and the [6,6-2H2]glucose infusion rate was decreased to 0.11 μmol ⋅ kg body wt−1 ⋅ min−1 during the clamp.
Blood samples (4 mL) were obtained before the tracer infusions began and at 60, 90, 120, 150, 180, 210, 220, 230, 240, 270, 300, 330, 360, 390, 400, 410, and 420 min to determine the labeling of phenylalanine and glucose in plasma and plasma substrate, hormone, and cytokine concentrations; plasma was separated and stored at −80°C until the final analyses. Additional blood (≈1 mL) was obtained every 10 min during the clamp to monitor plasma glucose concentrations. Muscle tissue (≈100 mg) was obtained under local anesthesia (lidocaine, 2%) from the quadriceps femoris by using a Tilley-Henkel forceps at 60 and 240 min to determine the basal rate of muscle protein synthesis (labeled phenylalanine incorporation into muscle protein; see section entitled “Calculations”) and the basal concentrations (240 min) of phosphorylated elements of intramuscular signal transduction proteins (Akt; mTOR; p70s6k) involved in the regulation of glucose uptake and muscle protein synthesis (21, 22). A third muscle biopsy sample was obtained at 420 min (ie, 3 h after the clamp procedure was started) to determine both the rate of muscle protein synthesis and the intracellular signaling responses to hyperaminoacidemia-hyperinsulinemia. The second and third biopsy samples were obtained from the same incision on the leg contralateral to that biopsied first; the forceps was directed in proximal and distal directions, respectively, so that the 2 biopsy samples were collected ≈5–10 cm apart. Muscle samples were initially frozen in liquid nitrogen and stored at −80°C until the final analysis.
Sample processing and analyses
Plasma glucose concentrations were measured with an automated glucose analyzer (Yellow Spring Instruments, Yellow Springs, OH). Plasma insulin concentrations were measured by radioimmunoassay (Linco Research, St Louis, MO). Commercially available enzyme-linked immunosorbent assay kits (R&D Systems Inc, Minneapolis, MN) were used to measure plasma concentrations of C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6).
Muscle phospholipid fatty acid composition was determined after the extraction of lipids from ≈30 mg muscle tissue with 2 mL chloroform/methanol (2:1, vol:vol) containing 0.01% butylated hydroxytoluene. Phospholipids were then isolated by using thin-layer chromatography (Whatman TLC LK 6D; Fischer Scientific, Pittsburgh, PA), fatty acids were converted to their methyl esters by reacting with 10% acetyl chloride in methanol, and their relative concentrations were measured by using gas chromatography–mass spectrometry (GC-MS; MSD 5973 System; Hewlett-Packard). The proportional contribution of each fatty acid to total fatty acid content was calculated by dividing the peak area of each fatty acid by the sum of all peak areas.
To determine the labeling of plasma glucose, plasma proteins were precipitated with ice-cold acetone, and hexane was used to extract plasma lipids. The aqueous phase, containing glucose, was dried by speed-vac centrifugation (Savant Instruments, Farmingdale, NY), glucose was derivatized with heptafluorobutyric acid, and the tracer-to-tracee ratio (TTR) was determined by using GC-MS as previously described (23).
To measure the plasma concentrations of phenylalanine and leucine (thought to be a major regulator of muscle protein synthesis; 24) and the labeling of phenylalanine in plasma, known amounts of [1-13C]phenylalanine and nor-leucine were added to an aliquot of each plasma sample, plasma proteins were precipitated, and the supernatant fluid (containing free amino acids) was collected to prepare the t-butyldimethylsilyl derivative of phenylalanine and leucine for analysis by GC-MS (25, 26). To determine phenylalanine labeling in muscle proteins and in tissue fluid, samples (≈20 mg) were homogenized in 1 mL trichloroacetic acid solution (3% wt:vol); proteins were precipitated by centrifugation and then hydrolyzed, and the supernatant fluid, containing free amino acids, was collected. Amino acids in the protein hydrolysate and supernatant fluid samples were purified on cation-exchange columns (Dowex 50W-X8-200; Bio-Rad Laboratories, Richmond, CA), and the t-butyldimethylsilyl derivative of phenylalanine prepared to determine its TTR by GC-MS analysis (25, 26).
Western analysis was used to measure the phosphorylation of Akt, mTOR, and p70s6k at 240 min (basal) and 420 min (clamp) as previously described (25–28). Briefly, frozen muscle tissue (≈20 mg) was rapidly homogenized in ice-cold buffer, proteins were extracted, and 50 μg protein from each sample was loaded onto 12% XT-Bis Tris gels, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and transferred on ice to methanol prewetted 0.2-μm PVDF membranes. Blots were then incubated sequentially with 5% (wt:vol) nonfat milk for 1 h, primary antibodies (AktThr308, mTORSer2448, p70s6kThr389, and pan-actin as a loading control; all purchased from New England Biolabs) overnight at 4°C, and then secondary antibody (anti-rabbit; New England Biolabs, Ipswich, MA) for 1 h. Data were expressed in relation to GAPDH.
Calculations
The muscle protein fractional synthesis rate (FSR) was calculated from the rate of incorporation of [ring-2H5]phenylalanine into muscle protein, by using a standard precursor-product model as follows:
where ΔEp is the change between 2 consecutive biopsy samples in extent of labeling (TTR) of protein-bound phenylalanine. Eic is the phenylalanine labeling in the precursor pool, and t is the time between biopsy sample collections. We used both the free phenylalanine labeling in muscle tissue fluid (at 240 and 420 min) and the average plasma phenylalanine labeling between 60 and 240 min (basal) and 270 and 420 min (clamp) as surrogates for the phenylalanine labeling in the precursor pool (ie, aminoacyl-t-RNA) (29).
Glucose and phenylalanine rates of appearance (Ra) in plasma were calculated by dividing the tracer infusion rate by the average TTR in plasma during the last 30 min of the basal period and the last 30 min of the clamp. Glucose and phenylalanine Ra during basal conditions represent endogenous glucose and phenylalanine production; during the clamp procedure, glucose and phenylalanine Ra represent the sum of endogenous glucose and phenylalanine production and the rate of infused glucose and phenylalanine, respectively. Endogenous Ra during the clamp was therefore calculated by subtracting the glucose (dextrose plus tracer) and phenylalanine (Travasol and tracer) infusion rate from total glucose and phenylalanine Ra, respectively.
Statistical analysis
Statistical analysis was performed by using SPSS version 13 software (SPSS Inc, Chicago, IL). All data sets were tested for normality, and skewed data sets were log transformed for data analysis or evaluated by using appropriate nonparametric testing procedures. Preliminary analysis of variance (ANOVA) was performed to examine whether differences between the control and omega-3 fatty acid groups existed in subject characteristics; plasma glucose, insulin, phenylalanine, and leucine concentrations; mixed muscle protein FSR; and the concentration of muscle intracellular signaling elements before the intervention. No differences were detected. For each group (corn oil compared with omega-3 fatty acid), repeated-measures ANOVA and Tukey's post hoc test were therefore used to evaluate before-to-after intervention changes (effect of time) in plasma glucose, insulin, phenylalanine, and leucine concentrations; mixed muscle protein FSR; and muscle intracellular signaling elements during basal and postabsorptive conditions and during the clamp procedure in the omega-3 fatty acid and corn oil supplementation groups. Differences between groups in the treatment-induced change, if they existed, were evaluated by using Student's t test for independent samples or the Mann-Whitney U test (for skewed data sets). Differences in measurements made only during the basal postabsorptive state (eg, systemic inflammatory markers, muscle phospholipid fatty acid composition) before and after omega-3 fatty acid or corn oil supplementation were evaluated by using ANOVA with group and time as the factors. A P value ≤0.05 was considered significant. Data are presented as means ± SEMs or medians with 25th and 75th percentiles for skewed data sets.
RESULTS
Subject characteristics, metabolic variables, and body composition
Subjects randomly assigned to receive omega-3 fatty acids and corn oil were similar in age, sex, BMI, and body composition, and their health and metabolic status (ie, blood pressure, plasma lipid panel, and plasma inflammatory markers) were similar both before and after corn oil and omega-3 fatty acid supplementation (Table 1). Only diastolic blood pressure was somewhat lower in the omega-3 fatty acid group (P < 0.05), and LDL-cholesterol and TNF-α concentrations slightly increased in response to both the corn oil and omega-3 fatty acid supplementation (main effect of time: P < 0.05), but no significant group × time interaction was observed.
TABLE 1.
Subject characteristics and basic metabolic variables before and after 8 wk of corn oil or omega-3 fatty acid supplementation1
| Corn oil (n = 7) |
Omega-3 fatty acids (n = 8) |
P value2 |
|||||
| Before | After | Before | After | Group | Time | Interaction | |
| Sex (M/F) | 5/2 | — | 5/3 | — | — | — | — |
| Age (y) | 71 ± 23 | — | 71 ± 1 | — | 0.894 | — | — |
| BMI (kg/m2) | 25.7 ± 1.6 | — | 25.6 ± 1.0 | — | 0.954 | — | — |
| Body mass (kg)5 | 77.3 ± 6.2 | — | 74.2 ± 4.6 | — | 0.694 | — | — |
| Fat-free mass (kg)5 | 54.3 ± 4.7 | — | 52.5 ± 4.4 | — | 0.784 | — | — |
| Appendicular MM (kg)5 | 21.7 ± 2.2 | — | 21.3 ± 2.1 | — | 0.904 | — | — |
| Systolic BP (mm Hg) | 133 ± 7 | 133 ± 5 | 121 ± 6 | 125 ± 5 | 0.17 | 0.59 | 0.73 |
| Diastolic BP (mm Hg) | 79 ± 26 | 75 ± 3 | 70 ± 3 | 70 ± 3 | 0.03 | 0.44 | 0.47 |
| HOMA-IR | 1.54 ± 0.34 | 1.41 ± 0.40 | 1.36 ± 0.23 | 1.33 ± 0.16 | 0.75 | 0.38 | 0.59 |
| Total plasma TG (mg/dL) | 72 (61, 80)7 | 84 (67, 1047) | 81 (65, 94) | 84 (73, 87) | 0.47 | 0.52 | 0.87 |
| Total plasma C (mg/dL) | 169 ± 12 | 169 ± 11 | 172 ± 7 | 183 ± 8 | 0.53 | 0.07 | 0.09 |
| LDL-C (mg/dL) | 95 ± 11 | 100 ± 11 | 103 ± 8 | 116 ± 8 | 0.39 | 0.04 | 0.32 |
| HDL-C (mg/dL) | 52 ± 5 | 52 ± 5 | 44 ± 4 | 49 ± 4 | 0.42 | 0.19 | 0.14 |
| CRP (mg/L) | 1.28 (0.90, 1.45) | 1.21 (0.61, 2.74) | 0.71 (0.38, 1.00) | 0.67 (0.24, 3.44) | 0.36 | 0.99 | 0.72 |
| TNF-α (pg/mL) | 0.67 ± 0.15 | 0.86 ± 0.21 | 0.50 ± 0.13 | 0.53 ± 0.13 | 0.26 | 0.03 | 0.09 |
| IL-6 (pg/mL) | 2.05 (0.88, 7.02) | 2.43 (1.83, 7.02) | 1.29 (1.02, 2.74) | 1.82 (1.35, 2.34) | 0.34 | 0.19 | 0.61 |
HOMA-IR, homeostasis model assessment of insulin resistance; BP, blood pressure; TG, triglycerides; LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; MM, muscle mass; TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; CRP, C-reactive protein; C, cholesterol.
P values determined by ANOVA with group (corn oil compared with omega-3 fatty acids) and time (before compared with after intervention) as factors after initial comparison of “before” values only by using Student's t or the Mann-Whitney U tests as appropriate.
Mean ± SEM (all such values).
Determined by using Student's t test for independent samples.
Measured by dual-energy X-ray absorptiometry as described in Subjects and Methods.
Significantly different from the corresponding “before” value in the omega-3 fatty acid group, P = 0.04 (initial Student's t test).
Median; quartile in parentheses (all such values).
Compliance with omega-3 fatty acid and corn oil supplementation and muscle phospholipid fatty acid composition
All subjects consumed ≥160 of the 224 pills assigned to them. Average compliance, as judged by the leftover pill count, was 100% and 96 ± 4% for the corn oil and omega-3 fatty acid groups, respectively. This was confirmed by analysis of the muscle phospholipid fatty acid profile. Omega-3 fatty acid supplementation approximately doubled (P < 0.01) the muscle phospholipid omega-3 fatty acid content, whereas corn oil supplementation had no effect on the muscle phospholipid fatty acid composition (Table 2).
TABLE 2.
Muscle phospholipid fatty acid profile before and after 8 wk of corn oil or omega-3 fatty acid supplementation1
| Corn oil (n = 7) |
Omega-3 fatty acids (n = 8) |
|||
| Before | After | Before | After | |
| Saturated fatty acids (% of fatty acids) | ||||
| 14:0 | 0.38 ± 0.02 | 0.35 ± 0.04 | 0.40 ± 0.09 | 0.32 ± 0.03 |
| 16:0 | 17.94 ± 2.14 | 17.16 ± 1.74 | 19.80 ± 1.72 | 18.53 ± 1.33 |
| 18:0 | 19.97 ± 0.82 | 19.14 ± 0.63 | 24.07 ± 1.79 | 21.28 ± 0.46 |
| Total | 38.24 ± 2.79 | 36.61 ± 2.11 | 44.17 ± 3.05 | 40.05 ± 1.49 |
| Monounsaturated fatty acids (% of fatty acids) | ||||
| 16:1n−7 | 0.35 ± 0.05 | 0.37 ± 0.10 | 0.34 ± 0.09 | 0.50 ± 0.09 |
| 18:1n−9 | 8.88 ± 0.55 | 10.71 ± 1.21 | 9.43 ± 0.54 | 8.77 ± 0.47 |
| Total | 9.23 ± 0.59 | 11.08 ± 1.24 | 9.77 ± 0.56 | 9.27 ± 0.50 |
| Omega-6 polyunsaturated fatty acids (% of fatty acids) | ||||
| 18:2n−6 | 30.72 ± 2.45 | 30.84 ± 2.50 | 25.56 ± 1.45 | 25.92 ± 0.49 |
| 20:3n−6 | 1.18 ± 0.12 | 1.31 ± 0.12 | 1.23 ± 0.12 | 1.30 ± 0.09 |
| 20:4n−6 | 16.11 ± 1.32 | 16.02 ± 0.98 | 14.24 ± 1.14 | 14.44 ± 0.85 |
| Total | 48.02 ± 3.122 | 48.17 ± 2.702 | 41.03 ± 2.41 | 41.65 ± 0.91 |
| Omega-3 polyunsaturated fatty acids (% of fatty acids) | ||||
| 20:5n−3 | 0.81 ± 0.10 | 0.84 ± 0.10 | 0.77 ± 0.15 | 2.41 ± 0.34 |
| 22:5n−3 | 1.62 ± 0.15 | 1.50 ± 0.13 | 2.23 ± 0.29 | 2.51 ± 0.27 |
| 22:6n−3 | 2.08 ± 0.25 | 1.79 ± 0.20 | 2.03 ± 0.28 | 4.11 ± 0.41 |
| Total3 | 4.52 ± 0.37 | 4.14 ± 0.31 | 5.04 ± 0.45 | 9.03 ± 0.9545 |
All values are means ± SEMs. The percentage of fatty acids was determined by dividing the peak area of each fatty acid by the sum of all peak areas after gas chromatographic analyses. There were no significant between-group differences in the “before” values, P ≥ 0.10 (Student's t tests).
Significant group (corn oil compared with omega-3 fatty acids) effect, P = 0.05 (ANOVA).
Significant group × time interaction (P < 0.05, ANOVA), which was followed by Tukey's post hoc analysis.
Significantly different from corresponding value before intervention, P < 0.01 (ANOVA).
Significantly different from the corresponding value in the corn oil group, P < 0.01 (ANOVA).
Plasma insulin, glucose, and amino acid concentrations and glucose and phenylalanine labeling in plasma and muscle
Plasma insulin concentrations were not significantly different between the corn oil and omega-3 fatty acid supplementation groups, either before or after supplementation during both basal, postabsorptive conditions (before: 6.3 ± 1.1 compared with 6.1 ± 1.0 μU/mL, respectively; after: 6.0 ± 1.5 compared with 5.6 ± 0.6 μU/mL, respectively) and during the hyperinsulinemic-hyperaminoacidemic clamp (before: 26.4 ± 1.9 compared with 31.7 ± 3.1 μU/mL, respectively; after: 28.1 ± 2.1 compared with 29.5 ± 2.2 μU/mL, respectively). ANOVA showed a significant effect of clamp (P < 0.001) but no significant effect of time or group × time interaction.
No differences between the corn oil and omega-3 fatty acid groups in baseline (before intervention) plasma concentrations of glucose and amino acids (all P ≥ 0.17; Tables 3 and 4) were observed. During the clamp, plasma glucose was successfully maintained at ≈5.5 mmol/L both before and after corn oil and omega-3 fatty acid supplementation; plasma phenylalanine and leucine concentrations rose by ≈80% and 45% above basal values, respectively (all P < 0.001). Omega-3 fatty acid supplementation led to a very small (≈6%) but significant (P < 0.05) increase in basal plasma glucose concentration (Tables 3 and 4). Plasma phenylalanine and leucine concentrations were not affected by either corn oil or omega-3 fatty acid supplementation during basal, postabsorptive conditions or during the hyperinsulinemic-hyperaminoacidemic clamp (Tables 3 and 4).
TABLE 3.
Plasma phenylalanine, leucine, and glucose concentrations and phenylalanine and glucose tracer-to-tracee ratios (TTR) during basal, postabsorptive conditions and during the hyperinsulinemic-hyperaminoacidemic clamp before and after corn oil supplementation (n = 7)1
| Concentration |
Enrichment (TTR) |
|||||||||
| Phenylalanine |
Leucine |
Glucose |
Phenylalanine |
Glucose |
||||||
| Time | Before | After | Before | After | Before | After | Before | After | Before | After |
| μmol/L | μmol/L | mmol/L | ||||||||
| Basal conditions | ||||||||||
| 60 min | 65 ± 6 | 68 ± 9 | 120 ± 7 | 120 ± 9 | 5.31 ± 0.17 | 5.26 ± 0.16 | 0.0928 ± 0.0086 | 0.0928 ± 0.0079 | — | — |
| 90 min | 65 ± 8 | 58 ± 5 | 117 ± 9 | 110 ± 8 | — | — | 0.1019 ± 0.0097 | 0.0962 ± 0.0091 | — | — |
| 120 min | 58 ± 8 | 65 ± 10 | 99 ± 6 | 122 ± 16 | 5.35 ± 0.20 | 5.18 ± 0.12 | 0.1128 ± 0.0120 | 0.0987 ± 0.0081 | — | — |
| 150 min | 64 ± 8 | 62 ± 10 | 114 ± 10 | 111 ± 14 | — | — | 0.1131 ± 0.0087 | 0.1085 ± 0.0097 | — | — |
| 180 min | 68 ± 9 | 69 ± 8 | 119 ± 8 | 131 ± 7 | 5.30 ± 0.19 | 5.09 ± 0.13 | 0.1132 ± 0.0069 | 0.1083 ± 0.0098 | — | — |
| 210 min | 69 ± 11 | 70 ± 4 | 120 ± 14 | 122 ± 5 | 5.30 ± 0.23 | 5.08 ± 0.14 | 0.1166 ± 0.0104 | 0.1023 ± 0.0073 | 0.0220 ± 0.0014 | 0.0222 ± 0.0014 |
| 220 min | — | — | — | — | 5.28 ± 0.22 | 5.08 ± 0.14 | — | — | 0.0218 ± 0.0015 | 0.0222 ± 0.0015 |
| 230 min | — | — | — | — | 5.26 ± 0.23 | 5.07 ± 0.13 | — | — | 0.0218 ± 0.0017 | 0.0225 ± 0.0016 |
| 240 min | 74 ± 11 | 75 ± 12 | 132 ± 13 | 137 ± 11 | 5.28 ± 0.23 | 5.10 ± 0.14 | 0.1192 ± 0.0102 | 0.1090 ± 0.0089 | 0.0224 ± 0.0019 | 0.0218 ± 0.0015 |
| Mean ± SEM | 66 ± 8 | 66 ± 8 | 117 ± 8 | 121 ± 7 | 5.30 ± 0.21 | 5.12 ± 0.13 | 0.1099 ± 0.0091 | 0.1030 ± 0.0087 | 0.0220 ± 0.0016 | 0.0222 ± 0.0015 |
| Hyperinsulinemic-hyperaminoacidemic clamp | ||||||||||
| 270 min | 101 ± 13 | 99 ± 18 | 184 ± 16 | 168 ± 11 | 5.42 ± 0.20 | 5.47 ± 0.07 | 0.1206 ± 0.0059 | 0.1132 ± 0.0110 | — | — |
| 300 min | 104 ± 13 | 118 ± 16 | 164 ± 11 | 197 ± 12 | 5.46 ± 0.19 | 5.52 ± 0.07 | 0.1193 ± 0.0075 | 0.0996 ± 0.0055 | — | — |
| 330 min | 113 ± 18 | 127 ± 20 | 166 ± 25 | 196 ± 25 | 5.64 ± 0.06 | 5.58 ± 0.07 | 0.1014 ± 0.0087 | 0.0996 ± 0.0049 | — | — |
| 360 min | 130 ± 17 | 126 ± 16 | 181 ± 15 | 177 ± 13 | 5.62 ± 0.06 | 5.60 ± 0.07 | 0.1019 ± 0.0062 | 0.0979 ± 0.0055 | — | — |
| 390 min | 117 ± 20 | 111 ± 11 | 151 ± 18 | 159 ± 19 | 5.57 ± 0.07 | 5.60 ± 0.06 | 0.0985 ± 0.0087 | 0.0983 ± 0.0054 | 0.0249 ± 0.0018 | 0.0254 ± 0.0012 |
| 400 min | — | — | — | — | 5.55 ± 0.06 | 5.59 ± 0.05 | — | — | 0.0247 ± 0.0016 | 0.0255 ± 0.0012 |
| 410 min | — | — | — | — | 5.55 ± 0.06 | 5.59 ± 0.08 | — | — | 0.0255 ± 0.0017 | 0.0260 ± 0.0011 |
| 420 min | 130 ± 20 | 118 ± 15 | 164 ± 18 | 155 ± 17 | 5.51 ± 0.06 | 5.60 ± 0.05 | 0.1015 ± 0.0057 | 0.0988 ± 0.0057 | 0.0253 ± 0.0016 | 0.0260 ± 0.0011 |
| Mean ± SEM | 116 ± 142 | 117 ± 142 | 168 ± 122 | 176 ± 132 | 5.54 ± 0.082 | 5.57 ± 0.042 | 0.1054 ± 0.005 | 0.1007 ± 0.0059 | 0.0251 ± 0.00162 | 0.0256 ± 0.00102 |
All values are means ± SEMs. ANOVA on the “before” values from Tables 3 and 4 with group (corn oil compared with omega-3 fatty acid) and study stage (basal compared with clamp) as the factors showed no significant between-group differences (all P ≥ 0.10) or group × study stage interactions (all P ≥ 0.36).
Significantly different from corresponding “basal” value, P < 0.001 [ANOVA on values from the corn oil group presented here with study stage (basal compared with clamp) and time (before compared with after intervention) as factors].
TABLE 4.
Plasma phenylalanine, leucine, and glucose concentrations and phenylalanine and glucose tracer-to-tracee ratios (TTR) during basal, postabsorptive conditions and during the hyperinsulinemic-hyperaminoacidemic clamp before and after omega-3 fatty acid supplementation (n = 8)1
| Concentration2 |
Enrichment (TTR) |
|||||||||
| Phenylalanine |
Leucine |
Glucose |
Phenylalanine |
Glucose |
||||||
| Time | Before | After | Before | After | Before | After | Before | After | Before | After |
| μmol/L | μmol/L | mmol/L | ||||||||
| Basal conditions | ||||||||||
| 60 min | 58 ± 10 | 61 ± 14 | 112 ± 11 | 117 ± 7 | 5.03 ± 0. 10 | 5.29 ± 0.07 | 0.0853 ± 0.0040 | 0.0914 ± 0.0093 | — | — |
| 90 min | 63 ± 9 | 57 ± 9 | 119 ± 8 | 111 ± 6 | — | — | 0.0854 ± 0.0078 | 0.0968 ± 0.0073 | — | — |
| 120 min | 56 ± 9 | 58 ± 10 | 107 ± 8 | 109 ± 7 | 5.06 ± 0.10 | 5.31 ± 0.08 | 0.0950 ± 0.0065 | 0.0987 ± 0.0071 | — | — |
| 150 min | 58 ± 9 | 57 ± 12 | 112 ± 10 | 103 ± 9 | — | — | 0.0962 ± 0.0057 | 0.0991 ± 0.0084 | — | — |
| 180 min | 54 ± 9 | 49 ± 5 | 104 ± 8 | 107 ± 6 | 5.06 ± 0.09 | 5.41 ± 0.10 | 0.0999 ± 0.0050 | 0.1086 ± 0.0062 | — | — |
| 210 min | 59 ± 8 | 52 ± 8 | 116 ± 8 | 106 ± 5 | 4.99 ± 0.10 | 5.30 ± 0.11 | 0.1014 ± 0.0047 | 0.1023 ± 0.0062 | 0.0220 ± 0.0008 | 0.0218 ± 0.0009 |
| 220 min | — | — | — | — | 4.99 ± 0.09 | 5.31 ± 0.10 | — | — | 0.0024 ± 0.0009 | 0.0218 ± 0.0009 |
| 230 min | — | — | — | — | 4.97 ± 0.09 | 5.28 ± 0.09 | — | — | 0.0225 ± 0.0009 | 0.0217 ± 0.0009 |
| 240 min | 55 ± 7 | 50 ± 7 | 110 ± 9 | 98 ± 7 | 4.97 ± 0.10 | 5.29 ± 0.11 | 0.1038 ± 0.0069 | 0.1044 ± 0.0055 | 0.0225 ± 0.0009 | 0.0217 ± 0.0009 |
| Mean ± SEM | 57 ± 8 | 56 ± 10 | 111 ± 7 | 108 ± 5 | 5.01 ± 0.09 | 5.31 ± 0.093 | 0.0955 ± 0.0052 | 0.0994 ± 0.0069 | 0.0223 ± 0.0090 | 0.0217 ± 0.0090 |
| Hyperinsulinemic-hyperaminoacidemic clamp | ||||||||||
| 270 min | 89 ± 9 | 91 ± 12 | 185 ± 10 | 183 ± 13 | 5.09 ± 0.18 | 5.37 ± 0.14 | 0.1015 ± 0.0030 | 0.0988 ± 0.0043 | ||
| 300 min | 91 ± 9 | 95 ± 11 | 165 ± 9 | 172 ± 14 | 5.30 ± 0.17 | 5.46 ± 0.11 | 0.0939 ± 0.0024 | 0.0937 ± 0.0031 | ||
| 330 min | 98 ± 13 | 99 ± 13 | 159 ± 12 | 158 ± 12 | 5.55 ± 0.06 | 5.48 ± 0.04 | 0.0917 ± 0.0032 | 0.0910 ± 0.0049 | ||
| 360 min | 113 ± 14 | 109 ± 20 | 178 ± 18 | 160 ± 19 | 5.62 ± 0.09 | 5.39 ± 0.10 | 0.0914 ± 0.0036 | 0.0881 ± 0.0041 | ||
| 390 min | 116 ± 16 | 108 ± 16 | 171 ± 10 | 146 ± 6 | 5.41 ± 0.07 | 5.39 ± 0.06 | 0.0866 ± 0.0076 | 0.0865 ± 0.0074 | 0.0260 ± 0.0005 | 0.0259 ± 0.0007 |
| 400 min | — | — | — | — | 5.46 ± 0.04 | 5.47 ± 0.07 | — | — | 0.0260 ± 0.0005 | 0.0264 ± 0.0007 |
| 410 min | — | — | — | — | 5.49 ± 0.06 | 5.48 ± 0.05 | — | — | 0.0264 ± 0.0005 | 0.0268 ± 0.0008 |
| 420 min | 103 ± 15 | 101 ± 11 | 152 ± 13 | 150 ± 10 | 5.43 ± 0.04 | 5.41 ± 0.06 | 0.0894 ± 0.0052 | 0.0905 ± 0.0040 | 0.0264 ± 0.0005 | 0.0266 ± 0.0007 |
| Mean ± SEM | 102 ± 124 | 101 ± 134 | 168 ± 94 | 161 ± 104 | 5.42 ± 0.054 | 5.41 ± 0.044 | 0.0924 ± 0.0037 | 0.0914 ± 0.0039 | 0.0262 ± 0.00504 | 0.0264 ± 0.00704 |
All values are means ± SEMs. ANOVA on the “before” values from Tables 3 and 4 with group (corn oil compared with omega-3 fatty acid) and study stage (basal compared with clamp) as the factors showed no significant between-group differences (all P ≥ 0.10) or group × study stage interactions (all P ≥ 0.36).
With the exception of the change in basal plasma glucose concentration in the omega-3 fatty acid group (P < 0.05 compared with the before-after change in basal plasma glucose concentration in the corn oil group), there were no significant differences in the before-after changes between the corn oil and the omega-3 fatty acid groups.
Significantly different from corresponding “before” value, P < 0.05 [ANOVA on values from the omega-3 fatty acid group presented here with study stage (basal compared with clamp) and time (before compared with after intervention) as factors and Tukey's post hoc analysis].
Significantly different from corresponding basal value, P < 0.001 [ANOVA on values from the omega-3 fatty acid group presented here with study stage (basal compared with clamp) and time (before compared with after intervention) as factors].
Plasma phenylalanine enrichment between 60 and 420 min and plasma glucose enrichment during the last 30 min of the basal state and the clamp before and after corn oil and omega-3 fatty acid supplementation are shown in Tables 3 and 4. The muscle free phenylalanine enrichments (TTR) before and after corn oil supplementation were 0.0612 ± 0.0076 and 0.0619 ± 0.0054, respectively, at the end of the basal, postabsorptive period and 0.0748 ± 0.0037 and 0.0739 ± 0.0031, respectively, at the end of the hyperinsulinemic-hyperaminoacidemic clamp; in the omega-3 fatty acid supplementation group, the respective values were 0.0679 ± 0.0030 and 0.0652 ± 0.0021 (at the end of the basal, postabsorptive period) and 0.0797 ± 0.0039 and 0.0762 ± 0.0029 (at the end of the hyperinsulinemic-hyperaminoacidemic clamp).
Muscle protein synthesis rate at baseline (before intervention)
The muscle protein synthesis rate at baseline was not different between the omega-3 fatty acid and corn oil groups, either during basal conditions (0.051 ± 0.005% compared with 0.051 ± 0.008%/h, respectively with muscle free phenylalanine as the precursor enrichment and 0.036 ± 0.003% compared with 0.029 ± 0.005%/h, respectively, with plasma phenylalanine as the precursor enrichment) or during hyperinsulinemia-hyperaminoacidemia (0.060 ± 0.007% compared with 0.063 ± 0.009%/h, respectively, with muscle free phenylalanine as the precursor enrichment and 0.051 ± 0.006% compared with 0.047 ± 0.007%/h, respectively with plasma phenylalanine as the precursor enrichment). ANOVA showed a significant effect of clamp (P < 0.001) but no significant effect of group (P ≥ 0.41) and no interaction between the 2 (P ≥ 0.60).
To confirm the existence of anabolic resistance in our older adults, we compared these baseline data with values we recently published (27) for healthy, nonobese, young and middle-aged adults who completed the same muscle protein metabolism study and experienced similar increases in plasma insulin and amino acid concentrations during the clamp as in our older subjects in the present study. Indeed, the hyperaminoacidema-hyperinsulinemia induced increase in the rate of muscle protein synthesis above basal values was twice as high (P < 0.05) in the young (0.022 ± 0.004%/h) than in the older (0.011 ± 0.003%/h) subjects (with muscle free phenylalanine as the precursor enrichment).
Effect of intervention on the muscle protein synthesis rate (plasma phenylalanine labeling as precursor pool enrichment)
Corn oil supplementation had no effect on either the basal muscle protein synthesis rate or the increase in the rate of muscle protein synthesis in response to amino acid and insulin infusion (change from basal values: 0.019 ± 0.004%/h before and 0.016 ± 0.005%/h after supplementation; P = 0.78; Figure 1). In contrast, omega-3 fatty acid supplementation had no effect on the basal rate of muscle protein synthesis but approximately doubled the anabolic response to amino acid and insulin infusion (change from basal values: 0.015 ± 0.004% compared with 0.036 ± 0.005%/h; P < 0.001), and the before-after change in the anabolic response in the omega-3 fatty acid group was significantly greater than in the corn oil group (P = 0.01).
FIGURE 1.
Mean (±SEM) mixed skeletal muscle protein fractional synthesis rate (FSR), calculated by using the average plasma free phenylalanine enrichment as the precursor pool enrichment, during basal, postabsorptive conditions and during the hyperaminoacidemic-hyperinsulinemic clamp before and after 8 wk of supplementation with either corn oil (n = 7) or omega-3 fatty acids (n = 8). There was no difference in the muscle protein FSR between the omega-3 fatty acid and corn oil groups before the intervention [ANOVA showed a significant effect of clamp (P < 0.001), no significant effect of group (P = 0.47), and no interaction (P = 0.60)]. aIn the corn oil group, ANOVA showed a significant main effect of clamp (P < 0.01). In the omega-3 fatty acid group, ANOVA showed a significant effect of clamp (P < 0.01) and an interaction (P < 0.001), which was followed by Tukey's post hoc analysis. bSignificantly different from the corresponding basal value, P < 0.01. cSignificantly different from the corresponding value before omega-3 fatty acid supplementation, P < 0.01. Furthermore, the before-after intervention change in the anabolic response (increase in the muscle protein FSR from basal values) was significantly greater in the omega-3 fatty acid group than in the corn oil group (P = 0.01, Student's t test for independent samples).
Effect of intervention on the muscle protein synthesis rate (muscle free phenylalanine labeling as precursor pool enrichment)
Corn oil supplementation had no effect on either the basal muscle protein synthesis rate or the increase in the rate of muscle protein synthesis in response to amino acid and insulin infusion (change from basal values: 0.013 ± 0.005%/h before and 0.013 ± 0.007%/h after supplementation; P = 0.94). In contrast, omega-3 fatty acid supplementation had no effect on the basal rate of muscle protein synthesis but significantly increased the anabolic response to amino acid and insulin infusion (change from basal values: 0.031 ± 0.003% compared with 0.009 ± 0.005%/h; P = 0.004).
Phosphorylation of signaling transduction proteins in muscle
At baseline (before supplementation), no differences between the omega-3 fatty acid and corn oil groups were observed in the basal, postabsorptive concentrations of AktThr308, mTORSer2448, and p70s6kThr389 in muscle and the increase in AktThr308, mTORSer2448, and p70s6kThr389 phopshorylation in response to hyperaminoacidemia-hyperinsulinemia. For each of these, ANOVA showed a significant effect of clamp (P ≤ 0.01), no significant effect of group (P ≥ 0.45), and no interaction between the 2 (P ≥ 0.17).
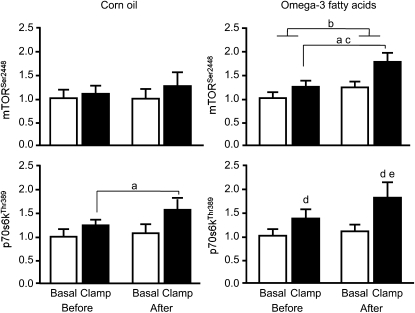
Neither corn oil nor omega-3 fatty acid supplementation affected the concentration of AktThr308 in muscle (P ≥ 0.28). The concentrations of AktThr308 during basal conditions and the clamp were as follows: 0.96 (0.51, 1.32) and 1.37 (1.27, 1.90) before and 0.94 (0.48, 1.74) and 1.41 (0.64, 1.78) after corn oil supplementation and 0.94 (0.62, 1.42) and 1.31 (1.09, 1.57) before and 0.96 (0.61, 1.27) and 1.46 (1.19, 2.07) after omega-3 fatty acid supplementation. Corn oil supplementation also had no effect on the concentrations of mTORSer2448 and p70s6kThr389 in muscle (Figure 2). In contrast, the concentrations of mTORSer2448 and p70s6kThr389 during the clamp were greater after than before omega-3 fatty acid supplementation (P < 0.05 for p70s6kThr389; P = 0.08 for mTORSer2448) (Figure 2), and the before-after changes in the insulin/amino acid–mediated increase in p70s6k and mTOR phosphorylation above basal values were greater in the omega-3 fatty acid group than in the corn oil group (P < 0.05 and P = 0.07, respectively).
FIGURE 2.
Mean (±SEM) concentrations (arbitrary units) of mTORSer2448 and p70s6kThr389 during basal, postabsorptive conditions and during the hyperaminoacidemic-hyperinsulinemic clamp before and after 8 wk of supplementation with either corn oil (n = 7) or omega-3 fatty acids (n = 8). aANOVA showed a significant main effect of clamp (P < 0.01). bANOVA showed a significant main effect of time (P < 0.05). cThere was a trend for a greater clamp-induced increase in mTORSer2448 after omega-3 fatty acid supplementation than before supplementation (interaction: P = 0.08). d,eANOVA showed a significant interaction (P < 0.05), which was followed by Tukey's post hoc analysis. dSignificantly different from corresponding basal value, P < 0.05. eSignificantly different from corresponding value before omega-3 fatty acid supplementation, P < 0.05. Furthermore, the before-after intervention changes in the insulin/amino acid–mediated increase in p70s6k and mTOR phosphorylation above basal values were greater in the omega-3 fatty acid group than in the corn oil group (P < 0.05 and P = 0.07, respectively; Mann-Whitney U test).
Whole-body glucose kinetics and phenylalanine rate of appearance
Whole-body glucose kinetics were not significantly different between the corn oil and omega-3 fatty acid groups at baseline and were not affected by either omega-3 fatty acid or corn oil supplementation (all P ≥ 0.3). Basal glucose Ra was 14.8 ± 1.0 μmol ⋅ kg FFM−1 ⋅ min−1 before and 15.0 ± 1.1 μmol ⋅ kg FFM−1 ⋅ min−1 after omega-3 fatty acid supplementation and 14.4 ± 1.2 μmol ⋅ kg FFM−1⋅ min−1 before and 14.5 ± 1.3 μmol ⋅ kg FFM−1 ⋅ min−1 after corn oil supplementation. During the clamp, endogenous glucose Ra decreased by 77 ± 5% before and 75 ± 5% after omega-3 fatty acid supplementation, and by 71 ± 6% before and 68 ± 4% after corn oil supplementation [no significant difference between groups (P = 0.34) and no significant effect of time (P = 0.44)]. Glucose Rd increased by 137 ± 24% before and 113 ± 13% after omega-3 fatty acid supplementation and by 113 ± 24% before and 109 ± 28% after corn oil supplementation [no significant difference between groups (P = 0.61) and no significant effect of time (P = 0.43)].
Whole-body phenylalanine Ra was also not different between the corn oil and omega-3 fatty acid groups at baseline and was not affected by either omega-3 fatty acid or corn oil supplementation (all P > 0.3). In the corn oil group, endogenous phenylalanine Ra was 41.3 ± 3.6 μmol/min before and 43.8 ± 4.0 μmol/min after supplementation during basal postabsorptive conditions and 24.6 ± 3.6 μmol/min before and 26.3 ± 4.3 μmol/min after supplementation during the clamp. ANOVA showed a main effect of clamp (P < 0.001), no effect of time (P = 0.31), and no clamp × time interaction (P = 0.57). In the omega-3 fatty acid group, the respective values were 42.1 ± 3.7 and 41.2 ± 2.9 μmol/min in the basal postabsorptive state and 30.8 ± 4.9 and 31.3 ± 4.7 μmol/min during the clamp. ANOVA showed a main effect of clamp (P = 0.005), no effect of time (P = 0.84), and no clamp × time interaction (P = 0.50).
DISCUSSION
The normal age-related loss of muscle mass is considered to be largely due to anabolic resistance (ie, an inadequate response to anabolic stimuli such as increased amino acid availability) (5–7). In the present study, we provide novel evidence that dietary omega-3 fatty acid supplementation augments the hyperaminoacidemia-hyperinsulinemia induced increase in the rate of muscle protein synthesis in older adults. Omega-3 fatty acids therefore probably attenuate the anabolic resistance and may potentially be useful as a therapeutic agent to treat sarcopenia.
The exact mechanisms by which omega-3 fatty acids act on the muscle protein synthesis process are not entirely clear, but our results indicate that it was at least partially mediated via increased activation of the mTOR-p70s6k signaling pathway. The mTOR-p70s6k signaling pathway is considered an integral control point for muscle cell growth (30–32), and Drummond et al recently showed that rapamycin, a potent inhibitor of mTOR, prevents the increase in the rate of muscle protein synthesis in response to feeding (33) and resistance exercise (34) in human subjects. Although we cannot determine the mechanism by which omega-3 fatty acids induced greater mTOR-p70s6k activation in response to increased amino acid and insulin supply, we can probably rule out Akt as the upstream signal because omega-3 fatty acid supplementation had no effect on Akt phosphorylation in our study.
It is thought that many of the well-known beneficial effects of omega-3 fatty acids (eg, reduction in plasma triglyceride concentration) are, at least partially, related to the antiinflammatory properties of omega-3 fatty acids (13, 35, 36). However, we were unable to discover significant differences in serum markers of inflammation and plasma triglyceride concentrations in response to omega-3 fatty acid supplementation, probably because we specifically selected healthy persons with low plasma concentrations of inflammatory markers and triglycerides for this study. Nevertheless, the fact that omega-3 fatty acids had anabolic effects in the absence of significant inflammatory activity or changes thereof indicates that the antiinflammatory properties of omega-3 fatty acids are likely not responsible for the muscle anabolic action.
The improvement in muscle protein metabolism was not accompanied by significant changes in whole-body glucose metabolism, which is thought to be responsive to omega-3 fatty acid intake (37–40). This is not entirely surprising, however. Although results from studies in animals indicate consistently that omega-3 fatty acids stimulate muscle glucose uptake (37–40), the results from studies in human subjects concerning the insulin-sensitizing effect of omega-3 fatty acids on glucose metabolism are less clear-cut, with some showing a positive effect, some no effect, and some an adverse effect (35, 41–43). In our study we consider it possible that increased activation of mTOR by omega-3 fatty acids during hyperaminoacidema-hyperinsulinemia may have prevented a potentially beneficial effect of omega-3 fatty acids on glucose uptake because there is some evidence for an amino acid induced decrease in insulin-mediated glucose transport via activation of the mTOR signaling pathway (44, 45).
In summary, we have shown that dietary omega-3 fatty acid, but not corn oil, supplementation increases muscle anabolic signaling activity and the insulin/amino acid–mediated increase in muscle protein synthesis above basal, postabsorptive values in older adults. Although the exact mechanisms by which omega-3 fatty acids stimulate muscle protein synthesis during hyperinsulinemia-hyperaminoacidemia remain to be resolved, our study provides compelling evidence of an interaction of omega-3 fatty acids and protein metabolism in human muscle and suggest that dietary omega-3 fatty acid supplementation could potentially provide a safe, simple, and low-cost intervention to combat sarcopenia.
Acknowledgments
We thank Hadia Jaffery, Rachel Burrows, and the staff of the Nutrition and Obesity Research Center and the Center for Applied Research Services for help in subject recruitment and technical assistance. We also thank the study subjects for their participation.
The authors’ responsibilities were as follows—GIS: involved in conducting the study, processing the study samples, collecting the data, performing the final data analyses, and writing the manuscript; PA and MJR: involved in processing the study samples, collecting the data, and writing the manuscript; DNR and BSM: involved in conducting the study; DR: involved in sample processing and sample analyses; and BM: involved in designing and conducting the study, processing the study samples, collecting the data, performing the final data analyses, and writing the manuscript. None of the authors had any potential conflicts of interest. No funding entity had any role in the design, implementation, analysis, or interpretation of the data.
REFERENCES
- 1.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr 2001;55:663–72 [DOI] [PubMed] [Google Scholar]
- 2.Ahmed N, Mandel R, Fain MJ. Frailty: an emerging geriatric syndrome. Am J Med 2007;120:748–53 [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52:80–5 [DOI] [PubMed] [Google Scholar]
- 4.Wolinsky FD, Callahan CM, Fitzgerald JF, Johnson RJ. The risk of nursing home placement and subsequent death among older adults. J Gerontol 1992;47:S173–82 [DOI] [PubMed] [Google Scholar]
- 5.Guillet C, Prod'homme M, Balage M, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J 2004;18:1586–7 [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–4 [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J 2006;20:768–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuthbertson DJ, Babraj J, Smith K, et al. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab 2006;290:E731–8 [DOI] [PubMed] [Google Scholar]
- 9.Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J 2001;15:475–82 [DOI] [PubMed] [Google Scholar]
- 10.Rieu I, Magne H, Savary-Auzeloux I, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 2009;587:5483–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gingras AA, White PJ, Chouinard PY, et al. Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol 2007;579:269–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander JW, Saito H, Trocki O, Ogle CK. The importance of lipid type in the diet after burn injury. Ann Surg 1986;204:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fetterman JW, Jr, Zdanowicz MM. Therapeutic potential of n−3 polyunsaturated fatty acids in disease. Am J Health Syst Pharm 2009;66:1169–79 [DOI] [PubMed] [Google Scholar]
- 14.Hayashi N, Tashiro T, Yamamori H, et al. Effect of intravenous omega-6 and omega-3 fat emulsions on nitrogen retention and protein kinetics in burned rats. Nutrition 1999;15:135–9 [DOI] [PubMed] [Google Scholar]
- 15.Wan JM, Istfan NW, Chu CC, Blackburn GL, Bistrian BR. Comparative effects of omega-3 and omega-6 polyunsaturated fatty acids on protein metabolism in rats bearing the mammary adenocarcinoma. Metabolism 1991;40:577–84 [DOI] [PubMed] [Google Scholar]
- 16.Smith HJ, Greenberg NA, Tisdale MJ. Effect of eicosapentaenoic acid, protein and amino acids on protein synthesis and degradation in skeletal muscle of cachectic mice. Br J Cancer 2004;91:408–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirschberg Y, Pomposelli JJ, Blackburn GL, Istfan NW, Babayan V, Bistrian BR. The effects of chronic fish oil feeding in rats on protein catabolism induced by recombinant mediators. Metabolism 1990;39:397–402 [DOI] [PubMed] [Google Scholar]
- 18.Sohal PS, Baracos VE, Clandinin MT. Dietary omega 3 fatty acid alters prostaglandin synthesis, glucose transport and protein turnover in skeletal muscle of healthy and diabetic rats. Biochem J 1992;286:405–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr 2007;86:451–6 [DOI] [PubMed] [Google Scholar]
- 20.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 1997;94:14930–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol 2009;106:1374–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 2004;66:799–828 [DOI] [PubMed] [Google Scholar]
- 23.Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab 2001;281:E1333–9 [DOI] [PubMed] [Google Scholar]
- 24.Rieu I, Balage M, Sornet C, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol 2006;575:305–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith GI, Villareal DT, Mittendorfer B. Measurement of human mixed muscle protein fractional synthesis rate depends on the choice of amino acid tracer. Am J Physiol Endocrinol Metab 2007;293:E666–71 [DOI] [PubMed] [Google Scholar]
- 26.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism 1997;46:943–8 [DOI] [PubMed] [Google Scholar]
- 27.Smith GI, Atherton P, Reeds DN, et al. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol 2009;107:1308–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith GI, Atherton P, Villareal DT, et al. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65-80 year old men and women. PLoS One 2008;3:e1875–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watt PW, Lindsay Y, Scrimgeour CM, et al. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: use in studies of human tissue protein synthesis. Proc Natl Acad Sci USA 1991;88:5892–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 1999;276:C120–7 [DOI] [PubMed] [Google Scholar]
- 31.O'Neil TK, Duffy LR, Frey JW, Hornberger TA. The role of phosphoinositide 3-kinase and phosphatidic acid in the regulation of mammalian target of rapamycin following eccentric contractions. J Physiol 2009;587:3691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 2001;3:1014–9 [DOI] [PubMed] [Google Scholar]
- 33.Dickinson JM, Drummond MJ, Fry CS, et al. Effect of rapamycin administration in humans on the skeletal muscle protein anabolic response to essential amino acid ingestion. FASEB J 2010; 24:740–3119858092 [Google Scholar]
- 34.Drummond MJ, Fry CS, Glynn EL, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 2009;587:1535–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Browning LM. n−3 Polyunsaturated fatty acids, inflammation and obesity-related disease. Proc Nutr Soc 2003;62:447–53 [DOI] [PubMed] [Google Scholar]
- 36.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep 2004;6:461–7 [DOI] [PubMed] [Google Scholar]
- 37.Liu S, Baracos VE, Quinney HA, Clandinin MT. Dietary omega-3 and polyunsaturated fatty acids modify fatty acyl composition and insulin binding in skeletal-muscle sarcolemma. Biochem J 1994;299:831–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science 1987;237:885–8 [DOI] [PubMed] [Google Scholar]
- 39.Borkman M, Chisholm DJ, Furler SM, et al. Effects of fish oil supplementation on glucose and lipid metabolism in NIDDM. Diabetes 1989;38:1314–9 [DOI] [PubMed] [Google Scholar]
- 40.Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J. N−3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab 2002;282:E664–71 [DOI] [PubMed] [Google Scholar]
- 41.Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n−3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J Nutr Biochem 2006;17:1–13 [DOI] [PubMed] [Google Scholar]
- 42.Carpentier YA, Portois L, Malaisse WJ. n−3 Fatty acids and the metabolic syndrome. Am J Clin Nutr 2006;83(suppl):1499S–504S [DOI] [PubMed] [Google Scholar]
- 43.Fedor D, Kelley DS. Prevention of insulin resistance by n−3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care 2009;12:138–46 [DOI] [PubMed] [Google Scholar]
- 44.Krebs M, Brunmair B, Brehm A, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Diabetes 2007;56:1600–7 [DOI] [PubMed] [Google Scholar]
- 45.Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. A negative feedback mechanism leading to insulin resistance in skeletal muscle cells. J Biol Chem 2001;276:38052–60 [DOI] [PubMed] [Google Scholar]
- 46.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]